 World Journal of Nano Science and Engineering, 2011, 1, 27-36 doi:10.4236/wjnse.2011.12005 Published Online June 2011 (http://www.SciRP.org/journal/wjnse) Copyright © 2011 SciRes. WJNSE Synthesis and Characterization of Soy P rotein Isolate/MMT Nanocomposite Film for the Control Release of th e Dru g Ofloxacin Preetishree Nayak, Sanjib Kumar Sahoo, Anamika Behera, Prativa Kumari Nanda, P. L. Nayak, B. C. Guru P. L. Nayak Research Foundation, Neelachal Bhavan, Odisha, India E-mail: plnayak@rediffmail.com Received March 2, 2011; revised March 24, 2011; ac ce pt e d A pr il 7, 2011 Abstract Nanocomposites were prepared by blending soy protein isolate with different percentage of MMT by melt extrusion technique. The nanocomposites were characterized by using, XRD, TEM, SEM a nd TGA methods. The XRD studies indicated the absence of diffraction peaks for the bio-nanocomposites. From the TEM stu- dies it was ascertained that the degree of exfoliation increased with increase in MMT content. The mor- phology of the nanocomposites was ascertained from the SEM studies. The degradation pattern of the nano- composites was evaluated from the TG analysis. The drug delivery system of the nanocmposites was invest- tigated by blending the nanocomposites with ofloxacin at different pH media. The various kinetic parameters were evaluated and the mechanism of drug delivery has been postulated based on the kinetic data. Keywords: Nanocomposites, Soy Protein, MMT, Drug Delivery, Ofloxacin 1. Introduction Carrier-mediated drug delivery has emerged as a power- ful methodology for the treatment of various pathologies. The therapeutic index of traditional and novel drugs is enhanced via the increase of specificity due to targeting of drugs to a particular tissue, cell or intracellular com- partment, the control over release kinetics, the protection of the active agent or a combination of the above [1]. Polymer composites were proposed as drug carriers over 30 years ago and have received growing attention since, mainly due to their stability, enhanced loading capabili- ties and control over physicochemical properties [2,3]. In addition to systemic administration, localized drug re- lease may be achieved using macroscopic drug depots close to the target site. In recent years, biodegradable polymers have attracted attention of researchers to be used as carriers for drug delivery systems [4-6]. Drug delivery plays an important role in the develop- ment of pharmaceutical dosage forms for the healthcare industry because often the duration of the drug release needs to be extended over a period of time [7]. This can be achieved by the incorporation of drugs into polymeric materials to control drug release at a pre-defined and reproducible rate for a prolonged duration. The majority of the drug delivery systems are fabricated from non- degradable polymers such as silicone, polyurethane and ethylene vinyl acetate copolymers, which are inexpen- sive, not biocompatible, and biologically inert and have received regulatory approval [8]. In recent years, the interest for biodegradable polymers as drug delivery systems, which control and prolong the action of thera- peutic agents, has attracted attention of researchers [9]. The reason being that delivery systems based on biode- gradable polymers do not require removal from the pa- tients at the end of the treatment period due to their deg- radation into physiologically occurring compounds that can be readily absorbed and further excreted from the body. This provides significant benefits such as reduce- tion of patient stress, no second surgery and reduction in cost in terms of ti me spent by the end-user s [10-12] .The most important biodegradable polymers which have been used for controlled drug delivery are chitosan, soy pro- tein, gelatin, sod iu m alginate, P LA, PC L, pol yanhydrides and polyorthoesters [13,14]. Soy protein isolate (SPI) is an abundant, inexpensive and renewable natural material. It is composed of almost exclusively of two globular protein fractions differenti-  P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE ated by sedimentation coefficient: 7S (b-conglycinin) and 11S (glycinin) [15]. Both fractions have the ability to form films by a two-step process. During the preheating step, proteins are unfolded and polymerized into soluble aggregates, followed by a cooling step and subsequent surface dehydration, which results in the formation of a film network through disulfide cross-linking and hydro- phobic bonds [16,17]. In general, protein fi lms a re effect- tive lipid, oxygen, and aroma barriers at low to inte rme- diate relative humidity (RH). The water exclusion prop- erties of SPI films are relatively poor due to the hydro- philic nature of soy proteins and to substantial amounts of added hygroscopic plasticizers [18,19]. Numerous studies have concentrated on improving the mechanical and water-excluding properties of soy protein-based films through physical, chemical and enzymatic treat- ments or compositing with hydrophobic materials in or- der to develop alternative resources for bio-plastics in packaging applications [20,21]. However, no investiga- tion of SPI film as a controlled d elivery system has been reported Recently, a new class of materials represented by bio-nanocomposites (biopolymer matrix including pro- teins reinforced with nanoparticles such as montmorillo- nite) has proven to be the promising option in improving mechanical and barrier properties of biopolymers [22-26]. The bio-nanocomposites consist of a biopolymer matrix reinforced with particles (nanoparticles) having at least one dimension in the nanometer range (1 - 100 nm) and exhibit much improved properties due to high aspect ratio and high surface area of nanoparticles [27-29]. T he most common class of materials used as nanoparticles are layered clay minerals such as montmorillonite (MMT), hectorite, sapnotite, and laponite. These clay minerals have been proven to be very effective due to their unique structure and properties. These clay minerals belong to the general family of 2:1 192 layered silicates indicating that they have 2 tetrahedral sheets sandwich- ing a central octahedral sheet [30]. MMT has a very high elastic modulus (178 GPa) as compared to most bio- po- lymer s. The hi gh value of e lastic modul us enable s MMT to improve mechanical properties of biopolymers by carrying a significant portion of the applied stress [31] There are four possible arrangements of layered clays dispersed in a polymer matrix – phase separated or im- miscible (microcomposite), intercalated, exfoliated, and disordered intercalated (partially exfoliated). In an im- miscible arrangement, platelets of layered clays exist as tactoids (stack o f platelets) and the po lymer encapsulates these tactoids. Intercalation occurs when a monolayer of extended polymer chains penetrates into the galleries (gap between layers of clay) of the layered silicates. In- tercalation results in finite expansion (2 - 3 nm) of the silicate layers. However, these silicate layers remain par- allel to each other. In the pre sent rese arc h progr am, na noco mposi tes were prepared by blending soy protein isolate with different percentage of MMT by melt extrusion technique. The nanocomposites were characterized by using SEM, TEM and XRD methods. The drug delivery system of the na- nocmposites were investigated by blending the nano- composites with ofloxacin at different pH media. The various kinetic parameters were evaluated and the me- chanism of drug delivery has been postulated based on the kinetic data. 2. Materials and Methods 2.1. Materials Soy protein isolate (Supro 760) with a protein content of 92.5% (dry basis) was obtained from Protein Technolo- gies International (St. Louis, MO). Two types of modi- fied montmorillonites (Cloisite 20A and Cloisite 30B) were obtained from Southern Clay Products (Austin, TX). 2.2. Preparation of SPI-MMT Nanocomposites The formulation consisted of SPI (70% - 85%, dry basis), glycerol (15%, dry basis), and MMT (0% - 15%, dry basis). All three types of clays (Cloisite Na+, Cloisite 20A, and Cloisi te 30B) were used at fo ur different levels (0, 5, 10, and 15%). The ingredients were mixed and left at room temperature for 2 hours for hydration. The mix- ture was subsequently extr uded in a twinscrew co-rotating extruder (ZSK 26, Coperion Corp., Ramsey, NJ). The extruder had screw diameter of 25 mm and length to di- ameter ratio (L/D) of 20. The extruder was operated at a screw speed of 100 rpm. The extruder had a 5 head bar- rel configuration. Temperatures in the 5 head barrel were maintained at 60˚C, 90˚C, 100˚C, 110˚C, and 90˚C re- spectively. The extrudate was dried in an oven at 50˚C for 48 hrs and grounded for use. 2.3. Film Casting Bio-nanocomposite powders (4% w/v) and deionized water were mixed for 30 min at room temperature. pH of the suspension was adjusted to 9 by adding 1 M NaOH. The suspensio n was heate d to 95 ˚C and held at that tem- perature for 20 min with continuous stirring. Subse- quently, the solution was cooled to 65˚C and 25 ml of the suspension was poured in 10 cm diameter petri dishes for casting nanocomposite films. The cast petri dishes were dried at ambient conditions for 48 hours. The dried films  P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE were peeled off the petri dish and pre-conditioned before further testing. 2.4. Structural Characterization of SPI-MMT Film s 2.4.1. X-Ray Diffraction (XRD) X-ray diffraction studies of nanocomposite powders were performed with a diffrac tion unit (MS Philips XLF ATPS XRD 100, Omni Scientific Instruments, Biloxi, MS) operating at 35 kV and 25 mA. The radiation was generated from a Cu-Kα source with a wavelength (λ) of 0.154 nm. The diffraction data was collected from 2θ values of 2.5 to 10˚ with a step size of 0.01˚, where θ is the angle of incidence of the X-ray beam on the sample. 2.4.2. Transmission Electron Microscopy (TEM ) The structure and morphology of nanocomposite pow- ders were visualized by a transmission electron micro- scope (Hitachi HF2000, Hitachi High-Technologies Eu- rope GmbH, Krefeld, Germany) operating at 200 kV. Samples of nanocomposite powders were prepared by suspending the powders in methanol. The suspension was sonicated for 5 min in an ultrasonic bath (Branson 1510, Branson Ultrasonics Co., Danbury, CT). A drop of the suspension was put on a fine-mesh carbon-coated TEM support grid (C-flatTM, Protochips Inc., Raleigh, NC). After drying in air, the nanocomposite powder re- mained attached to the grid and was viewed under the transmission electron microscop e . 2.4.3. Scanning Electron Microscopy (SEM) The morphology of the fracture surface (cross-sectional surface) of the nanocomposite films were visualized us- ing a field emission scanning electron microscope (JEOL 6400F, Japan Electron Optics Ltd., Tokyo, Japan) oper- ating at 5 kV. Small pieces (0.5 × 0.5 cm) of bio nano- composite films were frozen in liquid nitrogen, cut using a sharp razor blade, and mounted on specimen stubs with 2 sided carbon tape. The fracture surfaces of the films were sputter-coated with a thin layer (~8 - 10 nm) of gold-palladium (Au-Pd) using a sputter-coater (Hummer II, Anatech Ltd., Union City, CA). After coating, the samples were viewed under the scanning electron mi- croscope. 2.5. Thermal Stabil ity The thermal stability of nanocomposite films were inves- tigate d u sing a the rmogravi metric analyzer (Pyris 1 TGA, Perkin Elmer, Shelton, CT). The temperature of the sam- ple was increased from room temperature to 900˚C at a heating rate of 20˚C/mi n. Wei ght loss of the sa mple was measured as a function of temperature. Three parameters were determined from the TGA data: the temperature at 10% weight loss, the temperature at 50% weight loss, and the yield of charred residue at 850˚C. 2.6. Drug Loading Requi red a mount o f SPI/MMT was taken in 5 ml of ace- tic acid. The mixture was continuously stirred with a mechanical stirrer. Ofloxacin of different loadings, i.e., 10, 20, 30, 40 and 50 wt% were then added to the above mixture and stirred for 1 h and then the composites were kept at room temperature for drying. 3. Results and Discussion 3.1. XRD XRD patterns (Cloisite 20A and Cloisite 30 B (0%, 5%, 10%, and 15%) of bionanocomposite powders are shown in Figure 1. Powders of Cloisite 20A showed a diffract- tion peak at a 2θ angle of 3.56˚. Interlayer distance (d or d-spacing) between clay layers can be estimated from Bragg’s e quatio n as s hown belo w. π 2sin 180 d λ θ = where λ is t he wavelen gth of X-ray beam. The d-spacing of Cloisite 20A corresponding to the diffraction peak was calculated to be 2.48 nm. This is in close a greemen t with t he d-spacing value of 2.42 nm provided by the sup- plier. XRD patterns of Cloisite 3 0B and SPI -Cloisite 30B (0%, 5%, 10%, and 15%) nanocomposite powders are sho wn in Figure 2. T he d -spacing of Cloisite 30B corre- sponding to the diffraction peak at a 2θ angle of 5 .0˚ was calculated to be 1.77 nm. There was no diffraction peak in the 2θ range of 2.5˚ to 10˚ for the nanocomposites at all MMT contents of Cloisite 20A and Clois ite 30B. Ab- sence of diffraction peaks for SPI-MMT bio-nanocom- posit es su gge sts tha t t he layers o f MM T s ha ve a d -spacing of at least 3.53 nm (corresponding to a 2θ value o f 2.5˚) in all the bio-nanocomposites. 3.2. TEM TEM images of SPI-MMT nanocomposite powders with 5% and 15% contents of Cloisite 20A and Cloisite 30B are shown in Figure 3. The dark lines in the TEM im- ages correspond to MMT platelets and the gap between two adjacent lines is the d-spacing. It can be seen from Figures 3(a) and 3(c) that the MMT layers are exfoliated in nanocomposites with MMT content of 5%. At MMT 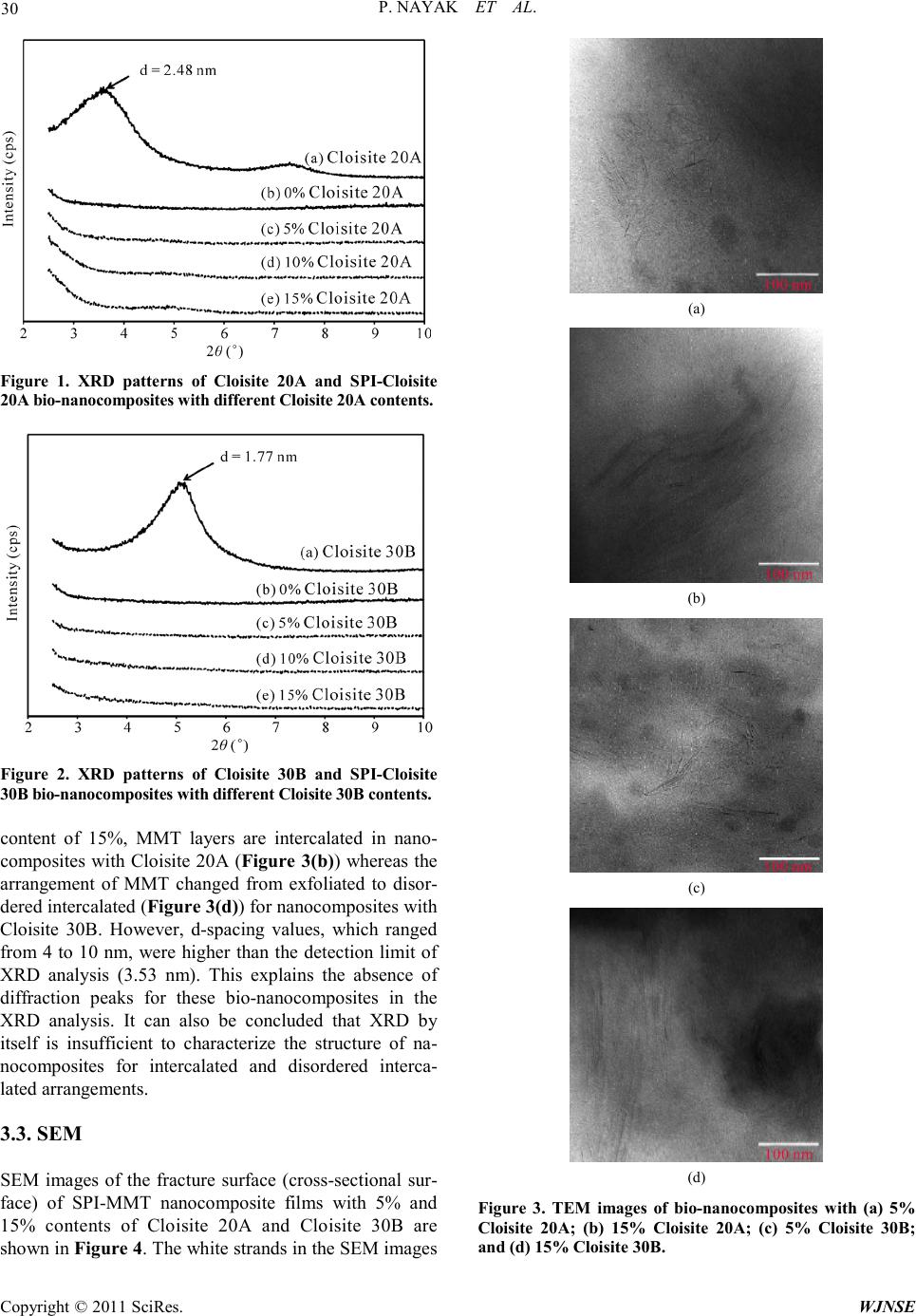 P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE Figure 1. XRD patterns of Cloisite 20A and SPI-Cloisite 20A bi o -nanocom pos ite s with differe nt Cl o isi te 20A contents. Figure 2. XRD patterns of Cloisite 30B and SPI-Cloisite 30B bio-nanocomposites with different Cloisite 30B content s. content of 15%, MMT layers are intercalated in nano- composites with Cloisite 20A (Figure 3(b)) whereas the arrangement of MMT changed from exfoliated to disor- dered intercalated (Figure 3(d)) for na noco mposites with Cloisite 30B. However, d-spacing values, which ranged from 4 to 10 nm, were higher than the detection limit of XRD analysis (3.53 nm). This explains the absence of diffraction peaks for these bio-nanocomposites in the XRD analysis. It can also be concluded that XRD by itself is insufficient to characterize the structure of na- nocomposites for intercalated and disordered interca- lated arrangements. 3.3. SEM SEM images of the fracture surface (cross-sectional sur- face) of SPI-MMT nanocomposite films with 5% and 15% contents of Cloisite 20A and Cloisite 30B are sho wn in Figure 4 . T he whit e str a nds in the SE M i ma ges (a) (b) (c) (d) Figure 3. TEM images of bio-nanocomposites with (a) 5% Cloisite 20A; (b) 15% Cloisite 20A; (c) 5% Cloisite 30B; and (d) 15% Cloisite 30B. 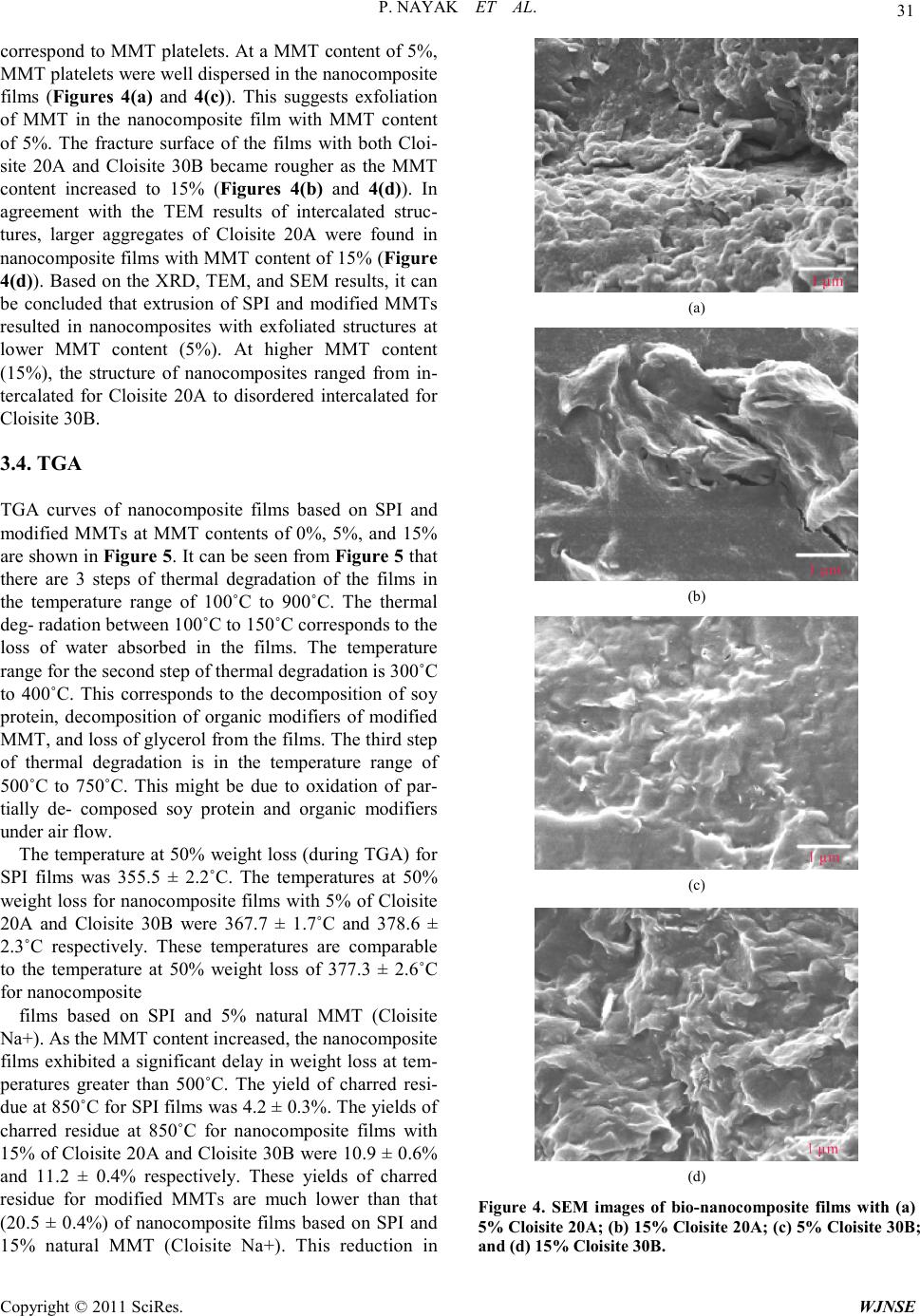 P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE correspond to MMT platelets. At a MMT content of 5%, MMT platelets were well dispersed in the nanocomposite films (Figures 4(a) and 4(c)). This suggests exfoliation of MMT in the nanocomposite film with MMT content of 5%. The fracture surface of the films with both Cloi- site 20A and Cloisite 30B became rougher as the MMT content increased to 15% (Figures 4(b) and 4(d)). In agreement with the TEM results of intercalated struc- tures, larger aggregates of Cloisite 20A were found in nanocomposite films with MMT content of 15% (Figure 4(d)). Based on the XRD, TEM, and SEM results, it can be concluded that extrusion of SPI and modified MMTs resulted in nanocomposites with exfoliated structures at lower MMT content (5%). At higher MMT content (15%), the structure of nanocomposites ranged from in- tercalated for Cloisite 20A to disordered intercalated for Cloisite 30B . 3.4. TGA TGA curves of nanocomposite films based on SPI and modified MMTs at MMT contents of 0%, 5%, and 15% are shown in Figure 5. It can be seen from Figure 5 that there are 3 steps of thermal degradation of the films in the temperature range of 100˚C to 900˚C. The thermal deg- radation between 100˚C to 150˚C corresponds to the loss of water absorbed in the films. The temperature range for the second step of thermal degradation is 300˚C to 400˚C. This corresponds to the decomposition of soy protein, decomposition of organic modifiers of modified MMT, and loss of glycerol from the films. The third step of thermal degradation is in the temperature range of 500˚C to 750˚C. This might be due to oxidation of par- tially de- composed soy protein and organic modifiers under air fl ow. The temperature at 50% weight loss (during TGA) for SPI films was 355.5 ± 2.2˚C. The temperatures at 50% weight loss for nanocomposite films wit h 5% of Cloisite 20A and Cloisite 30B were 367.7 ± 1.7˚C and 378.6 ± 2.3˚C respectively. These temperatures are comparable to the temperature at 50% weight loss of 377.3 ± 2.6˚C for nanocomposite films based on SPI and 5% natural MMT (Cloisite Na+). As the MMT content increased, the nanocomposite films exhibited a significant delay in weight loss at tem- peratures greater than 500˚C. The yield of charred resi- due a t 850 ˚C for SPI films was 4.2 ± 0.3%. The yields of charred residue at 850˚C for nanocomposite films with 15% of Cloisite 20A and Cloisite 30B were 10.9 ± 0.6% and 11.2 ± 0.4% respectively. These yields of charred residue for modified MMTs are much lower than that (20.5 ± 0.4%) of nanocomposite films based on SPI and 15% natural MMT (Cloisite Na+). This reduction in (a) (b) (c) (d) Figure 4. SEM images of bio-nanocomposite films with (a) 5% Cloisite 20A; (b) 15% Cloisite 20A; (c) 5% Cloisite 30B ; and (d) 15% Cloisite 30B. 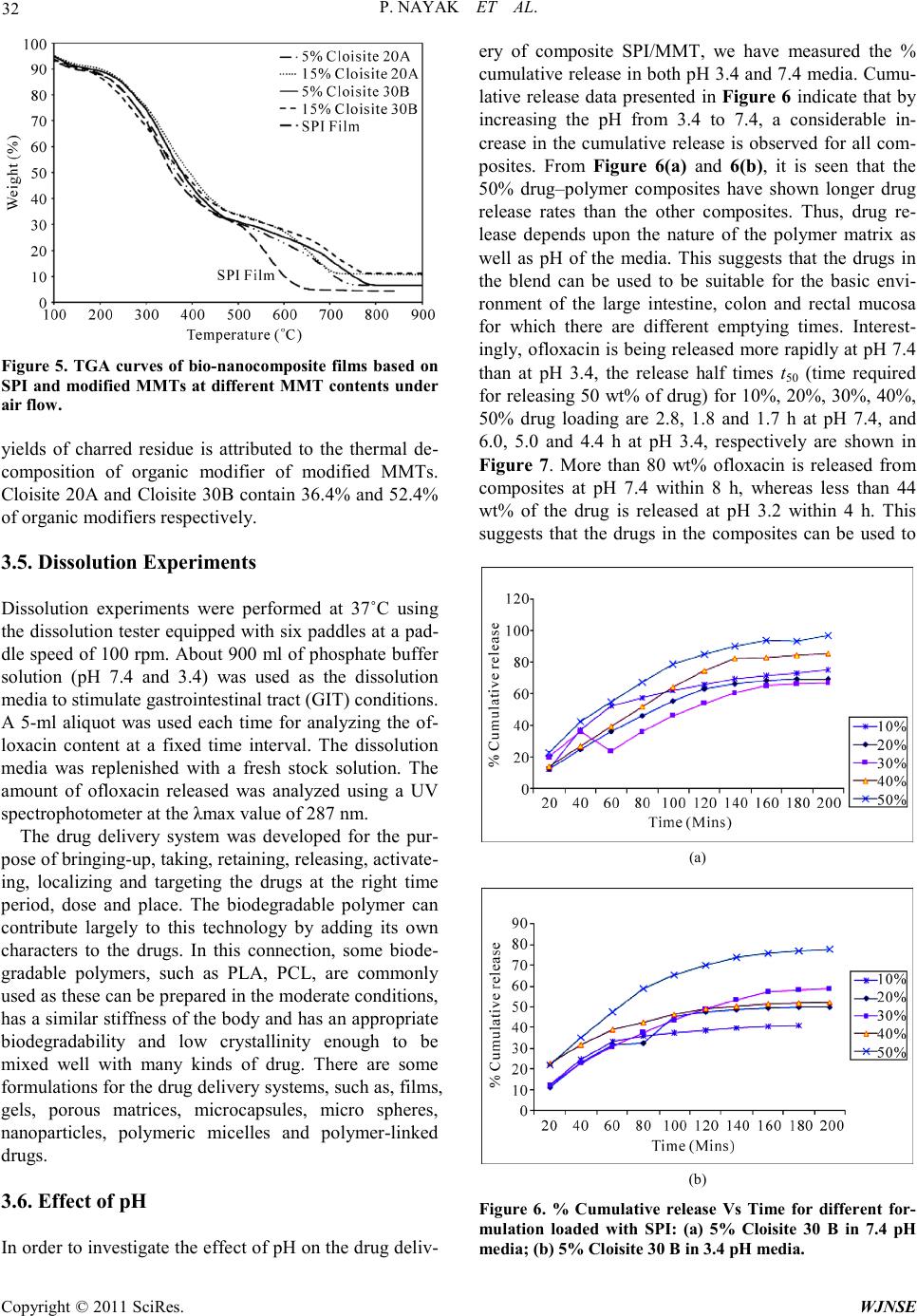 P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE Figure 5. TGA curves of bio-nanocomposite films based on SPI and modifie d MMTs at different MMT contents under air flow. yields of charred residue is attributed to the thermal de- composition of organic modifier of modified MMTs. Cloisite 20A and Cloisite 30B contain 36.4% and 52.4% of organic modifiers respectively. 3.5. Dissolution Experiments Dissolution experiments were performed at 37˚C using the dissolution tester e quipped with six padd les at a pad- dle speed of 100 rpm. About 900 ml of phosphate buffer solution (pH 7.4 and 3.4) was used as the dissolution media to stimula te gastrointestinal tract (GIT ) conditions. A 5-ml aliquot was used each time for analyzing the of- loxacin content at a fixed time interval. The dissolution media was replenished with a fresh stock solution. The amount of ofloxacin released was analyzed using a UV spectrophotometer at the λmax value of 287 nm. The drug delivery system was developed for the pur- pos e o f bri ngi ng -up, ta kin g, re ta ini ng, re le as i ng, activate- ing, localizing and targeting the drugs at the right time period, dose and place. The biodegradable polymer can contribute largely to this technology by adding its own characters to the drugs. In this connection, some biode- gradable polymers, such as PLA, PCL, are commonly used as these can be prepared in the moderate conditions, has a similar stiffness of the body and has an appropriate biodegradability and low crystallinity enough to be mixed well with many kinds of drug. There are some formulations for the drug deliver y syste ms, such as, fil ms, gels, porous matrices, microcapsules, micro spheres, nanoparticles, polymeric micelles and polymer-linked drugs. 3.6. Effect of pH In or der to i nvesti gate the ef fec t of p H on t he d ru g deliv- ery of composite SPI/MMT, we have measured the % cumulative release in both pH 3.4 and 7.4 media. Cumu- lative release data presented in Figure 6 indicate that by increasing the pH from 3.4 to 7.4, a considerable in- crease in the cumulative release is observed for all com- posites. From Figure 6(a) and 6(b), it is seen that the 50% drug–polymer composites have shown longer drug release rates than the other composites. Thus, drug re- lease depends upon the nature of the polymer matrix as well as pH of the media. This suggests that the drugs in the blend can be used to be suitable for the basic envi- ronment of the large intestine, colon and rectal mucosa for which there are different emptying times. Interest- ingl y, o floxaci n is being released more rapidly at pH 7.4 than at pH 3.4, the release half times t50 (time required for releasing 50 wt% of drug) for 10%, 20%, 30%, 40%, 50% drug loading are 2.8, 1.8 and 1.7 h at pH 7.4, and 6.0, 5.0 and 4.4 h at pH 3.4, respectively are shown in Figure 7. More than 80 wt% ofloxacin is released from composites at pH 7.4 within 8 h, whereas less than 44 wt% of the drug is released at pH 3.2 within 4 h. This suggests that the drugs in the composites can be used to (a) (b) Figure 6. % Cumulative release Vs Time for different for- mulation loaded with SPI: (a) 5% Cloisite 30 B in 7.4 pH me dia; (b) 5% Cloisite 30 B in 3.4 pH media . 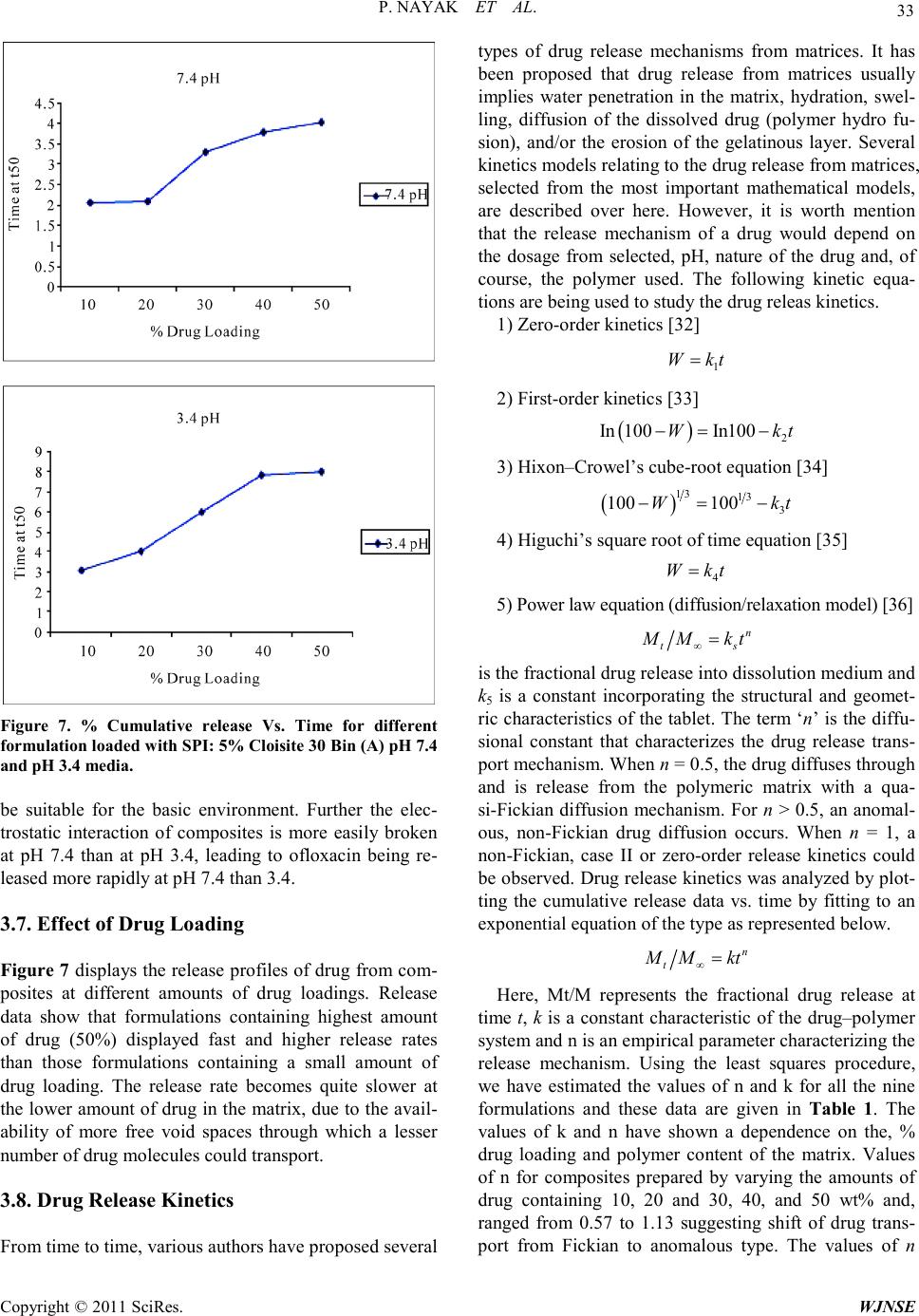 P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE Figure 7. % Cumulative release Vs. Time for different formulat i on l oa de d wit h SPI: 5% Cloisite 30 B in (A) pH 7.4 and p H 3.4 media. be suitable for the basic environment. Further the elec- trostatic interaction of composites is more easily broken at pH 7.4 than at pH 3.4, leading to ofloxacin being re- leased more rapidly at pH 7.4 than 3.4. 3.7. Effect of Drug Loading Figure 7 displays the release profiles of drug from com- posites at different amounts of drug loadings. Release data show that formulations containing highest amount of drug (50%) displayed fast and higher release rates than those formulations containing a small amount of drug loading. The release rate becomes quite slower at the lower a mount of drug in the ma trix, due to the a vail- ability of more free void spaces through which a lesser number of drug molecules could transport. 3.8. Drug Release Kinetics From time to time, various authors have proposed several types of drug release mechanisms from matrices. It has been proposed that drug release from matrices usually implies water penetration in the matrix, hydration, swel- ling, diffusion of the dissolved drug (polymer hydro fu- sion), and/or the erosion of the gelatinous layer. Several kinetics mod e ls relatin g to the drug release fro m matrice s, selected from the most important mathematical models, are described over here. However, it is worth mention that the release mechanism of a drug would depend on the dosage from selected, pH, nature of the drug and, of course, the polymer used. The following kinetic equa- tions are being used to study the drug releas kinetics. 1) Zero-order kinetics [32] 1 W kt= 2) First-order kinetics [33] 3) Hixon–Crowel’s cube-root equation [34] 4) Higuchi’s square root of time equa t ion [35] 5) Power law equ ati on (diff us ion / relaxa ti on mode l ) [36] is the fractional dr ug rele ase i nto dissolution mediu m and k5 is a constant incorporating the structural and geomet- ric characteristics of the tablet. The term ‘n’ is the diff u- sional constant that characterizes the drug release trans- por t mec ha ni s m. W he n n = 0.5, the drug diffuses through and is release from the polymeric matrix with a qua- si-Fickian diffusion mechanism. For n > 0.5, an anomal- ous, non-Fickian drug diffusion occurs. When n = 1, a non-Fickian, case II or zero-order release kinetics could be observed. Drug release kinetics was analyzed by plot- ting the cumulative release data vs. time by fitting to an expone ntial equation of the type as represented below. Here, Mt/M represents the fractional drug release at time t, k is a constant characteristic of the drug–polymer system and n is an empirical parameter characterizing the release mechanism. Using the least squares procedure, we have estimated the values of n and k for all the nine formulations and these data are given in Table 1. The values of k and n have shown a dependence on the, % drug loading and polymer content of the matrix. Values of n for composites prepared by varying the amounts of drug containing 10, 20 and 30, 40, and 50 wt% and, ranged from 0.57 to 1.13 suggesting shift of drug trans- port from Fickian to anomalous type. The values of n  P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE Table 1 . Kinetic parameters of different formulations at pH 7. 4 and pH 3.4. Sample Code(%) k n co-ordination coefficien t, R 7.4 (pH) 10 0.10 0.57 0.9872 20 0.17 0.63 0.9843 30 0.24 0.85 0.9675 40 0.26 1.07 0.9863 50 0.28 1.12 0.9765 3.4 (pH) 10 0.12 0.54 0.9785 20 0.17 0.87 0.9872 30 0.23 0.98 0.9832 40 0.25 1.03 0.9876 50 0.22 1.13 0.9865 more tha n 1 has also been recently reported This may be due to a reduction in the regions of low micro viscosity inside the matrix and closure of microcavities d uring t he swollen state of the polymer. Similar findings have been found elsewhere, wherein the effect of different polymer ratios on dissolutio n kinetics was investigated [37-39]. 4. Conclusions The last two decades of the twentieth century saw a pa- radigm shift from biostable biomaterials to biode-grada- ble (hydrolytically and enzymatically degradable) bio- materials for medical and related applications The cur- rent trend predicts tha t in the next co uple of years in the twenty first century, many of the permanent prosthetic devices used for temporary therapeutic applications will be replaced by biodegradable devices that could help the body to repair and regenerate the damaged tissues. Soy protein isolate (SPI) is a natural biodegrade- able, bio- compatible and nontoxic polymer. The blending of SPI with MMT has the advantage of enhancing some of the important properties of the base polymer. The nanocom- posites have been characterized by using XRD, TEM, SEM and TGA methods to ascertain the exact characte- ristic properties of the composite materials. The control drug d e livery application of t he nanoco mpiosite has been investigated by blending it with ofloxacin and the drug delivery kinetics has been monitored by usin g the kinet ic equations at two different PH media. The drug release is faster at pH 7.4 than at pH 3.4. The various kinetic pa- rameters have been computed a nd bas ed on the va lues o f the kinetic parameters such as k and n values the me- chanism of drug diffusion from the nanocomposite ma- trix has been postulated. From the computed k and n values it is concluded that the drug release takes the anomalous pa th rather than Fi ckian path. 5. Referen ces [1] S. N. Swain, K. K. Rao and P. L. Nayak, “Biodegradable Polymers: IV. Spectral, The rmal, and Mechanical Pro- perties of Cross-Linked Soy Protein Concentrate,” Poly- mer international, 20 05 , Vol . 54, No. , 2005, pp. 739. [2] P. K. Nanda, K. K. Rao and P. L. Nayak, “Spectral, Thermal, Morphological, 548 and Biodegradability Prop- erties of Environment-Friendly Green Plastics of Soy Protein Modified with Thiosemicarbazide,” Journal of Applied Polymer Science, Vol. 103, No. 5, March 2007, pp. 3134-3142. doi:10.1002/app.24590 [3] P. K. Nanda, K. K. Rao and P. L. Nayak, “Thermal De- gradation Analysis of 550 Biodegradable plastics from Urea-Modified Soy Protein Isolate,” Polymer-Plastics Technology and Engineering, Vol. 46, No. , 2007, pp. 207. doi:10.1080/03602550601152713 [4] C. P. Path ak, A. S. Sawhney and J. A. R. Hubbell, “Photo Polymerization of Immuno Protective Gels in Contact with Cells and Tissue,” Journal of American Ch emical Society, Vol. 114, No. 21, October 1992, pp. 8311-8312. doi:10.1021/ja00047a065 [5] G. Lambert, E. Fatale and P. Couvreur, “Nanoparticulate Systems for the Delivery of Antisense Oligonucleotides,” Advanced Drug Delivery Review s, Vol. 47, No. 1, March 2001, pp . 99-112. doi:10.1016/S0169-409X(00)00116-2 [6] K. M cAllister, P. Sanzan i, M. Adam and M. J. Rubi nstein, “Polymeric Nanogels Produced via Inverse Microemul- sion Polymerization as Po tential Gene and Antisen se De- livery Agents,” Journal of American Chemical Society, Vol. 124, No. 51, 2002, pp. 15198-15207. doi:10.1021/ja027759q [7] P. Legrand and G. Barratt, “Polymeric Nanocapsules as Drug Delivery System ,” STP Pharma Sciences, Vol. 9, No. , 1991, pp. 411-418. [8] J Jagur-Grodzinski, “Biomedical Application of Func- tional Polymers,” Reactive and Functional Polymers, Vol. 39, No. 2, February 1999, pp . 99-138. doi:10.1016/S1381-5148(98)00054-6 [9] S Ramakri sh na and J. Maye r , “Biomedical Application of Polymer Composite Materials,” Composites Science and Technology, Vol. 61, No. 9, July 2001, pp . 1189-1224. doi:10.1016/S0266-3538(00)00241-4 [10] D. Allen, Maysinger and A. Eisenberg, “Nano-Engi- neering Block Co-Polymers Aggregates for Drug Deli- very,” Colloids and Surfaces B: Biointerfaces, Vol. 16, No. 1-4, November 1999, pp. 3-27. doi:10.1016/S0927-7765(99)00058-2 [11] H. Maeda, T Sawa and T. K onno, “M echanism of Tumor Targeted Delivery Macromolecular Drugs including the EPR Effect in Solid Tumor and Clinical Overview of the  P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE Prototyoe Polymeric Drugs,” Journal of Controlled Re- lease, Vol. 74, No. 1-3, July 2001, pp. 47-61. doi:10.1016/S0168-3659(01)00309-1 [12] V. B. Pokharkar and S. Sivaram, “Permeabilty Studies across Poly (alkylene carbon ate) Membranes,” Journal of Controlled Release, V ol. 41, No. 3, September 1996, pp. 157-162. doi:10.1016/0168-3659(96)01325-9 [13] C. J. Goodwin, M. Braden, S. Downes and N. J. Marshall, “Release of Bioactive Human Growth Hormone from a Biodegradable Material Poly(epsilon-caprolactone) ,” Journal of Biomedical Materials Research, Vol. 40, No. 2, May 1998, pp. 204-13. doi:10.1002/(SICI)1097-4636(199805)40:2<204::AID-JB M5>3.0.CO;2-P [14] C. G. Pitt, M. M. Gratzl, A. R. Jeffcoat, R. Zweidinger and A. Schindler, “Sustained Drug Delivery System II: Factor s Affectin g Release Rat e f ro m P oly(e caprolacto ne) , and Related B iodegrad able Polyesters,” Journal of Phar- maceut ical Sciences, Vol. 68, No. 12, December 1979, pp. 1534-1538. doi:10.1002/jps.2600681219 [15] E. Tomlinson and J. J. Burger, “Incorporation of Water Soluble Drugs in Albumin Microspheres,” In: J. Widder and R. Green, Eds., Methods in Enzymology, Academic Press , New York, 1985, pp. 27-43. [16] A. Gennadios and C. L. Weller, “Edible Films and Coat- ings from Soymilk and Soy Protein,” Cereal Foods World, Vol. 36, No. , 1991, pp. 1004-1009. [17] M. Subirade, I. Kelly, J. Gueguen and M. Pezolet, “Mo- lecular Basis of Film Formation from a Soybean P rotein: Comparison between the Conformation of Glycinin in Aqueous Solution and in Films,” International Journal of Biological Macromolecules, Vo l. 23, No. 4, November 1998, pp . 241-249. doi:10.1016/S0141-8130(98)00052-X [18] A. Gennadios, T. H. McHugh, C. L. Weller and J. M. Krochta, “Edible Coatings and F ilms Based on Proteins,” In: J. M. Krochta, E. A. Baldwin and M. Nisperos-Car- riedo, Eds., Edible Coatings and Films to Improve Food Quality, Technomic Publishing Company, Lancaster, 1994. pp. 201-277. [19] J. M. Krochta and C. D. De Mulder-Johnston, “Edible and Biodegradable Polymer Films: Challenges and Op- portunities,” Food Technology, Vol. 51, No. , 1997, pp. 61-74. [20] A. H. Brandenburg, C. L. Weller, R. F. Testin, “Edible Films and Coatings from Soy Protein,” Journal of Food Scien ce, Vol . 58, No. 5, September 1993, pp. 1086-1089. doi:10.1111/j.1365-2621.1993.tb06120.x [21] A. Gennadios, V. M. Ghorpade, C. L. Weller and M. A. Hanna, “Increase in Water Vapor Barrier Properties of Biopolymer-Based Edible Films and Coatings by Com- positing Lipid Materials,” Food Science and Biotechnol- ogy, Vol. 13, No. , 1996, pp. 528-535. [22] J. W. Rhim, A. Gennadios, C. L. Well er, C. Cezeirat and M. A. Hanna, “Soy Protein Isolatedialdehyde Starch Films,” Industrial Crops and Products, Vol. 8, No. 3, September 1998, pp. 195-203. doi:10.1016/S0926-6690(98)00003-X [23] P. L. Nayak and J. Macromol, “Biodegradable Polymer: Opportunities and Challenges,” Sci.Rev.Ma cromol. Chem. Phys., Vo l. 39, No. , 199 9, pp. 481-505. [24] P. L. Nayak and J. Macromol, “Natural Oil Based Poly- mers: Opportunities and Challenges,” Sci.Rev.Macromol. Chem. Phys., Vol. 40, No. 1, 2000, pp. 1-21. [25] S. N. Swai n, S. M. Biswal, P. K. Nanda and P. L. N a ya k , “Biodegradable So y-Based Plastics: Opportunities and Challenges,” Journal of Polymers and the Environment, Vol. 12, No. 1, 2004, pp. 35-42. doi:10.1023/B:JOOE.0000003126.14448.04 [26] S. N. Swain, K. K. Rao and P. L. Nayak, “Biodegradable Polymers. III. Spectral, Thermal, Mechanical, and Mor- phological Properties of Cross-Linked Furfural–Soy P ro- tein Concentrate,” Journal of Thermal Analysis and Ca- lorimetry, Vo l . 93, No. , 2004, pp. 2590. [27] S. S. Ray and M. Bousmina, “Biodegradable Polymers and Their Layered Silicate Nanocomposites: In Greening the 21st Century Material s World,” Progress in Materials Scien ce, Vol . 50, No. 8, November 2005, pp. 962-1079. doi:10.1016/j.pmatsci.2005.05.002 [28] J. W. Rhim and P. K. W. Ng, “Natural Biopolymer-Based Nanocomposite Films for Packaging Applications,” Crit- ical Reviews in Food S cience and Nutrition, Vo l . 47, No. 4, 2007, pp. 411-433. doi:10.1080/10408390600846366 [29] R. Zhao, P. Torl ey and P. J. Halley, “Emerging Biode- gradable Materials: Star ch- and Proteinbased Bio-Nano- composites,” Journal of Materials Science, V ol. 43, No. , 2008, pp . 3058-3071. doi:10.1007/s10853-007-2434-8 [30] T. D. Fornes and D. R. Paul, 2003. “Modeling Properties of Nylon 6/Clay Nanocomposites Using Composite Theories,” Polymer, Vol. 44, No. 17, August 2003, pp. 4993-5013. doi:10.1016/S0032-3861(03)00471-3 [31] H. R. Dennis, D. L. Hunter, D. Chang, S. Kim, J. L. White, J. W. Cho an d D. R. Paul, “Effect of Melt Processing Conditions on the Extent of Exfoliation in Organoclay-Based Nanocomposites,” Polymer , Vol. 42, No. 23, November 2001, pp. 9513-9522. doi:10.1016/S0032-3861(01)00473-6 [32] J. W. Rhim, Y. Wu, C. L. Weller and M. Schnepf, “Physical Characteristics of a Composite Films of Soy Protein Isolate and Propyleneglycol Alginate,” Journal of Food Science, Vol. 64, No. 1, 1999, pp. 149-152. doi:10.1111/j.1365-2621.1999.tb09880.x [33] K. Dean an d L. Yu, “Biodegradable Protein-Nanopar- ticles Composites,” In: R. Smith, Ed ., Biodegradable Po- lymers for Industrial Applications, Woodhead Publishing Ltd ., UK, 2005, pp. 289-312. doi:10.1533/9781845690762.2.289 [34] A. K. Singla and D. K. Medirata, “Influence of sodium Lauryl Sulfate on Indomethacin Release Patterns,” Drug Development and Industrial Pharmacy, Vol. 14, No. , 1988, pp . 1883-1888. doi:10.3109/03639048809151994 [35] T. Higuchi, “Mechanism of Rate of Sustained -Action Medication,” Journal of Pharmaceutical Sciences, Vol. 52, No. 12, December 1963, pp. 1145-1149. doi:10.1002/jps.2600521210 [36] A. R. Kulkarni, K. S. Soppimath and T. M. Aminabhavi,  P. NAYAK ET AL. Copyright © 2011 SciRes. WJNSE “Controlled Release of Diclofenac Sodium from Sodium Alginate Beads Crosslinked with Glutaraldehyde,” Phra- maceut ica Acta Hel vita e, Vol. 74, No. 1, December 1999, pp. 29-36. doi:10.1016/S0031-6865(99)00015-1 [37] T. M. Aminabhavi and H. G. Naik, “Chemical Compati- bility Study of Geomembranes-Sorption/Desorption, Diffusion and Swelling Phenomena,” Journal of Ha- zardous Materials, Vol. 60, No. 2, June 1998, pp. 175- 203. doi:10.1016/S0304-3894(98)00090-9 [38] S. P. Lyu , R. Sparer, C. Hobot and K. Dang, “Adjusting Drug Diffusivity Using Miscible Polymer Blends,” Jour- nal of Controlled Release, Vol. 102, No. 3, February 2005, pp . 679-687. doi:10.1016/j.jconrel.2004.11.007 [39] R. L Ritger and N. A. Pep pas, “A Simple Equation for Disposition of So lute Rel ease—II,” Journal of Controlled Release, Vol. 5, No. 1, June 1987, pp. 37-42. doi:10.1016/0168-3659(87)90035-6
|