 American Journal of Plant Sciences, 2011, 2, 190-201 doi:10.4236/ajps.2011.22021 Published Online June 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice Akkareddy Srividhya1#, Lakshminarayana R. Vemireddy1*,#, Puram Venkata Ramanarao1, Sakile Sridhar1, Mudduluru Jayaprada1, Ghanta Anuradha1, Battiprolu Srilakshmi1, Hariprasad K. Reddy2, Arramsetty Subramanyam Hariprasad3, Ebrahimali Abubackar Siddiq1 1Institute of Biotechnology, Acharya NG Ranga Agricultural University, Rajendranagar, Hyderabad, India; 2Department of Genetics and Plant Breeding S. V. Agricultural College, Tirupati, India; 3Directorate of Rice Research, Rajendranagar, Hyderabad, India. Email: *vlnreddi@gmail.com Received February 15th, 2011; revised March 11th, 2011; accepted March 18th, 2011. ABSTRACT Differential response of seedling characteristics under water stress conditions is known to be associated with drought resistance in rice and elucidation of its genetics could b e of help in breeding for tolerance to the stress. A recombinant inbred population derived from the cross between a semi-dwarf variety IR64 and landrace INRC10192 was grown in hydroponic culture and phenotyped for varied responses of seedlings to water deficit imposed by poly ethylene glycol (PEG). The ratio between mean value of seedling trait under stress and control conditions was used for assessing drought tolerance. In all 19 putative QTLs relating to five seedling traits viz., shoo t length, maximum root length, shoot dry weight, root dry weight and root to shoot dry weight ratio under PEG induced stress conditio ns were id entified con - firms that the traditional tall landraces as the one chosen for the study posses hitherto unexploited drought tolerant genes and utiliza tion o f them as poten tial don ors in br eeding fo r yield en han cement wou ld be rewarding. They might be useful for improving drought resistance of rice by marker assisted selection/breeding. Keywords: Ory z a Sat iva L., QTL Mapping, RIL Population, Seedling Morphology, PEG, Hydroponic Culture 1. Introduction Over one half of the world’s rice area is rainfed and breeding for drought resistance has been the research priority in these areas [1,2]. Many morpho-physiological traits of seedlings have been reported to influence the performance of the crop under rainfed upland condition. Root and shoot systems, for instance, affect growth and development of seedlings. In rice, several root character- istics viz., root length, depth of rooting, root thickness and root to shoot ratio are considered to play an impor- tant role in resisting water deficit. Earlier studies showed varieties with longer, thicker and bigger ro ot systems are to be drought resistant and associated, in some cases, with higher grain yield under drought [1,3,4]. As re- ported by O’Toole (1982) [5] greater rooting depth and density could result in more available soil water and hence better resistance to moisture stress. Molecular marker technology has now been found to facilitate better understanding of the genetic basis of the indices of tolerance, most of which are quantitatively inherited. Quantitative trait loci (QTL) approach has helped to identify QTL for the stress tolerance related traits including length, weight and thickness of root and shoot height, leaf rolling and osmotic adjustment under hydroponics [6-8] and other artificially created stress conditions [7,9-16]. The root-related characters have recently been used in breeding for tolerance to the stress [17,18] using marker assisted selection. Although all such investigation enriched our under- standing of plant response to water deficit, genetic mecha- nisms underlying the expression of drought resistance are poorly unders tood because of incons istency in the choice of testing environ ments and cu ltiv ars, timing and sev erity of the stress imposed and difficulties in measuring root-related traits. In field grown experiments, screening for responses of root-related traits to the stress is not easy besides being time-consuming and imprecise. The Poly ethylene glycol (PEG ) of higher molecular weight (4000 - #These authors contributed equally to this work.  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice191 8000) is quite commonly used in hydroponics as an al- ternative for judging the performance of plants against water stress under field conditions [19]. Generally high molecular weight PEG absorbed by the plants is accu- mulated in roots and leaves results in osmotic adju stment besides imposing water-de ficit conditions [16]. Although water stress induced by the PEG-6000 develops faster in creating an osmotic shock, the responses of plants to such osmotic treatment is ind icative of the relative po ten- tial of the different rice varieties to tolerate water stress at physiological and biochemical level [20]. Reports of Yoshida and Hasegawa (1982) [21] show response of root related traits viz., overall size and maximum depth of the system and individual root thickness in plants grown under field and hydroponic condition to be positively related to the level of drought resistance. Price et al. (1997) [3] too confirmed that root-related traits of rice grown hydroponically viz., maximum root length and adventi- tious root thickness were related to resistance to water- deficit in the field. Therefore, hydroponic culture has been employed to study root related traits in the present study. As of now, most reported drought-related QTL map- ping experiments in rice has employed only five popula- tions [7]. Many of the upland parents used in these populations (e.g., CT9993-5-10-1-M and Azucena) are not considered to be highly drought-tolerant in terms of grain yield under severe drought conditions. Many tradi- tional and improved cultivars from drought-prone areas on the other hand are known to be tolerant to the stress [22]. But they too have rarely been used as parents in mapping studies. Recent studies at IRRI and elsewhere suggest the possibilities of d etecting stress tolerance QTL in parental sources not known to be tolerant [23,24]. Keeping the foregoing, the present study was initiated with the objective of detecting hidden drought response QTLs in mapping population derived from the cross in- volving the primitive landrace INRC10192 which is not known to possess drought tolerance and IR64 as the par- ents. The mapping population consisting of 140 recom- binant inbred lines derived from the cross was used to assess the response of the lines and parents to water defi- cit induced by PEG and map genetic regions associated with seedling traits. 2. Materials and Methods 2.1. Choice of Parents and Development of Mapping Population The popular semi-dwarf high yielding variety IR64 was used as female parent while INRC10192 a tall, lodging prone, photosensitive, medium duration and long root landrace accession from the Assam Rice Collection (ARC), India as male parent. Recombinant inbred lines (RILs) comprising 140 lines derived from the cross by selfing and advancing to F7 generation at the Biotech- nology Unit (BTU), ARI, Acharya N G Ranga Agricul- tural University (ANGRAU), Hyderabad, India by Single Seed Descent method f ormed the mapping pop ula ti on . 2. 2 . Phen otypic Evaluation Approximately 150 seeds each of the 140 RILs along with parents and IR20 and Moroberekan as drought sus- ceptible and drought tolerant checks respectively, were sown on moistened filter paper in petri dishes (5 cm di- ameter and 2 cm depth) and kept in an incubator main- tained at 30˚C for 48 h. The germinated seeds were re- moved from the incubator and kept under room tempera- ture (27˚C ± 1˚C) for three days. The seedlings were al- lowed then to grow in Yoshida’s nutrient solution [25] under greenhouse conditions for three days. The seed- lings of each of the lines were evenly distributed in four petri dishes, two of which for water deficit (stress) treat- ment and two for control. The random complete block design (RCBD) with two rep lications was followed. Wa- ter deficit was created by using half-strength Yoshida’s nutrient solution containing 15% PEG-6000 (W/W) with osmotic potential (OP) of –2.36 to –2.95 bars at 25˚C - 30˚C, while the control was continued with normal nu- trient solution. Five days later, OP level of the stress treatment was increased to –4.04/–4.91 bars by replacing 15% with 20% PEG-6000 to the nutrient solution and maintained for two weeks. Though this is considered to be the critical concentration for early seedling stage screening of rice for drought tolerance [16,26,27], the seedlings were subjected to still higher stress level the range being –6.15/–7.35 bars (PEG-6000 at 25% of nu- trient solution) for four days. The nutrient solution was replenished once a week in control; while for keeping the water potential stable, the solution containing PEG was changed on alternate days in the stress treatment. The pH of both the treatments was adjusted to 5.0 by adding 1 mol/l HCl or NaOH solution ever y 24 hours. Response to the treatment was observed 29 days after sowing (DAS). Immediately after removing from nutrient solution, the seedlings were thoroughly washed with distilled water and blotted with tissue paper to remove excess water before observing for root and shoot length (Figure 1). The same root and shoot samples were used for measur- ing root and shoot dry weights, after drying them at 70˚C for 48 h [23,28]. For study of secondary traits related to drought tolerance, observations were made on six seed- lings per replication and averaged them under control (C) and stress (S) conditions and of their relative parameters (R) (Ratio of the parameter under stress to the control). Standard procedures were followed for recording the observations as follows: shoot length (SL)—length (cm) Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice 192 Figure 1. Root and shoot pictures of parents (INRC10192 and IR64) and control (Azucena and MTU1061). The age of the seedlings was 18 days. from node/collar region to the tip of the shoot, maximum root length (MRL)—length (cm) of the seminal root (Seminal root is the longest root at early seedling stage), shoot dry weight (SDW)—weight (mg) of shoot after oven-drying at 70˚C for 48 h and for 24 h, root dry weight (RDW)—weight (mg) of root after oven-drying at 70˚C for 48 h and root shoot weight ratio (RS)—ratio of root dry weight to the shoot dry w e ight. 2.3. Construction of Linkage Map and QTL Mapping DNA was isolated from fresh leaf samples of tagged plants using the modified CTAB method [29]. The PCR was performed with 10 µl final volume containing 25 - 50 ng of genomic DNA, 10X buffer, 0.125 mM final concentration of each dNTPs, 0.2 µM of each forward and reverse primer and 1U of Biogene Taq DNA Poly- merase. The PCR was set up with an initial denaturation of 94˚C for 5 min, followed by 35 cycles of denaturation at 94˚C for 45 secs, annealing at 55˚C for 45 secs, exten- sion at 72˚C for 1 min, followed by the final extension of 72˚C for 10 min. PCR samples were run on a 3% agarose gel containing ethidium bromide along with the marker 50 bp ladder (MBI Fermentas, Canada) at 5.3 V/cm (Bio‐Rad Power Pack 300) for an hour in 0.5x Tris ‐Ace- tic acid‐EDTA (TAE) buffer. The resolved PCR bands were documented using Bio‐Rad Molecular Imager Gel Doc XR System. Linkage map was constructed using MAPMAKER/EXP v.3. QTLs were identified using Simple Interval Mapping (SIM) and Composite Interval Mapping (CIM) methods of QTL Cartographer 2.5 [30] (Wang et al., 2007). The significant threshold was esti- mated by performing 1000 permutations of each meas- urement (p < 0.05) using QTL cartographer [31]. 3. Results 3.1. Phenotypic Performance Under both control and stress conditions, the parents, IR64 and INRC10192 manifested significant differences in respect of all the traits studied, except RDW in the control (Table 1). Significant differences in RDW and RS were however observed between the two treatments in the case of both the parents. In respect of SDW and MRL, INRC10192 only showed significant differences under the stress. Relative parameters measured under stress revealed significant differences between parents for MRL(R), RDW(R) and RS(R). INRC10192 show ed higher mean values than IR 64 for all the traits studied (Supl Table 1). Study of RILs under controlled conditions revealed transgressive segregants ranging from 48.5% (MRL) to 93.3% (RS), while under stress it ranged from 49.4% (RDW) to 99.8% (RS). The relative parameters showed the range to be between 69.4% (RDW) and 92.2% (RS). All the traits showed continuous variation fitting well normal distribution ex- cept relative SDW and RDW (Supl Table 1, Figure 2). Those traits which are not distributed normally were subjected to transformation using skew-normal interval mapping method developed by Fernandez et al. (2007) [32]. Correlation coefficients revealed that maximum root length (MRL) under control had significant positive cor- relation with shoot length and shoo t dry weig ht (Tab le 2 ), while under stress it had highly significant positive cor- relation with both SL and SDW. In contrast, MRL showed significant negative correlation with RDW and RS under stress. Root dry weight under control, had strong significant positive asso ciation with SL and SDW, but under stress, it had significant negative association with SL, MRL and SDW. As expected, RS had strong Table 1. Test of significance of the parents for shoot and root traits and of their relative parameters measured in control and stress treatments. IR IN IR/IN IR/IN Rel. par. Trait C/S C/S C S S SL 0.19 0.55 0.02 0.01 0.37 MRL 0.73 0.02 0.01 0.05 0.03 SDW 0.06 0.03 0.04 0.01 0.22 RDW 0.05 0.01 0.20 0.01 0.02 RS 0.09 0.01 0.04 0.001 0.01 C-Control; S-Stress; Rel. par. = relative parameter. Note: Values in bold are ignificant probabil ity levels, IR-IR64; IN-INRC10 1 92. s Copyright © 2011 SciRes. AJPS 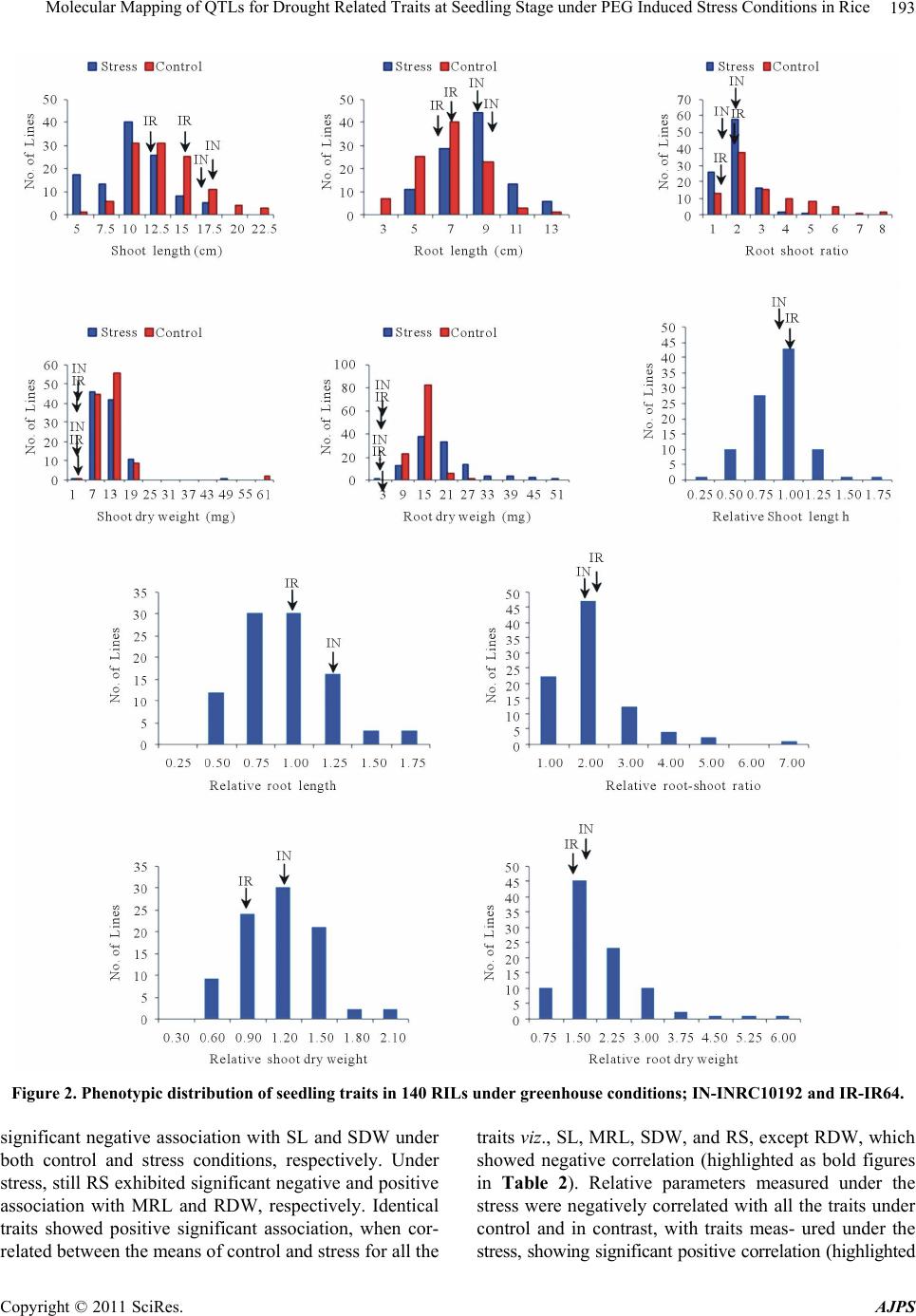 Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice Copyright © 2011 SciRes. AJPS 193 Figure 2. Phenotypic distribution of seedling traits in 140 RILs under greenhouse conditions; IN-INRC10192 and IR-IR64. significant negative association with SL and SDW under both control and stress conditions, respectively. Under stress, still RS exhibited significant negative and positiv e association with MRL and RDW, respectively. Identical traits showed positive significant association, when cor- related between the means of control and stress for all the traits viz., SL, MRL, SDW, and RS, except RDW, which showed negative correlation (highlighted as bold figures in Table 2). Relative parameters measured under the stress were negatively correlated with all the traits under control and in contrast, with traits meas- ured under the stress, showing significant positive correlation (highlighted  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice 194 as bold figures in Table 2). 3.2. Construction of Linkage Map and QTL Mapping Of 412 rice microsatellite primers used to screen the parents, 113 found to be polymorphic and they are dis- tributed throughout the rice genome. The linkage map covered 1978.9 cM employing Kosambi mapping func- tion, resulting in an averag e marker interval of 31.41 cm. The linear order of microsatellite markers reported in the present genetic map is not in total agreement with the physical map. Such observed discrepancies in the map distances are most likely to occur on account of different parental strains, number of markers, differences in the size and type of mapping populations and levels of polymorphism. Nevertheless, for exploratory mapping, resolution and genome coverage of the present linkage map may be adequate at least for some of the chromosomes to detect QTLs relating to drought related traits. A total of 19 pu- tative QTLs associated with the five seedling traits were detected (Figure 3). Chromosomes 1 and 8 harbor most of the QTLs. Phenotypic variance of the detected QTLs ranged from 7.9% to 29.8%. For most of the QTLs the increasing effect was contributed by the landrace INRC10192 (68.4 %). A single QTL designated as qsl1.1 on chromosome 1was identified for sho ot length under control con dition s. It was located in the marker interval of RM1-RM495 with a LOD score of 4.24, explai ning 18. 4% of phenotypic variance. The allele effect was contributed by INRC10192. Two QTLs of minor effect qmrl3.1 and qmrl3.2 influ- encing maximum root length on chromosome 3 were identified only under control condition. They had same LOD value of 2.52 and explained comparable phenotypic variance 11.74% and 11.51% QTL. The increased allele effect of these QTLs was contributed by INRC10192. For shoot dry weight, in all four QTLs were identified, of which three viz., qsdw2.1, qsdw2.2 and qsdw9.1 were identified on chromosomes 2 and 9 under stress condi- tions. The LOD score and phenotypic variance explained by individual QTLs ranged from 2.51 to 3.03, and 18.7% to 23.7%, respectively. One QTLs, qsdw1.1 identified on chromosome 1 in the marker interval of RM1-RM495 with a LOD score of 3.48 explained a phenotypic vari- ance of 13.69%. INRC10192 had allele effect for qsdw1.1, qsdw2.1 and qsdw2.2, while IR64 contributed at qsdw9.1 locus. Five QTLs for root dry weight were identified on three chromosomes under the stress condition and for relative parameter. Four QTLs, (qrdw1.1, qrdw1.2, qrdw8.1 and qrdw8.2) mapped two each on chromosomes 1 and 8, explained phenotypic variation ranging from 7.9 to 25.9% and LOD score in the range of 2.74 - 6.33. Three QTLs (qrdw1.1, qrdw7.1 and qrdw8.2) were identified for relative root dry weight with LOD score of 3.89 - 5.79 and phenotypic variation explaining 13.62% - 29.29%. Of these, two qrdw1.1 and qrdw8.2 were identi- fied simultaneously for root dry weight under the stress and for relative root dry weight measured under the stress. IR64 contributed positive allele effect for qrdw1.1 and qrdw1.2; while, for the remaining three QTLs, INRC10192 contributed favorable allele effect. Seven QTLs were found associated with root shoot r a- tio. Of them, five namely qrs1.1, qrs1.2, qrs2.1, qrs8.1 and qrs8.2 were detected under the stress condition, while qrs1.2 and qrs8.2 were found as well for relative root shoot ratio measured under the stress condition. Further, qrs1.3 and qrs7.1 were also found for relative RS under the stress. Besides, under the stress, the QTL qrs8.1 was as well detected under control condition with main effect. All these QTLs showed LOD score in the Table 2. Correlation coefficients between the traits under control (C) and stress (S) conditions and of the relative parameters (R) (* = p < 0.05; **= p < 0.01). Copyright © 2011 SciRes. AJPS 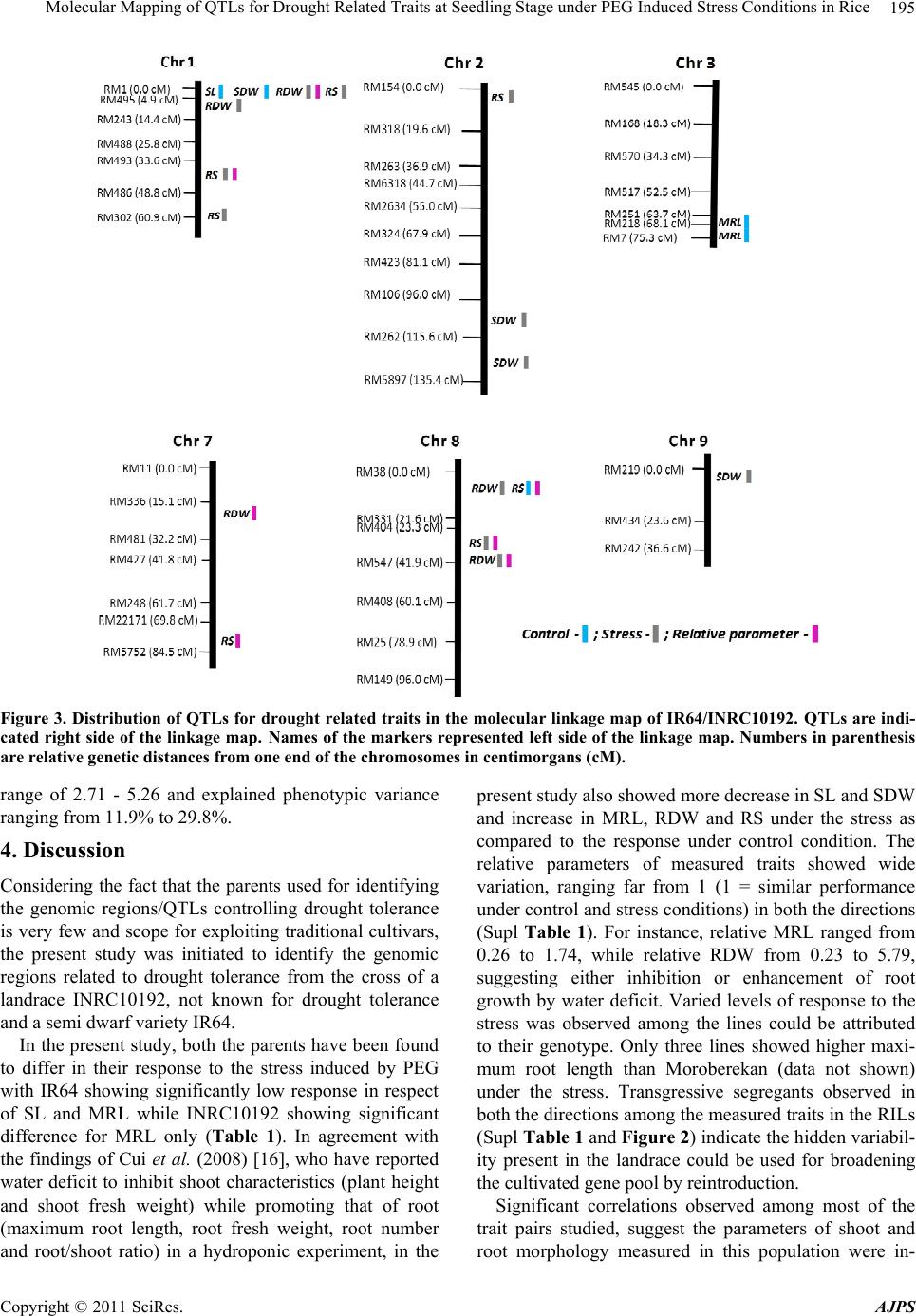 Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice195 Figure 3. Distribution of QTLs for drought related traits in the molecular linkage map of IR64/INRC10192. QTLs are indi- cated right side of the linkage map. Names of the markers represented left side of the linkage map. Numbers in parenthesis are relative genetic distances from one end of the chromosomes in centimorgans (cM). range of 2.71 - 5.26 and explained phenotypic variance ranging from 11.9% to 29.8%. 4. Discussion Considering the fact that the parents used for identifying the genomic regions/QTLs controlling drought tolerance is very few and scope for exploiting traditional cultivars, the present study was initiated to identify the genomic regions related to drought tolerance from the cross of a landrace INRC10192, not known for drought tolerance and a semi dwarf variety IR64. In the pr esent study, both the parents h ave been found to differ in their response to the stress induced by PEG with IR64 showing significantly low response in respect of SL and MRL while INRC10192 showing significant difference for MRL only (Table 1). In agreement with the findings of Cui et al. (2008) [16], who have reported water deficit to inhibit shoot characteristics (plant height and shoot fresh weight) while promoting that of root (maximum root length, root fresh weight, root number and root/shoot ratio) in a hydroponic experiment, in the present study also showed more decrease in SL and SDW and increase in MRL, RDW and RS under the stress as compared to the response under control condition. The relative parameters of measured traits showed wide variation, ranging far from 1 (1 = similar performance under control and stress conditions) in both the directions (Supl Table 1). For instance, relative MRL ranged from 0.26 to 1.74, while relative RDW from 0.23 to 5.79, suggesting either inhibition or enhancement of root growth by water deficit. Varied levels of response to the stress was observed among the lines could be attributed to their genotype. Only three lines showed higher maxi- mum root length than Moroberekan (data not shown) under the stress. Transgressive segregants observed in both the directions among the measured traits in the RILs (Supl Table 1 and Figure 2) indicate the hidden variabil- ity present in the landrace could be used for broadening the cultivated gen e poo l by reintroduction. Significant correlations observed among most of the trait pairs studied, suggest the parameters of shoot and root morphology measured in this population were in- Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice 196 ter-related. SL, MRL, and SDW showing positive and significant correlation with each other under control and stress conditions indicate indirect selection of one by the other trait possible. These results are in conformity with the earlier findings [10,33,34]. Whereas under control condition, MRL was positively correlated with RDW, while under the stress it showed negative correlation with RDW. These results are in agreement with those of Asch et al. (2005) [33], on the basis of “quantification of the effects of different levels of drought stress on dry matter partitioning and root development in rice”. They con- cluded assimilate partitioning between root and shoot was affected under low moisture stress conditions but not to affect under severe stress by drastically decreasing the partitioning to the root as in the present study. Thus MRL showed negative correlation with RDW under stress. Shoot length and shoot dry weight show significant positive association with many of the traits and selection based on these two could greatly help simultaneous se- lection of other traits governing drought tolerance. Since the root trait MRL has been found to be significant and positively correlated with SL and SDW under both con- trol and stress conditions selection based on the shoot traits would enable selection of genotypes with MRL, without taking the trouble of observing the roots as re- ported by Mane et al. (2003) and Zheng et al. (2003) [14,35]. Relative parameters used for different indices or drought tolerance under stress showed significant nega- tive associations with their respective characters under control, indicating that the lines that performed under control need not to do so under the stress. On the con- trary relative parameters measured under the stress had strong positive and significant correlation with traits measured. The region RM1-RM495 on chromosome 1 found in the present study to harbor five QTLs for shoot and root related traits which has been reported by many earlier workers. For instance, Li et al. (2005) [13] have repo rted QTLs for root thickness, root number and root dry weight, while length of mesocotyl by Redona and Mack- ill (1996) [36] in the same region. Further, Xu et al. (2004) [37] and Price et al. (2000) [38] have reported QTLs for PH and root number, respectively in the same region. Cui et al. (2008) [16] have identified a QTLs cluster in the same region for plant height and shoot fresh weight under well watered conditions. Interestingly, in the present study also under well-watered conditions, QTLs for shoot length and shoot dry weight have been mapped to the same region. Thus, this region appears to be a good candidate for breeding for drought tolerance through MAS as well as for fine mapping and positional cloning of the un derlying ge ne(s). Two QTLs for shoot dry weigh t under stress condition have been located at the interval RM106-RM5897 on chromosome 2. In a study by Cui et al. (2008) [16] the region near RM262 on the same chromosome has been detected to harbor QTL for shoot fresh weight. However, Xu et al. (2001) [39] have detected a QTL for root weight in the same region. The vicinity of RM570- RM251 on chromosome 3 was observed to harbor two QTLs for MRL under control conditions. The region, RM38-RM331 on chromosome 8 was observed to have effects on RDW under stress conditions and RS under control conditions and of its relative parameter measured under stress. Another region i.e., RM404-RM547 on chromosome 8 controlled RDW and RS under stress conditions and of their relative parameters measured un- der stress. It is noteworthy that alleles at 5 of 6 regions were contributed by the parent INRC10192 (Table 3). Among six intervals on four chromosomes identified to harbor multiple QTLs, two interv als (RM1-RM495 on chromosome 1 and RM38-RM331 on chromosome 5) were found to affect related traits under the two water supply conditions and one interval (RM570-RM251 on chromosome 3) to affect traits under well watered condi- tions. Three intervals (RM493-RM302 on chromosome 1, RM106-RM5897 on chromosome 2 and RM404-RM547 on chromosome 8) were observed to be water sup- ply-specific regions and had effects only under stress conditions, suggesting that water supply-specific regions or QTLs might be closely associated with the responses of lines to water deficit. Particularly, this also suggests that water deficit promoted the expression of QTLs lo- cated in these regions. It is assumed that inductive expression of new genes would permit adaptation to stresses. Several studies have documented that gene expression is induced by stresses [40-42]. For instance, Rabbani et al. (2003) [42] have found 62 genes were induced by drought in rice. Salek- deh et al. (2002) [43] have shown concentrations of sev- eral leaf proteins have been increased significantly dur- ing drought and declined on re-watering. Genes induced in drought stress generally involved in protection of cells from water deficit by producing metabolic proteins and regulation of genes for signal transduction [44]. Very recently, Rabello et al. (2008) [45] identified drought responsive genes in roots of upland rice and observed that the genes exclusively expressed in the tolerant genotypes were related to the maintenance of turgor and cell integrity. Hence it can be interpreted that QTLs de- tected only under drought or well-watered conditions perhaps involved in the responses of plants to stress. Some of the candidate genes controlling drought stress were identified based on previous reports in the marker intrval of RM1 and RM495 on chromosome1 which e Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice Copyright © 2011 SciRes. AJPS 197 Table 3. QTLs for drought related traits and of the relative parameters under stress (PEG) and control conditions. Chr, refers to Chromosome number. LOD, refers to log10-Likelihood value PVE, Phenotypic variance explained by single QTL. a0, Additive effect. Al.ef., Allele effect of substituting a single allele from one parent to another. Positive values show that allelic contribution is from IR64 and negative values from INRC10192. asignificant only in composite interval mapping. bsignificant only in simple interval mapping. *Repeated/Stable QTLs across control/stress treat- ments. P-Pos ition of the QTL fr o m left flanking marker in cM. harbours important QTLs for SL, SDW, RDW and RS (Suppl Table 2). Related traits are often due to pleiotropic effect of a gene(s) or QTLs, which may enable selection for a com- plex trait via an easily observable related trait. In the present study, six loci distributed over chromosomes 1, 2, 3 and 8 have been found to harbor multiple QTLs affect- ing the same or different traits (Ta ble 4). The number of QTLs in each of the clusters ranges from 2 to 5. Out of 24 QTLs detected, only five have been identi- fied as stable (Table 3), while the remaining have been detected either under stress (10 QTLs) or control (7 QTLs) conditions only. Among these five stable QTLs also, only one QTL viz., qrs8.1 has been identified under control as well as stress conditions, while the remaining four QTLs viz., qrdw1.1, qrdw8.2, qrs1.2 and qrs8.2 for traits measured under stress and of their relative parame- ters measured under the stress. Very low percentage of common (stable) QTLs detected across water supply con- ditions is in agreement with the significant responses of RILs to water deficit strong ly suggesting QTLs detection is depend on specific environment and water deficit to induce or inhibit some new genes to express simultane- ously [13,16]. This lower coincidence of QTLs across two water regimes has as well been reported by Ka- moshita et al. (2002a and 2002b) [12,46]. They could find only two stable QTLs among 31 detected for seven traits in two experiments with different sowing dates under anaerobic conditions, suggesting a large effect of the phenotyping environment as defined by tempera- ture and solar radiation on QTsL identification for root traits. Further, Zhang et al. (2001b) [47] showed that QTLs and epistatic loci for seminal root length detected in solution culture were different from those detected in paper culture and revealed a different genetic system responsible for seminal root growth under different water supply conditio ns. Recently, MacMillan et al. (2006) [48] mapped QTL for six root- and shoot-related traits in four treatment environments (a control, low light, low soil nitrogen and low soil water), and most QTLs for an iden tical trait in th e four environments were different. Generally, it is consid ered that QTLs × environment interaction is an important Table 4. QTL clusters identified for shoot and root related traits. S.No.Marker interval Chr Traits No. of QTLs 1 RM1-RM495 1 SL, SDW, RDW(2), RS 5 2 RM493-RM3021 RS(3) 3 3 RM106-RM58972 SDW(2) 2 4 RM570-RM2513 MRL(2) 2 5 RM38-RM331 8 RDW, RS(2) 3 6 RM404-RM5478 RDW(2), RS(2) 4 Values in parenthesis indicates no. of QTLs for the respective traits.  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice 198 component for genetic determination of root growth [13,48]. Similar results were observed by Zheng et al. (2003) [14], who used flooding and upland as different water supply conditions, and by Li et al. (2005) [13], who carried out phenotype scoring under lowland (flooded), upland (aerobic soil) and polyvinyl chloride (PVC)-pipe aerobic conditions. The differences in the experimental conditions and mapping populations em- ployed often result in marked differences in resistance traits and consistency of QTLs [12]. No QTL was detected for some of the traits like shoot length and maximum root length under stress cond itions, although segregation for these traits was obvious in the RILs. Earlier studies have found epistasis and G × E in- teractions plays major role in determining yield and its components and most of the phenology traits, especially under drought conditions [49-52]. This finding probably explains why no QTL could be detected for such traits. In the present study, no epistatic interaction was found for shoot length and maximu m root length ind icating th e G × E play crucial role to determine QTLs for these traits. Keeping in view the objective of the present investig a- tion i.e., discovering new QTLs of promise, it is impor- tant to identify QTLs with enhancing favorable alleles from the landrace for introducing in the crop improve- ment program. Though the landrace is agronomically unattractive, the results suggested to con tribute favorable alleles for 65.86% of the QTLs, by enhancing the level of tolerance to drought through various seedling indices. These findings are in agreement with those of earlier workers many who had detected QTLs with trait value enhancing alleles in agronomically phenotypically infe- rior parental sources in rice [53-55]. McCouch and Do- erge (1995) [56] have identified more than 50% of the QTLs for root morphology in the RIL population of the cross CO39/ Moroberekan and all the alleles that had positive effect were from Moroberekan, the japonica donor parent. It is thus possible to detect more number of novel and major QTLs with favorable alleles in mapping populations derived of crosses involving primitive land- races. It is encouraging that a high percentage of transgres- sive segregants could be detected for nearly all the drought tolerance related traits in the mapping popu lation involving a pa rent i.e., the landrace INRC10192 which is not known for its superior agronomic traits. Recovery, however, of useful transgressive segregants in such population could be due to result of interaction between the trait enhancing alleles of the landrace INRC10192 with those of the high yielding variety IR64. The ge- nomic regions harboring drought tolerant QTLs could be of great targets to identify the candidate genes by fine- mapping. However, further investigations are required to identify the promising candidate genes underlying the major drought tolerant QTLs and the tightly linked or gene specific markers can be used for development of drought resistance rice cultivars through marker-assisted selection. Pyramiding of the stress tolerance QTL/gene(s) coupled with genes governing high yield might help to evolve high yielding varieties ideally suited to drought prone rainfed rice ecologies. 5 Acknowledgements We thank DRR for maintaining the rice material under National Professors Project. Thanks to the Biotechnology unit, ANGRAU for supporting the research work and fellowship supported by CSIR, New Delhi to AS. REFERENCES [1] S. Fukai and M. Cooper, “Development of Drought-Re- sistant Cultivars Using Physio-Morphological Traits in Rice,” Field Crops Research, Vol. 40, No. 2, 1995, pp. 67-87. doi:10.1016/0378-4290(94)00096-U [2] H. T. Nguyen, R. C. Babu and A. Blum, “Breeding for Drought Resistance in Rice: Physiology and Molecular Genetics Considerations,” Crop Science, Vol. 37, No. 5, 1997, pp. 1426-1434. doi:10.2135/cropsci1997.0011183X003700050002x [3] A. H. Price, A. D. Tomos, and D. S. Virk, “Genetic Dis- section of Root Growth in Rice (Oryza Sativa L.) I: A Hydroponic Screen,” Theoretical and Applied Genetics, Vol. 95, No. 1-2, 1997, pp. 132-142. doi:10.1007/s001220050541 [4] H. R. Lafitte, A. H. Price and B. Courtois, “Yield Re- sponse to Water Deficit in an Upland Rice Mapping Population: Associations among Traits and Genetic Markers,” Theoretical and Applied Genetics, Vol. 109, 2004, pp. 1237-1246. doi:10.1007/s00122-004-1731-8 [5] J. C. O’Toole, “Adaptation of Rice to Drought-Prone Environments,” In: IRRI, Ed., Drought Resistance in Crops with the Emphasis on Rice, International Rice Re- search Institute, Manila, 1982, pp 195-213. [6] A. H. Price and A. D. Tomos, “Genetic Dissection of Root Growth in Rice (Oryza Sativa L.). II: Mapping Quantitative Trait Loci Using Molecular Markers,” Theoretical and Applied Genetics, Vol. 95, No. 1-2, 1997, pp. 143-152. doi:10.1007/s001220050542 [7] A. H. Price, K. A. Steele, B. J. Moore and R. G. W. Jones, “Upland Rice Grown in Soil-Filled Chambers and Ex- posed to Contrasting Water-Deficit Regimes II Mapping Quantitative Trait Loci for Root Morphology and Distri- bution,” Field Crops Research, Vol. 76, No. 1, 2002, pp. 25-43. doi:10.1016/S0378-4290(02)00010-2 [8] Y. Kato, S. Hirotsu, K. Nemoto and J. Yamagishi, “Iden- tification of QTLs controlling Rice Drought Tolerance at Seedling Stage in Hydroponic Culture,” Euphytica, Vol. 160, No. 3, 2008, pp. 423-430. Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice199 doi:10.1007/s10681-007-9605-1 [9] M. C. Champoux, G. Wang, S. Sarkarung, D. J. Mackill, J. C. O’Toole, N. Huang and S. R. McCouch, “Locating Genes Associated with Root Morphology and Drought Avoidance in Rice via Linkage to Molecular Markers,” Theoretical and Applied Genetics, Vol. 90, No. 7-8, 1995, pp. 969-981. doi:10.1007/BF00222910 [10] R. Yadav, B. Courtois, N. Huang and G. McLaren, “Map- ping Genes Controlling Root Morphology and Root Dis- tribu Tion in a Double Haploid Population of Rice,” Theoretical and Applied Genetics, Vol. 94, No. 5, 1997, pp. 619-632. doi:10.1007/s001220050459 [11] J. Zhang, H. G. Zheng, A. Alarti, G. Pantuwan, T. T. Nguyen, J. N. Tripathy, A. K. Sarial, S. Robin, R. C. Ba- bu, B. D. Nguyen, S. Sarkarung, A. Blum and H. T. Nguyen, “Locating Genomic Regions Associated with Components of Drought Resistance in Rice: Comparative Mapping within and Across Species,” Theoretical and Applied Genetics, Vol. 103, No. 1, 2001a, pp. 19-29. doi:10.1007/s001220000534 [12] A. Kamoshita, L. J. Wade, M. L. Ali, M. S. Pathan, J. Zhang, S. Sakarung and H. T. Nguyen, “Mapping QTLs for Root Morphology of a Rice Population Adapted to Rainfed Lowland Conditions,” Theoretical and Applied Genetics, Vol. 104, No. 5, 2002a, pp. 880-893. doi:10.1007/s00122-001-0837-5 [13] Z. Li, P. Mu, C. Li, H. Zhang, Z. Li, Y. Gao and X. Wang, “QTL Mapping of Root Traits in a Doubled Haploid Population from a Cross between Upland and Lowland Japonica Rice in Three Environments,” Theoretical and Applied Genetics, Vol. 110, No. 7, 2005, pp. 1244-1252. doi:10.1007/s00122-005-1958-z [14] B. S. Zheng, L. Yang, W. P. Zhang, C. Z. Mao, Y. R. Wu, K. K. Yi, Y. Liu and P. Wu, “Mapping QTLs and Candi- date Genes for Rice Root Traits under Different Wa- ter-Supply Conditions and Comparative Analysis Across Three Populations,” Theoretical and Applied Genetics, Vol. 107, No. 8, 2003, pp. 1505-1515. doi:10.1007/s00122-003-1390-1 [15] B. Zheng, L. Yang, C. Mao, Y. Huang and P. Wu, “Comparison of QTLs for Rice Seedling Morphology under Different Water Supply Conditions,” Journal of Genetics and Genomics, Vol. 3 5 , No . 8, 200 8, pp. 473-484. doi:10.1016/S1673-8527(08)60065-X [16] K. Cui, J. Huang, Y. Xing, S. Yu, C. Xu and S. Peng, “Mapping QTLs for Seedling Characteristics under Dif- ferent Water Supply Conditions in Rice (Oryza Sativa L.),” Physiologia Plantarum, Vol. 132, No. 1, 2008, pp. 53-68. [17] K. A. Steele, A. H. Price, H. E. Shashidhar and J. R. Wit- combe, “Marker-Assisted Selection to Introgress Rice QTLs Controlling Root Traits into an Indian Upland Rice Variety,” Theoretical and Applied Genetics, Vol. 112, 2006, pp. 208-221. doi:10.1007/s00122-005-0110-4 [18] L. Liu, P. Mu, X. Li, Y. Qu, Y. Wang and Z. Li, “Local- ization of QTL for Basal Root Thickness in Japonica Rice and Effect of Marker-Assisted Selection for a Major QTL,” Euphytica, Vol. 164, No. 3, 2008, pp. 729-737. doi:10.1007/s10681-008-9695-4 [19] A. Sinhababu and R. K. Kar, “Comparative Responses of Three Fuel Wood Yielding Plants to PEG-Induced Water Stress at Seedling Stage,” Acta Physiologiae Plantarum, Vol. 25, No. 4, 2003, pp. 403-409. doi:10.1007/s11738-003-0022-3 [20] S. Basu, A. Ro ychoudhury, P. P. Saha and D. N. Sengupta, “Comparative Analysis of Some Biochemical Responses of Three Indica Rice Varieties during Polyethylene Gly- col-Mediated Water Stress Exhibits Distinct Varietal Dif- ference,” Acta Physiologiae Plantarum, Vol. 32, No. 3, 2010, pp. 551-563. doi:10.1007/s11738-009-0432-y [21] S. Yoshida and S. Hasega wa, “The Rice Root System: It s Development and Function,” In: Irri, Ed., Drought Resis- tance in Crops with the Emphasis on Rice, International Rice Research Institute, Manila, 1982, pp. 83-96. [22] L. Liu, R. Lafitte and D. Guan, “Wild Oryza Species as Potential Sources of Drought-Adaptive Traits,” Euphytica, Vol. 138, No. 2, 2004, pp. 149-161. doi:10.1023/B:EUPH.0000046801.27042.14 [23] A. J. Ali, J. L. Xu, A. M. Ismail, B. Y. Fu, C. H. M. Vi- jaykumar, Y. M. Gao, J. Domingo, R. Maghirang, S. B. Yu, G. Gregorio, S. Yanaghihara, M. Cohen, B. Carmen, D. Mackill and Z. K. Li, “Hidden Diversity for Abiotic and Biotic Stress Tolerances in the Primary Gene Pool of Rice Revealed by a Large Backcross Breeding Program,” Field Crops Research, Vol. 97, No. 1, 2006, pp. 66-76. doi:10.1016/j.fcr.2005.08.016 [24] H. R. Lafitte, C. H. M. Vijayakumar, Y. M. Gao, Y. Shi, J. L. Xu, B. Y. Fu, S. B. Yu, A. J. Ali, J. Domingo, R. Maghirang, R. Torres, D. Mackill and Z. K. Li, “Im- provement of Rice Drought Tolerance through Backcross Breeding: Evaluation of Donors and Selection in Drought Nurseries,” Field Crops Research, Vol. 97, No. 1, May 2006, pp. 77-86. doi:10.1016/j.fcr.2005.08.017 [25] A. Blum, “Osmotic Adjustment and Growth of Barley Genotypes under Drought,” Crop S cience, Vol. 29, 1989, pp. 230-233. doi:10.2135/cropsci1989.0011183X002900010052x [26] S. Yoshida, D. A. Forno, J. H. Cock and K. A. Gomez, “Laboratory Manual for Physiological Studies of Rice,” 3rd Edition, the International Rice Research Institute, Manila, 1976. [27] S. C. Gloria, O. Ito and A. A. Arcelia, “Physiological Evaluation of Responses of Rice (Oryza Sativa L.) to Water Deficit,” Plant Science, Vol. 163, No. 3, 2002, pp. 815-827. [28] G. B. Gregorio, D. Sendhira, R. D. Mendoza, N. L. Manigbas, J. P. Roxas and C. Q. Guerta, “Progress in Breeding for Salinity Tolerance and Associated Abiotic Stresses in Rice,” Field Crops Research, Vol. 76, No. 2, 2002, pp. 91-101. doi:10.1016/S0378-4290(02)00031-X [29] M. G. Murray and W. F. Thompson, “Rapid Isolation of High Molecular Weight Plant DNA,” Nucleic Acids Re- search, Vol. 8, No. 19, 1980, pp. 4321-4325. doi:10.1093/nar/8.19.4321 [30] S. Wang, C. J. Basten and Z. B. Zeng, “Windows QTL Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice 200 Cartographer 2.5,” North Carolina State University, Ra- leigh, 2006. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm [31] G. A. Churchill and R. W. Doerge, “Empirical Threshold Values for Quantitative Trait Mapping,” Genetics, Vol. 138, No. 3, 1994, pp. 963-971. [32] E. Fernandes, A. Pacheco and C. Penha-gonçalves, “Map- ping of Quantitative Trait Loci Using the Skew-Normal Distribution,” Journal of Zhejiang University Science, Vol. 8, No. 11, 2007, pp. 792-801. doi:10.1631/jzus.2007.B0792 [33] T. M. Gireesha, H. E. Shashidhar and S. Hittalmani, “Genetics of Root Morphology and Related Traits in an Indica-Indica Based Mapping Population of Rice (Oryza Sativa L.),” Research on Crops, Vol. 1, No. 2, 2000, pp. 208-215. [34] S. P. Mane, H. E. Shashidhar, A. Kanbar and S. Hittal- mani, “Molecular Marker Analysis for Root Length in a Diverse Germplasm of Rice (Oryza Sativa L.),” Indian Journal of Genetics, Vol. 63, No. 3, 2003, pp. 197-201. [35] F. Asch, M. Dingkuhn, A. Sow and A. Audebert, “Drou- ght-Induced Changes in Rooting Patterns and Assimilate Partitioning between Root and Shoot in Upland Rice,” Field Crops Research, Vol. 93, No. 2-3, 2005, pp. 223-236. doi:10.1016/j.fcr.2004.10.002 [36] E. D. Redona and D. J. Mackill, “Mapping Quantitative Trait Loci for Seedling-Vigor in Rice Using RFLPs,” Theoretical and Applied Genetics, Vol. 92, No. 3-4, 1996, pp. 395-402. doi:10.1007/BF00223685 [37] C. G. Xu, X. Q. Li, Y. Xue, Y. W. Huang, J. Gao and Y. Z. Xing, “Comparison of Quantitative Trait Loci Control- ling Seedling Characteristics at Two Seedling Stages Us- ing Rice Recombinant Inbred Lines,” Theoretical and Applied Genetics, Vol. 109, 2004, pp. 640-647. doi:10.1007/s00122-004-1671-3 [38] A. H. Price, K. A. Steele, B. J. Moore, P. B. Barraclough and L. J. Clark, “A Combined RFLP and AFLP Linkage Map of Upland Rice (Oryza Sativa L.) Used to Identify QTLs for Root-Penetration Ability,” Theoretical and Ap- plied Genetics, Vol. 100, No. 1, 2000, pp. 49-56. doi:10.1007/s001220050007 [39] J. C. Xu, J. Z. Li, X. W. Zheng, L. X. Zou and L. H. Zhu, “QTL Mapping of the Root Traits in Rice Seedling,” Acta Genetica Sinica, Vol. 28, 2001, pp. 433-438. [40] E. A. Bray, “Plant Responses to Water Deficit,” Trends in Plant Science, Vol. 2, No. 2, 1997, pp. 48-54. doi:10.1016/S1360-1385(97)82562-9 [41] A. Kathiresan, H. R. Lafitte, J. Chen, L. Mansueto, R. Bruskiewich and J. Bennett, “Gene Expression Microar- rays and Their Application in Drought Stress Research,” Field Crops Research, Vol. 97, No. 1, 2006, pp. 101-110. doi:10.1016/j.fcr.2005.08.021 [42] M. A. Rabbani, K. Maruyama, H. Abe, A. Khan, K. Ka- tsura, Y. Ito, K. Yoshiwara, M. Seki, K. Shinozaki, Ya- maguchi and K. Shinozaki, “Monitoring Expression Pro- files of Rice Genes under Cold, Drought and High-Salin- ity Stresses and Abscisic Acid Application Using Cdna Microarray and RNA Gel-Blot Analyses,” Plant Physi- ology, Vol. 133, No. 4, 2003, pp.1755-1767. doi:10.1104/pp.103.025742 [43] G. H. Salekdeh, J. Siopongco, L. J. Wade, B. Ghareyazie and J. Bennett, “A Proteomic Approach to Analyzing Drought and Salt Responsiveness in Rice,” Field Crops Research, Vol. 76, No. 2-3, 2002, pp. 199-219. doi:10.1016/S0378-4290(02)00040-0 [44] M. Rodriguez, E. Canales, C. J. Borroto, E. Carmona, J. Lopez, M. Pujol and O. Borras-Hidalgo, “Identification of Genes Induced upon Water-Deficit Stress in a Drought Tolerant Rice Cultivar,” Journal of Plant Physiology, Vol. 163, No. 5, 2006, pp. 577-584. doi:10.1016/j.jplph.2005.07.005 [45] A. R. Rabello, C. M. Guimaraes, P. H. N. Rangel, F. R. Silva, D. Seixas, E. D. Souza, A. C. M. Brasileiro, C. R. Spehar, M. E. Ferreira and A. Mehta, “Identification of Drought-Responsive Genes in Roots of Upland Rice (Oryza Sativa L),” BMC Genomics, Vol. 9, No. 1, 2008, p. 485. doi:10.1186/1471-2164-9-485 [46] A. Kamoshita, J. X. Zhang, J. Siopongco, S. Sakarung, H. T. Nguyen and L. J. Wade, “Effects of Phenotyping En- vironment on Identification of Quantitative Trait Loci for Rice Root Morphology under Anaerobic Conditions,” Crop Science, Vol. 42, No. 1, 2002, pp. 255-265. doi:10.2135/cropsci2002.0255 [47] W. P. Zhang, X. Y. Shen, P. Wu, B. Hu and C. Y. Liao, “QTLs and Epistasis for Seminal Root Length under a Different Water Supply in Rice (Oryza Sativa L.),” Theoretical and Applied Genetics, Vol. 103, 2001, pp. 118-123. doi:10.1007/s001220100561 [48] K. MacMillan, K. Emrich, H. P. Piepho, C. E. Mullins and A. H. Price, “Assessing the Importance of Genotype × Environment Interaction for Root Traits in Rice Using a Mapping Population II: Conventional QTL Analysis,” Theoretical and Applied Genetics, Vol. 113, No. 5, 2006, pp. 953-964. doi:10.1007/s00122-006-0357-4 [49] S. B. Yu, J. X. Li, C. G. Xu, Y. F. Tan, Y. J. Gao, X. H. Li, Q. Zhang and M. A. S. Maroof, “Importance of Epis- tasis as the Genetic Basis of Heterosis in an Elite Rice Hybrid,” Proceedins of the National Academy of Sciences, USA, Vol. 94, No. 17, 1997, pp. 9226-9231. doi:10.1073/pnas.94.17.9226 [50] Z. K. Li, L. J. Luo, H. W. Mei, Q. Y. Shu, D. L. Wang, R. Tabien, D. B. Zhong, C. S. Ying, J. W. Stancel, G. S. Khush and A. H. Paterson, “Overdominant Epistatic Loci Are the Primary Genetic Basis of Inbreeding Depression and Heterosis in Rice: I Biomass and Grain Yield,” Ge- netics, Vol. 158, No. 4, 2001, pp. 1737-1753. [51] Y. Z. Xing, Y. F. Tan, J. P. Hua, X. L. Sun, C. G. Xu and Q. Zhang, “Characterization of the Main Effects Epistatic Effects and Their Environmental Interactions of QTLs on the Genetic Basis of Yield Traits in Rice,” Theoretical and Applied Genetics, Vol. 105, No. 2-3, 2002, pp. 248-257. doi:10.1007/s00122-002-0952-y [52] J. C. Lanceras, G. Pantuwan, B. Jongdee and T. Toojinda, “Quantitative Trait Loci Associated with Drought Toler- ance at Reproductive Stage in Rice,” Plant Physiology, Copyright © 2011 SciRes. AJPS  Molecular Mapping of QTLs for Drought Related Traits at Seedling Stage under PEG Induced Stress Conditions in Rice Copyright © 2011 SciRes. AJPS 201 Vol. 135, No. 1, 2004, pp. 384-399. doi:10.1104/pp.103.035527 [53] C. Brondani, P. H. N. Rangel, R. P. V. Brondani and M. E. Ferreira, “QTL Mapping and Introgression of Yield- Related Traits from Oryza Glumaepetula to Cultivated Rice (Oryza Sativa L). Using Microsatellite Makers,” Theoretical and Applied Genetics, Vol. 104, 2002, pp. 1192-1203. doi:10.1007/s00122-002-0869-5 [54] C. Li, A. Zhou and T. Sang, “Genetic Analysis of Rice Domestication Syndrome with the Wild Annual Species, Oryza Nivara,” New Phytology, Vol. 170, No. 1, 2006, pp. 185-193. doi:10.1111/j.1469-8137.2005.01647.x [55] S. R. McCouch, M. Sweeney, J. Li, H. Jiang, M. Thom- son, E. Septiningsih, J. Edwards, P. Moncada, J. Xiao, A. Garris, T. Tai, C. Martinez, J. Tohme, M. Sugiono, A. McClung, L. P. Yuan and S. N. Ahn, “Through the Ge- netic Bottleneck: O. Rufipogon as a Source of Trait-En- hancing Alleles for O. Sativa L.,” Euphytica, Vol. 154, No. 3, 2007, pp. 317-339. doi:10.1007/s10681-006-9210-8 [56] S. R. McCouch and R. W. Doerge, “QTL Mapping in Rice,” Trends in Genetics, Vol. 11, No. 8, 1995, pp. 482- 487. doi:10.1016/S0168-9525(00)89157-X
|