Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 706-709 doi:10.4236/msa.2011.26097 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Materials Sciences and Applicatio ns, 2011, 2, 706-709 doi:10.4236/msa.2011.26097 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Catalytic Esterification of Oleic Acid over /MCM-41 Nanostructured Materials 2 4 SO Alexsandra Rodrigues do Nascimento1, Gicélia Rodrigues1, Joselaine Carvalho Santana2, Anne Michelle Garrido Pedrosa2, Marcelo José Barros de Souza1 1Universidade Federal de Sergipe, Departamento de Engenharia Química, São Cristóvão-SE, Brazil; 2Universidade Federal de Sergipe, Departamento de Química, São Cristóvão-SE, Brazil. Email: marcelojbs@ufs.br Received June 4th, 2010; revised August 10th, 2010; accepted May 19th, 2011. ABSTRACT This paper deals a study concerning the synthesis and catalytic application of a series of 4/MCM-41 catalysts with different 4/Si ratios, in the catalytic esterification of oleic acid, aiming biofuels production. The catalysts were characterized by XRD and FT-IR. The catalytic tests were carried out in a batch reactor and the obtained results showed good catalytic activity with high degrees of conversions of oleic acid. 2 SO 2 SO Keywords: MCM-41, Sulphate, Esterification, Oleic Acid 1. Introduction The esterification is a reaction that occurs between the carboxylic acids of vegetable oils with methanol or ethanol with the production of esters and water [1,2] is a widely used route for the production of biofuel. Esterifi- cation reactions are classic examples of reversible reac- tions and typically are catalyzed by acids. Heterogeneous catalysts such as mesoporous M41S (Classical types Mobil Mesoporous materials) with acidic properties, have been studied in the literature and used successfully in reactions involving molecules of high molecular weight [3,4]. The silica b ased MCM-41 (Mobil Compos- ite of Matter) is the main mesoporous material of the M41S family, discovered by researchers in Mobil Oil Corporation [5]. The formation of the MCM-41 phase occurs according to the liquid crystal template (LCT) mechanism, in which SiO4 tetrahedra react with the sur- factant template under hydrothermal conditions [5,6]. A typical preparation of the MCM-41 hexagonal array needs basically a solvent, a template (surfactan t molecule) and a silica source. These materials present larger pores compared to other catalysts and is appropriate with the structure of the fatty acid, which need a larger area of contact. The number of acid sites on the surface of the catalyst can be modified in large quantities by ion exchang e or by treatment with acids. The MCM-41 has typically low surface acidity. This acidity is important to catalyze the reactions of esterification. Thus, it is an important route to the esterification reactions to acidify the surface of the material. Some papers are found in the literature con- cerning the surface modification of mesoporous materials with acid treatment [3]. In this work, the MCM-41 was impregnated with different concentrations of sulphate in order to obtain acid catalysts. These catalysts can be ap- plied in the acid organic reactions as esterification. 2. Experimental The MCM-41 was synthesized starting from silica gel (VETEC), sodium silicate (VETEC), cethyltrimethyl- ammonium bromide (CTMABr, vetec) and distilled wa- ter. The pH level was performed by Micronal pHmetter and after adjusted in a range of 9.5 - 10 using a 30% ace- tic acid solution. The chemicals were mixed in order to obtain a gel with the following molar composition: 4.58SiO2 : 0.437Na2O : 1CTMABr : 200H2O. The proce- dure used to obtain ca. 1.6 g of calcined MCM-41 was: 1) 0.911 g of silica, 0.705 g of sodium silicate and 8.34 g of water were placed into a 100 mL teflon beaker and stirred at 60˚C for 2 h in order to obtain a clear solution; 2) a solution prepared from 1.743 g of cethyltrimethyl- ammonim bromide and 8.34 g of distilled water was added to the above mentioned mixture and aged for 30 minutes at room temperature. The hydrogel was placed 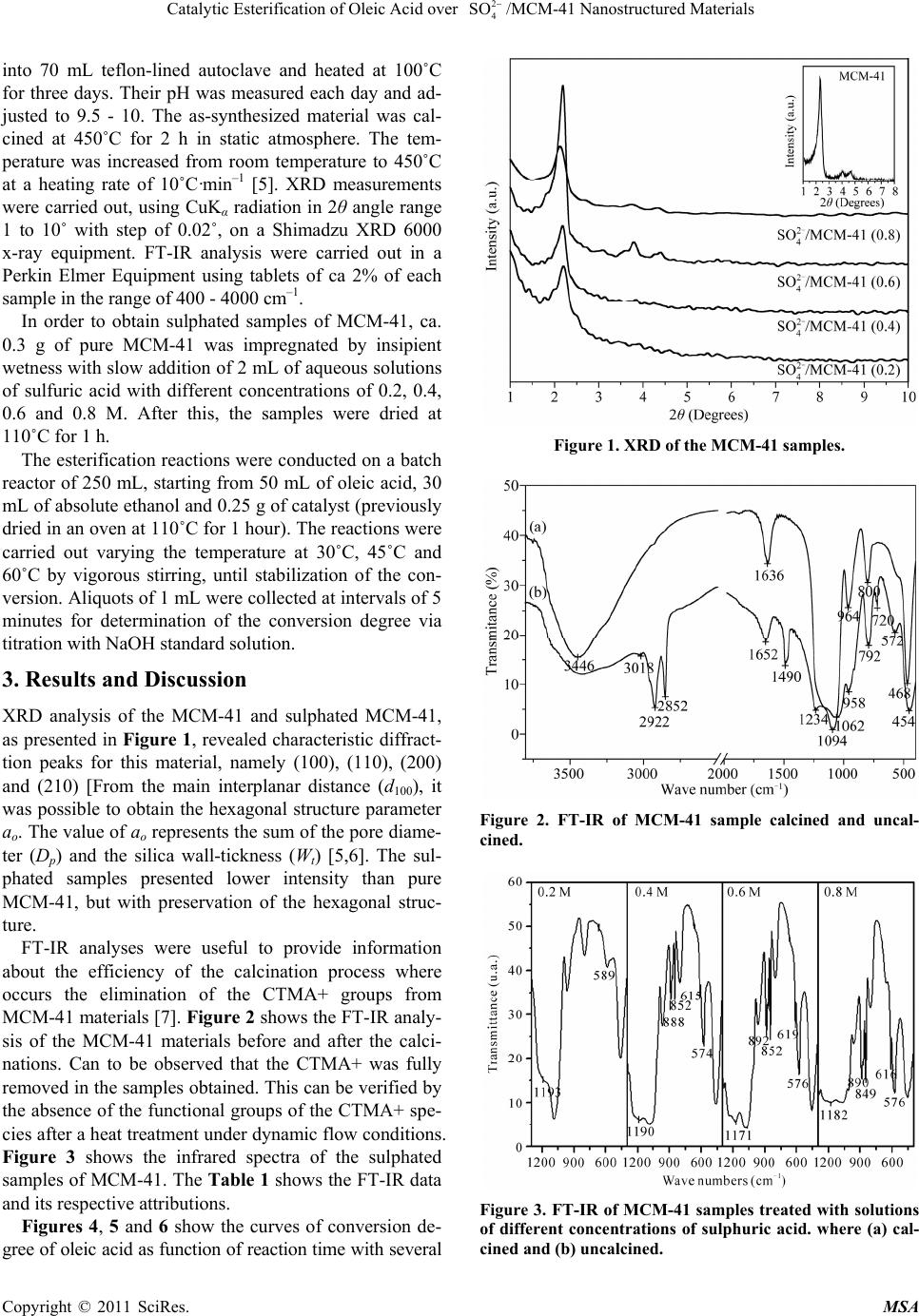 707 /MCM-41 Nanostructured Materials Catalytic Esterification of Oleic Acid over 2 4 SO into 70 mL teflon-lined autoclave and heated at 100˚C for three days. Their pH was measured each day and ad- justed to 9.5 - 10. The as-synthesized material was cal- cined at 450˚C for 2 h in static atmosphere. The tem- perature was increased from room temperature to 450˚C at a heating rate of 10˚C·min –1 [5]. XRD measurements were carried out, using CuKα radiation in 2θ angle range 1 to 10˚ with step of 0.02˚, on a Shimadzu XRD 6000 x-ray equipment. FT-IR analysis were carried out in a Perkin Elmer Equipment using tablets of ca 2% of each sample in the range of 400 - 4000 cm–1. In order to obtain sulphated samples of MCM-41, ca. 0.3 g of pure MCM-41 was impregnated by insipient wetness with slow addition of 2 mL of aqueous solutions of sulfuric acid with different concentrations of 0.2, 0.4, 0.6 and 0.8 M. After this, the samples were dried at 110˚C for 1 h. The esterification reactions were conducted on a batch reactor of 250 mL, starting from 50 mL of oleic acid, 30 mL of absolute ethanol and 0.25 g of catalyst (previously dried in an oven at 110˚C for 1 hour). The reactions were carried out varying the temperature at 30˚C, 45˚C and 60˚C by vigorous stirring, until stabilization of the con- version. Aliquots of 1 mL were collected at intervals of 5 minutes for determination of the conversion degree via titration with NaOH standard solu tion. 3. Results and Discussion XRD analysis of the MCM-41 and sulphated MCM-41, as presented in Figure 1, revealed characteristic diffract- tion peaks for this material, namely (100), (110), (200) and (210) [From the main interplanar distance (d100), it was possible to obtain the hexagonal structure parameter ao. The value of ao represents the sum of the pore diame- ter (Dp) and the silica wall-tickness (Wt) [5,6]. The sul- phated samples presented lower intensity than pure MCM-41, but with preservation of the hexagonal struc- ture. FT-IR analyses were useful to provide information about the efficiency of the calcination process where occurs the elimination of the CTMA+ groups from MCM-41 materials [7]. Figure 2 shows the FT-IR analy- sis of the MCM-41 materials before and after the calci- nations. Can to be observed that the CTMA+ was fully removed in the samples obtained. This can be verified by the absence of the functional groups of the CTMA+ spe- cies after a heat treatment under dynamic flow conditions. Figure 3 shows the infrared spectra of the sulphated samples of MCM-41. The Table 1 shows the FT-I R data and its respective attribution s . Figures 4, 5 and 6 show the curves of conversion de- gree of oleic acid as function of reaction time with several Figure 1. XRD of the MCM-41 samples. Figure 2. FT-IR of MCM-41 sample calcined and uncal- cined. Figure 3. FT-IR of MCM-41 samples treated with solutions of different concentrations of sulphuric acid. where (a) cal- cined and (b) uncalci ne d. Copyright © 2011 SciRes. MSA 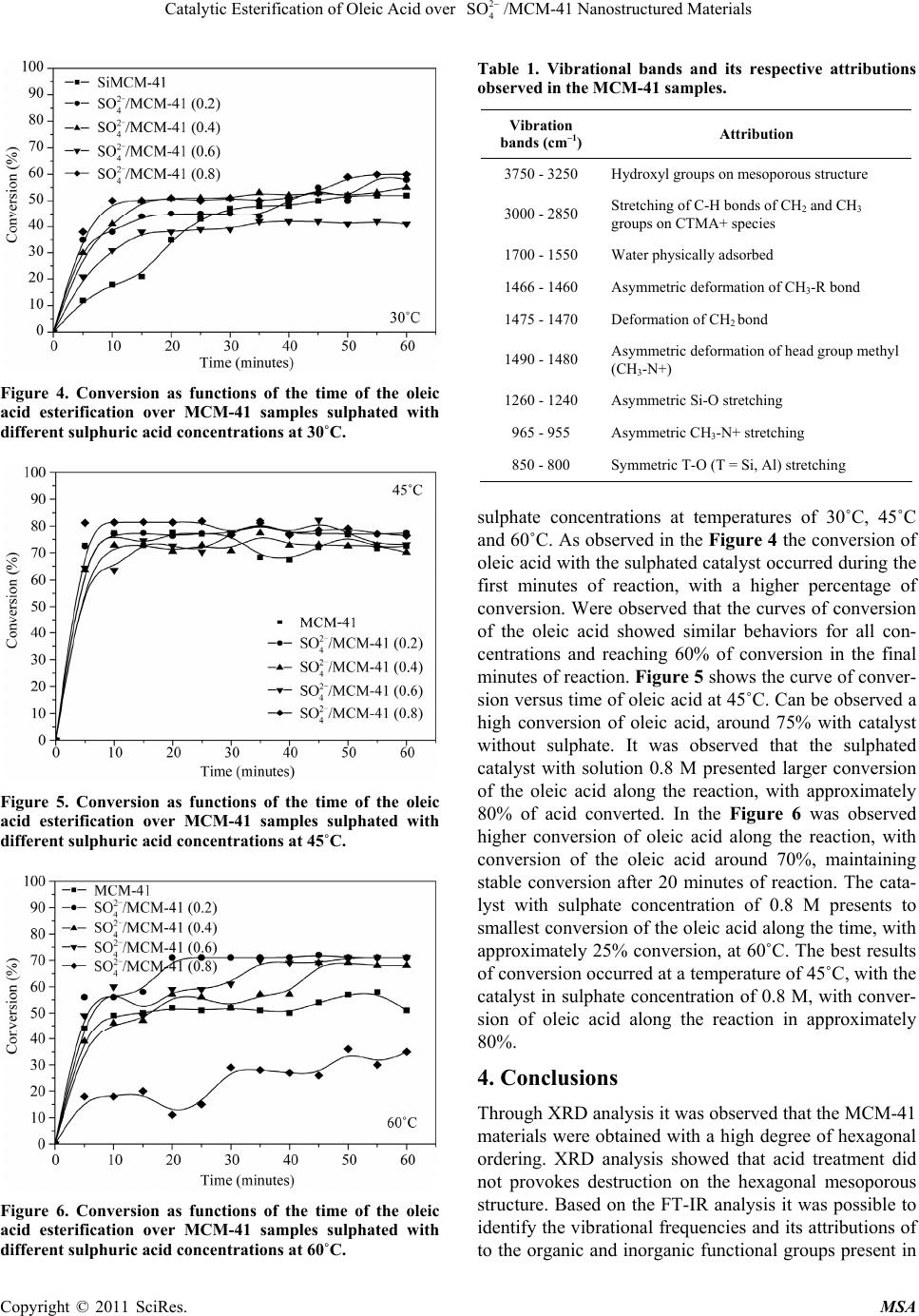 Catalytic Esterification of Oleic Acid over 2 4 SO 708 /MCM-41 Nanostructured Materials Figure 4. Conversion as functions of the time of the oleic acid esterification over MCM-41 samples sulphated with different sulphuric acid concentrations at 30˚C. Figure 5. Conversion as functions of the time of the oleic acid esterification over MCM-41 samples sulphated with different sulphuric acid concentrations at 45˚C. Figure 6. Conversion as functions of the time of the oleic acid esterification over MCM-41 samples sulphated with different sulphuric acid concentrations at 60˚C. Table 1. Vibrational bands and its respective attributions observed in the MCM-41 samples. Vibration bands (cm–1) Attribution 3750 - 3250 Hydroxyl groups on mesoporous structure 3000 - 2850 Stretching of C-H bonds of CH2 and CH3 groups on CTMA+ species 1700 - 1550 Water physically adsorbed 1466 - 1460 Asymmetric deformation of CH3-R bond 1475 - 1470 Deformation of CH2 bond 1490 - 1480 Asymmetric deformation of head group methyl (CH3-N+) 1260 - 1240 Asymmetric Si-O stretching 965 - 955 Asymmetric CH3-N+ stretching 850 - 800 Symmetric T-O (T = Si, Al) stretching sulphate concentrations at temperatures of 30˚C, 45˚C and 60˚C. As observed in the Figure 4 the conversion of oleic acid with the sulphated catalyst occurred during the first minutes of reaction, with a higher percentage of conversion. Were observed that the curves of conversion of the oleic acid showed similar behaviors for all con- centrations and reaching 60% of conversion in the final minutes of reaction. Figure 5 shows the curve of conver- sion versus time of oleic acid at 45˚C. Can be observed a high conversion of oleic acid, around 75% with catalyst without sulphate. It was observed that the sulphated catalyst with solution 0.8 M presented larger conversion of the oleic acid along the reaction, with approximately 80% of acid converted. In the Figure 6 was observed higher conversion of oleic acid along the reaction, with conversion of the oleic acid around 70%, maintaining stable conversion after 20 minutes of reaction. The cata- lyst with sulphate concentration of 0.8 M presents to smallest conversion of the oleic acid along the time, with approximately 25% conversion, at 60˚C. The best results of conversion occurred at a temperature of 45˚C, with the catalyst in sulphate concentration of 0.8 M, with conver - sion of oleic acid along the reaction in approximately 80%. 4. Conclusions Through XRD analysis it was observed that the MCM-41 materials were obtained with a high degree of hexagonal ordering. XRD analysis showed that acid treatment did not provokes destruction on the hexagonal mesoporous structure. Based on the FT-IR analysis it was possible to identify the vibr ational frequencies and its attribution s of to the organic and inorganic functional groups present in Copyright © 2011 SciRes. MSA  Catalytic Esterification of Oleic Acid over 2 4 SO /MCM-41 Nanostructured Materials Copyright © 2011 SciRes. MSA 709 the catalysts. The esterification reactions were very effi- cient taking in consideration that were happened at am- bient pressure and low temperatures, reaching relatively high conversions. 5. Acknowledgments The authors acknowledge LABCAT/UFS (Catalysis Laboratory of the Federal University of Sergipe), CAPES (Coordenação de Aperfeiçoamento de pessoas de Nível Superior) and CNPq (Conselho Nacional de Desen- volvimento Científico e Tecnológico) for the financial support. REFERENCES [1] K. D. Maher and D. C. Bressler, “Pyrolysis of Triglyc- eride Materials for the Production of Renewable Fuels and Chemicals,” Bioresource Technology, Vol. 98, No. 12, 2007, pp. 2351-2368. doi:10.1016/j.biortech.2006.10.025 [2] A. Corma, “Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions,” Chemical Re- views, Vol. 95, No. 3, 1995, pp. 559-624. doi:10.1021/cr00035a006 [3] A. S. Araujo, J. M. F. B. Aquino, C. D. R. Souza and M. J. B. Souza, “Isopropanol Dehydration over Nanostructured Sulfated MCM-41,” Studies in Surfaace Science and Catalysis, Vol. 141, 2002, pp. 531-536. doi:10.1016/S0167-2991(02)80586-9 [4] A. S. Araujo and M. Jaroniec, “Determination of the Sur- face Area and Mesopore Volume for Lanthanide-Incor- porated MCM-41 Materials by Using High Resolution Thermogravimetry,” Thermochimica Acta, Vol. 345, No. 2, 2000, pp. 173-177. doi:10.1016/S0040-6031(99)00374-3 [5] J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D Schmitt, C. T. W. Chu, D. H. Olson and E. W. Sheppard, “A New Family of Mesoporous Mo- lecular Sieves Prepared with Liquid Crystal Templates,” Journal of the American Chemical Society, Vol. 114, No. 27, 1992, pp. 10834-10843. doi:10.1021/ja00053a020 [6] X. S. Zhao, M. G. Q. Lu and G. J. Millar, “Advances in Mesoporous Molecular Sieve MCM-41,” Industrial & Engineering Chemistry Research, Vol. 35, No. 7, 1996, pp. 2075-2090. doi:10.1021/ie950702a [7] A. S. Araujo, V. J. Fernan des Jr., M. J. B. Souza , A. O. S. Silva and J. M. F. B. Aquino, “Model Free-Kinetics Ap- plied to CTMA+ Removal of AlMCM-41 Molecular Sieves,” Thermochimica Acta, Vol. 413, No. 1-2, 2004, pp. 235-240. doi:10.1016/j.tca.2003.10.002 |

