Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 692-699 doi:10.4236/msa.2011.26095 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Evaluation of Lignin-Calcium Complex as Thermal Stabilizer for Poly Vinyl Chloride Hussein Ali Shnawa Polymer Research Center, University of Basrah, Basrah, Iraq. Email: hussanqi@yahoo.com Received December 27th, 2010; revised March 18th, 2011; accepted May 19th, 2011. ABSTRACT Chemical modification of lignin was carried out by reacted it with HI acid, then the modified lignin treated with cal- cium hydroxide to prepare calcium-lignin chelating complex, this derivative was examined as thermal stabilizer for PVC, thermal degradation of PVC neat as blank and containing three weight percents (1, 2, and 4) into polymer was accelerated by heat treatment at 190˚C for 2 h then PVC films were casting from THF solvent with thickn ess 0.03 mm. Thermal stabilization activity of this derivative was investigated by using infrared spectroscopy, according to the re- sults obtained Calcium-lign in complex have suitable activity to in creased PVC stability at lo w concentration depending on it’s ability to reaction with HCl as well as the chemical stru cture of lignin that contain phenolic properties. Keywords: Lignin, Modification of Lignin, Poly (Vinyl Chloride), Thermal Degradation, IR Study 1. Introduction Lignin has a highly branched chemical structure consist- ing of phenol propane unites which are connected mainly together by ether or C–C linkages [1,2]. Because of vari- ety functional groups in lignin that provide many poten- tial reactive sites for chemical modifications and applica- tions, one of these modification method that used to in- crease lignin activity were by reacted it with formalde- hyde in alkaline solution to form methylol groups into lignin matrix [3,4], or by synthesized polymer from lig- nin on the basic of some functional groups such as phenylene, hydroxyl and methoxyl [5]. There are other studies show that the modification lig- nin by oxidation reaction (obtained through several sources and methods) can be used as chelating agent for some type of metal ions [6,7]. German C. Quintana and his co-worker study the capacity for removal of heavy metals from liquid streams by formation of complexes with lignin’s oxidized by acid treatment and their con- clusions refer to lignin capable of adsorbing Cadmium ions from aqueous solution and a slight increase in ad- sorption capacity when lignin was oxidized [8]. Another study show the used of native lignin as will as modifica- tion lignin as additive for low density polyethylene which can act as antioxidant and UV-stabilizers [9]. On the other hand, Poly (vinyl chloride), PVC, is one of the leading thermoplastic materials. It stands second in the world after polyethylene so far as production is con- cerned. However, PVC shows low thermal stability [10]. It is generally accepted that poly(vinyl chloride), PVC, is an unstable polymer when exposed to high temperatures during it’s moldings and applications. Therefore, the poor thermal stability of PVC still remains one of its main problems [11]. The purpose of this work was to study the chemical modification of lignin by chelated it with Ca ion then evaluated its effect on thermal stability of poly (vinyl chloride). 2. Experimental 2.1. Modification of Lignin 15 g Lignin (Kraft lignin) from paper industries—Basrah was modified by refluxed it with 60 ml of 30% solution of HI acid (May & Baker Ltd.) for 3 h at the end or the reaction period the solution was allowed to cool before filtrated. Modified lignin washed with distillation water several times and finally dried under reduced pressure for 24 h. 2.2. Preparation of Calcium-Lignin Chelating Complex Modified lignin 10 g was dissolved in 250 ml water and added periodically 10% of Ca(OH)2 as clear and fil- 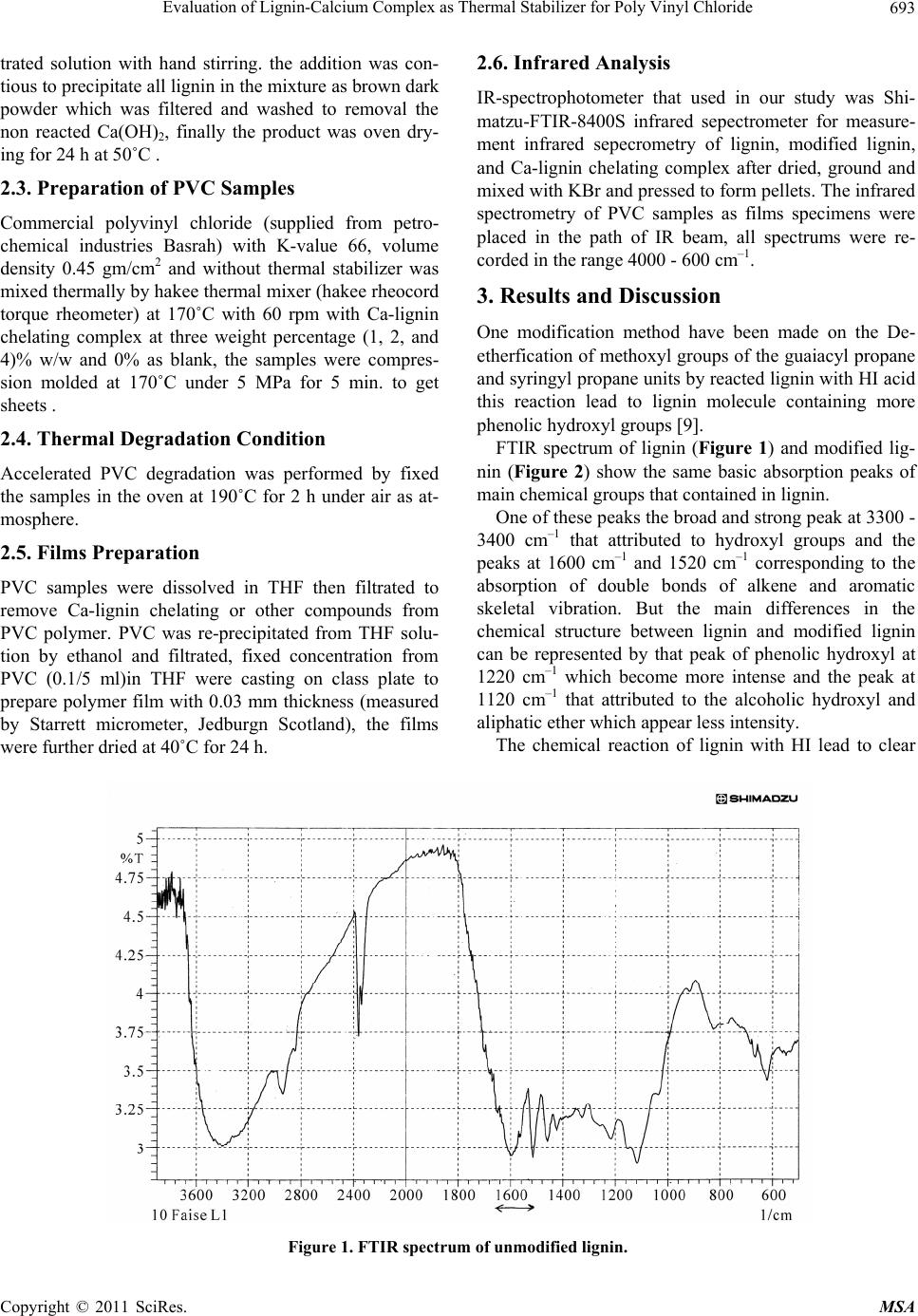 Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride693 trated solution with hand stirring. the addition was con- tious to precipitate all lignin in the mixture as brown dark powder which was filtered and washed to removal the non reacted Ca(OH)2, finally the product was oven dry- ing for 24 h at 50˚C . 2.3. Preparation of PVC Samples Commercial polyvinyl chloride (supplied from petro- chemical industries Basrah) with K-value 66, volume density 0.45 gm/cm2 and without thermal stabilizer was mixed thermally by hakee thermal mixer (hakee rheocord torque rheometer) at 170˚C with 60 rpm with Ca-lignin chelating complex at three weight percentage (1, 2, and 4)% w/w and 0% as blank, the samples were compres- sion molded at 170˚C under 5 MPa for 5 min. to get sheets . 2.4. Thermal Degradation Condition Accelerated PVC degradation was performed by fixed the samples in the oven at 190˚C for 2 h under air as at- mosphere. 2.5. Films Preparation PVC samples were dissolved in THF then filtrated to remove Ca-lignin chelating or other compounds from PVC polymer. PVC was re-precipitated from THF solu- tion by ethanol and filtrated, fixed concentration from PVC (0.1/5 ml)in THF were casting on class plate to prepare polymer film with 0.03 mm thickness (measured by Starrett micrometer, Jedburgn Scotland), the films were further dried at 40˚C for 24 h. 2.6. Infrared Analysis IR-spectrophotometer that used in our study was Shi- matzu-FTIR-8400S infrared sepectrometer for measure- ment infrared sepecrometry of lignin, modified lignin, and Ca-lignin chelating complex after dried, ground and mixed with KBr and pressed to form pellets. The infrared spectrometry of PVC samples as films specimens were placed in the path of IR beam, all spectrums were re- corded in the range 4000 - 600 cm–1. 3. Results and Discussion One modification method have been made on the De- etherfication of methoxyl groups of the guaiacyl propane and syringyl propane units by reacted lignin with HI acid this reaction lead to lignin molecule containing more phenolic hydroxyl gr oups [9]. FTIR spectrum of lignin (Figure 1) and modified lig- nin (Figure 2) show the same basic absorption peaks of main chemical groups that contained in lignin. One of these peaks the broad and strong peak at 3300 - 3400 cm–1 that attributed to hydroxyl groups and the peaks at 1600 cm–1 and 1520 cm–1 corresponding to the absorption of double bonds of alkene and aromatic skeletal vibration. But the main differences in the chemical structure between lignin and modified lignin can be represented by that peak of phenolic hydroxyl at 1220 cm–1 which become more intense and the peak at 1120 cm–1 that attributed to the alcoholic hydroxyl and aliphatic ether which appe ar less intensity. The chemical reaction of lignin with HI lead to clear Figure 1. FTIR spectrum of unmodified lignin. Copyright © 2011 SciRes. MSA 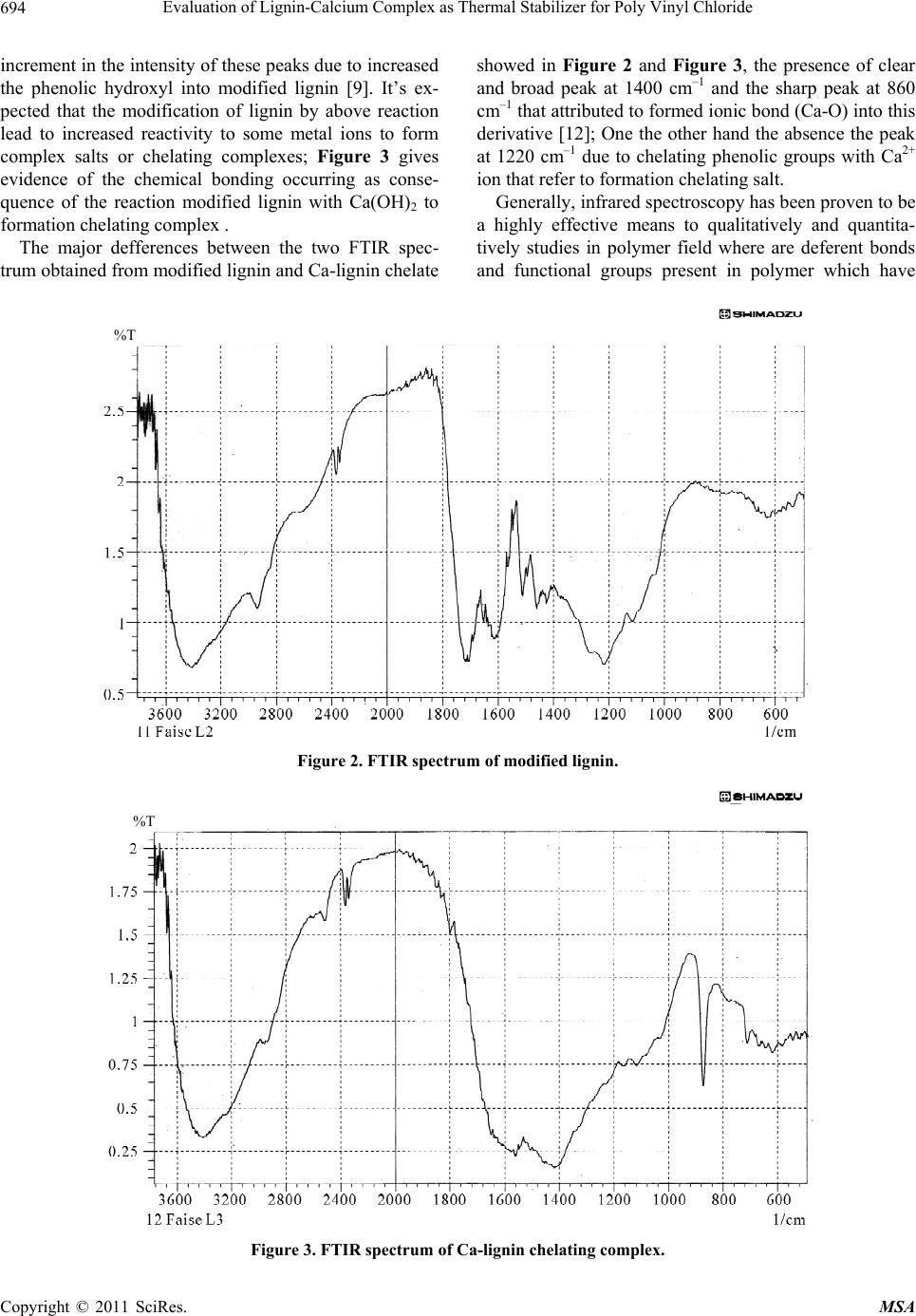 Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride 694 increment in the intensity o f these peaks due to increased the phenolic hydroxyl into modified lignin [9]. It’s ex- pected that the modification of lignin by above reaction lead to increased reactivity to some metal ions to form complex salts or chelating complexes; Figure 3 gives evidence of the chemical bonding occurring as conse- quence of the reaction modified lignin with Ca(OH)2 to formation chelating complex . The major defferences between the two FTIR spec- trum obtained from modified lignin and Ca-lignin chelate showed in Figure 2 and Figure 3, the presence of clear and broad peak at 1400 cm–1 and the sharp peak at 860 cm–1 that attributed to formed ionic bond (Ca-O) into this derivative [12]; One the other hand the absence the peak at 1220 cm–1 due to chelating phenolic groups with Ca2+ ion that refer to formation chelating salt. Generally, infrared spectroscopy has been proven to be a highly effective means to qualitatively and quantita- tively studies in polymer field where are deferent bonds and functional groups present in polymer which have %T Figure 2. FTIR spectrum of modified lignin. %T Figure 3. FTIR spectrum of Ca-lignin chelating complex. Copyright © 2011 SciRes. MSA  Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride695 different vibration frequencies, and by identifying char- acteristic frequencies as absorption peaks can be detected and monitoring many functional groups in polymer structure and composites [13,14]. The application of in- frared spectroscopy in polymer are concerned with the range 650 - 4000 cm–1; many works used IR or FTIR to study the decomposition and thermal degradation of PVC as well as evolution the thermal, photo-stabilizer and antioxidants additives for this polymer [11, 15-19]. Matuana L. M. (2001) classified the characteristic IR bands of PVC into three regions the first is called the (C–Cl) stretching region in the range from 600 - 700 cm–1, the second region is attributed to (C–C) stretching in the region from 900 - 1200 cm–1 and the third region is at 1250 - 2970 cm–1 assigned to numerous C–H mode. The IR spectrum of stander PVC without added thermal stabilizer or heat treatment is presented in Figure 4; ab- sorption bands of this polymer evidenced at about 2950 cm which attributed to C–H stretching from CH2, 1440 cm–1 wagging of CH2, 1300 cm–1 of CH2 deformation, 1250 cm–1 C–H stretching from CHCl, 1070 cm–1 C–C, 930 cm–1 rocking of CH2, 700 cm–1 and 600 cm–1 stretch- ing of C–Cl. After degradation of PVC by heated it for 2 h. at 190˚C main differences occur in the chemical structure lead to found new chemical groups such as polyene, hy- droxyl, carbonyl, cyclic compound and other [20,21], this new chemical structure can be observed and monitored by IR spectrometry; Figure 5 shows the IR spectra of PVC after decomposed with out any thermal stabilizer. This spectrum contain clear and new deference peaks at several position where are not found in IR spectrum of stander PVC; (Figure 4) one of these peaks that appear at 3650 cm–1 which attributed to th e free hydrox yl g roup s stretching vibration which can formed as a result to thermal-oxidation process, while in the state of PVC contain deferent percent from Ca-lignin chelating com- plex; Figure 6, Figure 7, and Figure 8, where in this samples the peak became much less intense or not found. Additionally, clear peak can be detected in Figure 5, in the region between 320 0 - 3400 cm–1 corresponding to absorption of hydroxyl with hydrogen bond, but this not found in the presence on lignin derivative at all %wt. that mean increased PVC stability to thermal degradation by this additive where appear that the Ca-lignin chelating complex can be play a main role in the PVC stabilizing mechanism by it’s chemical structure which can ab- sorbed HCl. The intensified new strong and sharp at 1730 cm–1 in Figure 5 attributed to formation carbonyl groups as ali- phatic ketone in PVC after heat treatment and this not found in PVC contain 1% Figure 6 and became less in- tense in 2 wt% sample, Figure 7; other significant peak used to study the thermal degradation of PVC, polyene 40060080010001200140016001800200030004000 1/c m 37.5 45 52.5 60 67.5 75 82.5 90 97.5 %T 33 Faise hussen PVCS %T Figure 4. IR spectrum of pure PVC (blank). Copyright © 2011 SciRes. MSA  Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride 696 40060080010001200140016001800200030004000 1/cm 10 20 30 40 50 60 70 80 %T 34 Faise hussen PVCO %T Figure 5. IR spectrum of pure PVC after degradation. 40060080010001200140016001800200030004000 1/cm 37.5 45 52.5 60 67.5 75 82.5 90 %T 32 Faise hussen pvc-1 %T Figure 6. IR spectrum of PVC contain 1% Ca-lig.chelating complex. that formed in the first state of degradation process (Owen 1984) at 1602 - 1640 cm–1 which appear in Fig- ures 5, 7 and 8 and not found in Figure 6, that mean the best activity can be achieved at less concentration from this derivative. The absorption of the polyene and hydroxyl groups are used to follow the extend of polymer degradation by calculation polyene index (IPe) and hydroxyl index (IOH) and carbonyl index (ICO); as seen from Figure 9, Figure 10 and Figure 11 the presence of lignin derivative lead Copyright © 2011 SciRes. MSA 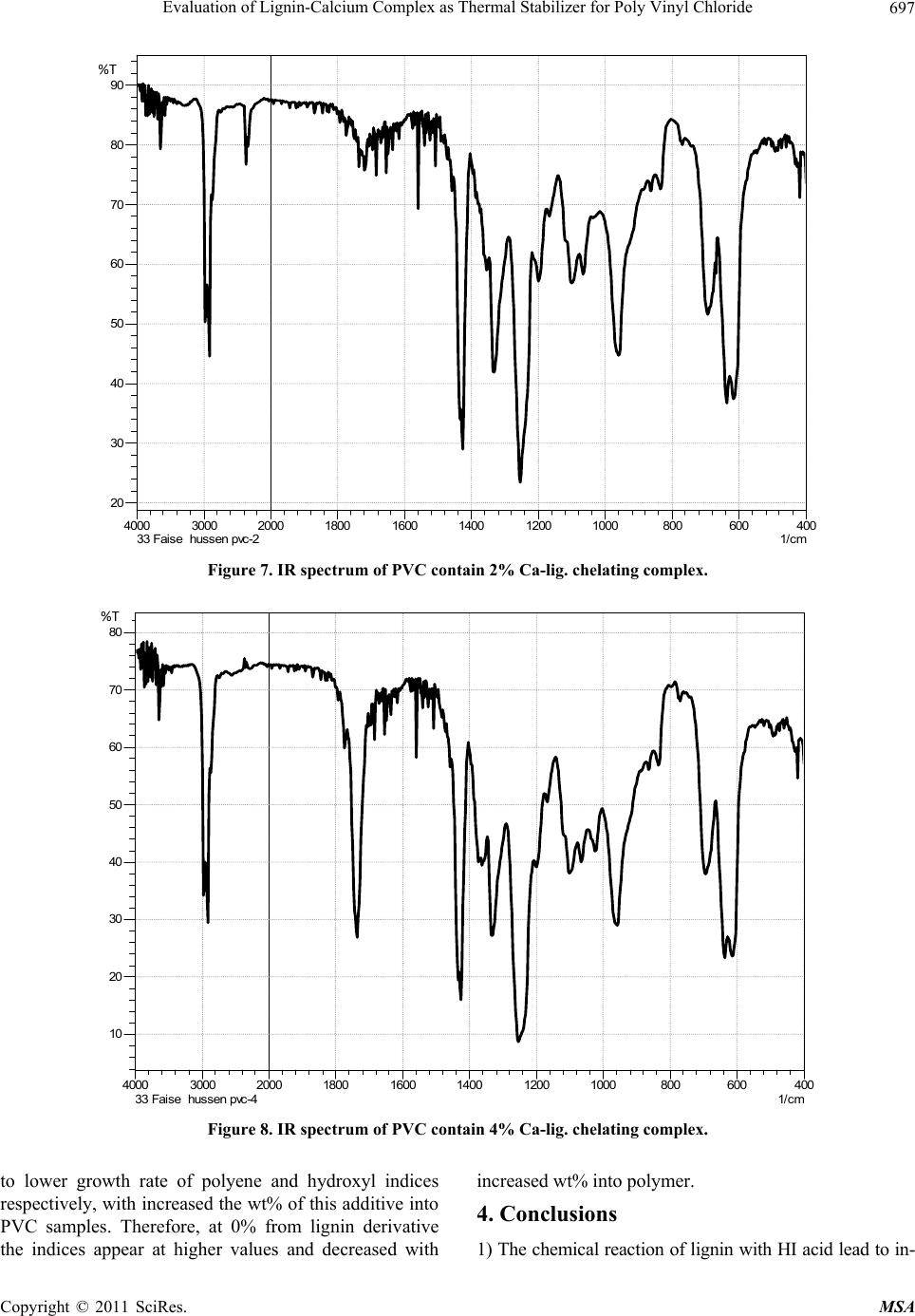 Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride 697 40060080010001200140016001800200030004000 1/cm 20 30 40 50 60 70 80 90 %T 33 Faise hussen pvc-2 %T Figure 7. IR spectrum of PVC contain 2% Ca-lig. chelating complex. 40060080010001200140016001800200030004000 1/cm 10 20 30 40 50 60 70 80 %T 33 Faise hussen pvc-4 %T Figure 8. IR spectrum of PVC contain 4% Ca-lig. chelating complex. to lower growth rate of polyene and hydroxyl indices respectively, with increased the wt% of this add itive into PVC samples. Therefore, at 0% from lignin derivative the indices appear at higher values and decreased with increased wt% into polymer. 4. Conclusions 1) The chemical reaction of lignin with HI acid lead to in - Copyright © 2011 SciRes. MSA 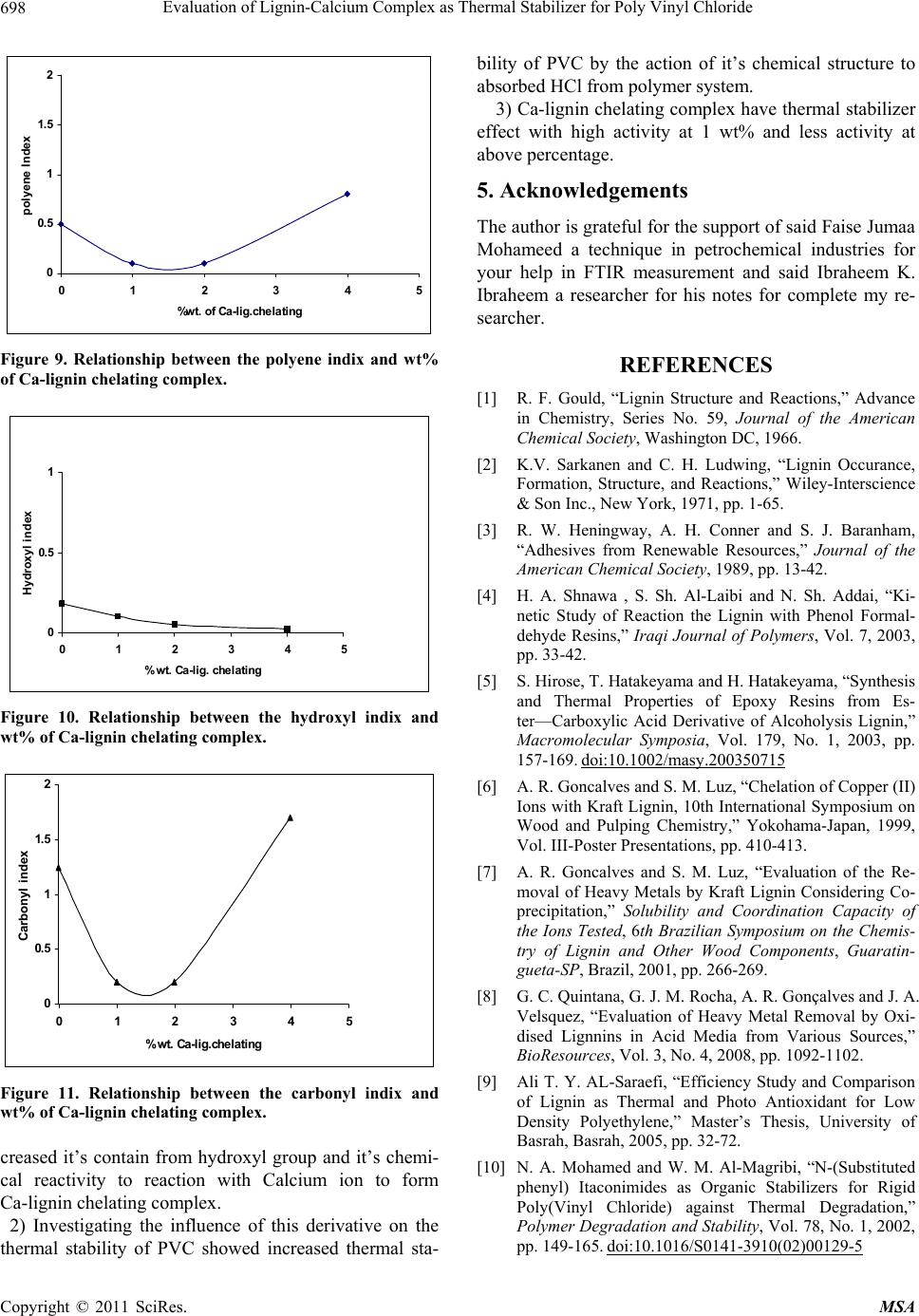 Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride 698 0 0.5 1 1.5 2 01234 %wt. of Ca-lig.chelating polyene Index 5 Figure 9. Relationship between the polyene indix and wt% of Ca-lignin chelating complex. 0 0.5 1 012345 % wt. Ca-lig. chelating Hydroxyl in d ex Figure 10. Relationship between the hydroxyl indix and wt% of Ca-lignin chelating complex. 0 0.5 1 1.5 2 012345 % wt. Ca- lig.chelating Carbonyl index Figure 11. Relationship between the carbonyl indix and wt% of Ca-lignin chelating complex. creased it’s contain from hydroxyl group and it’s chemi- cal reactivity to reaction with Calcium ion to form Ca-lignin chelating complex. 2) Investigating the influence of this derivative on the thermal stability of PVC showed increased thermal sta- bility of PVC by the action of it’s chemical structure to absorbed HCl from polymer system. 3) Ca-lignin chelating complex have thermal stabilizer effect with high activity at 1 wt% and less activity at above percentag e. 5. Acknowledgements The author is grateful for the suppo rt of said Faise Jumaa Mohameed a technique in petrochemical industries for your help in FTIR measurement and said Ibraheem K. Ibraheem a researcher for his notes for complete my re- searcher. REFERENCES [1] R. F. Gould, “Lignin Structure and Reactions,” Advance in Chemistry, Series No. 59, Journal of the American Chemical Society, Washington DC, 1966. [2] K.V. Sarkanen and C. H. Ludwing, “Lignin Occurance, Formation, Structure, and Reactions,” Wiley-Interscience & Son Inc., New York, 1971, pp. 1-65. [3] R. W. Heningway, A. H. Conner and S. J. Baranham, “Adhesives from Renewable Resources,” Journal of the American Chemical Society, 1989, pp. 13-42. [4] H. A. Shnawa , S. Sh. Al-Laibi and N. Sh. Addai, “Ki- netic Study of Reaction the Lignin with Phenol Formal- dehyde Resins,” Iraqi Journal of Polymers, Vol. 7, 2003, pp. 33-42. [5] S. Hirose, T. Ha takey a ma a nd H. Hatakeyama, “Synthesis and Thermal Properties of Epoxy Resins from Es- ter—Carboxylic Acid Derivative of Alcoholysis Lignin,” Macromolecular Symposia, Vol. 179, No. 1, 2003, pp. 157-169. doi:10.1002/masy.200350715 [6] A. R. Goncalves and S. M. Luz, “Chelation of Copper (II) Ions with Kraft Lignin, 10th International Symposium on Wood and Pulping Chemistry,” Yokohama-Japan, 1999, Vol. III-Poster Presentations, pp. 410-413. [7] A. R. Goncalves and S. M. Luz, “Evaluation of the Re- moval of Heavy Metals by Kraft Lignin Considering Co- precipitation,” Solubility and Coordination Capacity of the Ions Tested, 6th Brazilian Symposium on the Chemis- try of Lignin and Other Wood Components, Guaratin- gueta-SP, Brazil, 2001, pp. 266-269. [8] G. C. Quintana, G. J. M. Rocha, A. R. Gonçalves and J. A. Velsquez, “Evaluation of Heavy Metal Removal by Oxi- dised Lignnins in Acid Media from Various Sources,” BioResources, Vol. 3, No. 4, 2008, pp. 1092-1102. [9] Ali T. Y. AL-Saraefi, “Efficiency Study and Comparison of Lignin as Thermal and Photo Antioxidant for Low Density Polyethylene,” Master’s Thesis, University of Basrah, Basrah, 2005, pp. 32-72. [10] N. A. Mohamed and W. M. Al-Magribi, “N-(Substituted phenyl) Itaconimides as Organic Stabilizers for Rigid Poly(Vinyl Chloride) against Thermal Degradation,” Polymer Degradation and Stability, Vol. 78, No. 1, 2002, pp. 149-165. doi:10.1016/S0141-3910(02)00129-5 Copyright © 2011 SciRes. MSA  Evaluation of Lignin-Calcium Complex as Thermal Stab ilizer for Poly Vinyl Chloride Copyright © 2011 SciRes. MSA 699 [11] H. F. Alfred and W. H. Raymond, “The Mechanism of Poly(Vinyl Chloride) Tabilization by Barium, Cadmium, and Zinc Carboxylates. Infrared Studies,” Journal of Po- lymer Science, Vol. 40, No. 137, 1959, pp. 419-431. doi:10.1002/pol.1959.1204013712 [12] J. F. Rabek, “Experimental Methods in Poly mer Chemis- try,” John Wiley & Sons, New York, 1970, pp: 221-253. [13] E. Yousif, A. Hameed, A. Kamil, Y. Farina, N. Asaad and A. Graisa, “Synthesis of New Polymers Derived from Poly (Vinyl Chloride) and Study Their Biological Evaluation,” Australian Journal of Basic and Applied Sciences, Vol. 3, 2009, pp. 1786-1794. [14] H. Mekki and M. Belbachir, “Preparation of Vinyl Chlo- ride—Vinyl Ether Copolymers via Partial Etherification from PVC,” EXPRESS Polymer Letters, Vol. 11, 2007, pp. 495-498. [15] A. V. Karyakin, G. V. Grishin and B. D. Kurykin, “A Study of Photodegradation of PolyvinylChloride by In- frared Spectroscopy,” Polymer Science USSR, Vol. 7, No. 3, 1965, pp. 389-393. doi:10.1016/0032-3950(65)90077-8 [16] H. Kaczmarek, A. Felczak, D. Bajer and D. Bajer, “Photooxidative Degradation of Carboxylated Poly(Vinyl Chloride),” Polymer Bulletin, Vol. 62, No. 4, 2009, pp. 503-510. doi:10.1007/s00289-008-0030-y [17] M. Giurginca and Traian Zaharescu, “Thermo-Oxidative Degradation of Some Polymer Couples Containing HNBR,” Polymer Bulletin, Vol. 49, No. 5, 2003, pp. 357- 362. doi:10.1007/s00289-002-0115-y [18] R. Rasheed, H. Mansoor, E. Yousif, A. Hameed, Y. Fa- rina and A. Graisa, “Photostabilizing of PVC Films by 2-(Aryl)-5-[4-(Aryloxy)-Phenyl]-1,3,4-Oxadiazole Com- pounds,” European Journal of Scientific Research, Vol 30, 2009, pp. 464-477. [19] M. T. Taghizadeh, N. Nalbandi and A. Bahadori, “Stabi- lizing Effect of Epoxidized Sunflower Oil as a Secondary Stabilizer for Ca/Hg Stabilized PVC,” EXPRESS Polymer Letters, Vol. 2, 2008, pp. 65-76. [20] D. Braun and E. Bezdadea, “Theory of Degradation and Stabilisation Mechanisms,” In: L. I. Nass and C. A. Heiberger, Eds., Encyclopedia of PVC, Vol. 1, Mercel Deckker Inc., New York and Basel, 1986, pp. 397-429 . [21] E. D. Owen, “Degradation and Stabilisation of PVC,” Elsevier Applied Science Puplishers Ltd., London & New York, 1984, pp: 21-252. |

