Using Kaolinitic Clay for Preparation of a Hydrotalcite-Like Compound
690
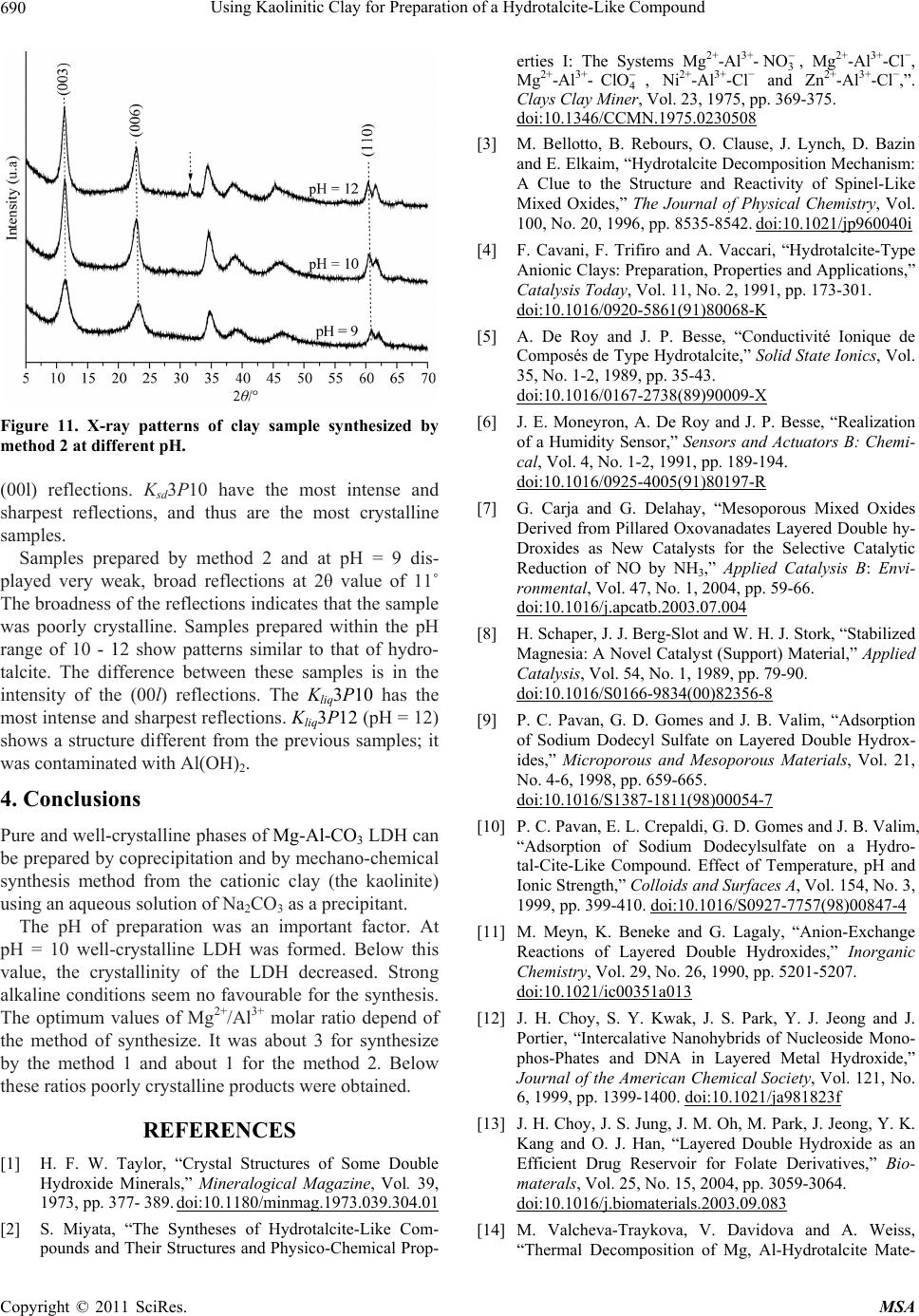
Figure 11. X-ray patterns of clay sample synthesized by
method 2 at different pH.
(00l) reflections. Ksd3P10 have the most intense and
sharpest reflections, and thus are the most crystalline
samples.
Samples prepared by method 2 and at pH = 9 dis-
played very weak, broad reflections at 2θ value of 11˚
The broadness of the reflections indicates that the sample
was poorly crystalline. Samples prepared within the pH
range of 10 - 12 show patterns similar to that of hydro-
talcite. The difference between these samples is in the
intensity of the (00l) reflections. The Kliq3P10 has the
most intense and sharpest reflections. Kliq3P12 (pH = 12)
shows a structure different from the previous samples; it
was contaminated with Al(OH)2.
4. Conclusions
Pure and well-crystalline phases of Mg-Al-CO3 LDH can
be prepared by coprecipitation and by mechano-chemical
synthesis method from the cationic clay (the kaolinite)
using an aqueous solution of Na2CO3 as a precipitant.
The pH of preparation was an important factor. At
pH = 10 well-crystalline LDH was formed. Below this
value, the crystallinity of the LDH decreased. Strong
alkaline conditions seem no favourable for the synthesis.
The optimum values of Mg2+/Al3+ molar ratio depend of
the method of synthesize. It was about 3 for synthesize
by the method 1 and about 1 for the method 2. Below
these ratios poorly crystalline products were obtained.
REFERENCES
[1] H. F. W. Taylor, “Crystal Structures of Some Double
Hydroxide Minerals,” Mineralogical Magazine, Vol. 39,
1973, pp. 377- 389. doi:10.1180/minmag.1973.039.304.01
[2] S. Miyata, “The Syntheses of Hydrotalcite-Like Com-
pounds and Their Structures and Physico-Chemical Prop-
erties I: The Systems Mg2+-Al3+-3
NO , Mg2+-Al3+-Cl−,
Mg2+-Al3+-4
ClO
, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−,”.
Clays Clay Miner, Vol. 23, 1975, pp. 369-375.
doi:10.1346/CCMN.1975.0230508
[3] M. Bellotto, B. Rebours, O. Clause, J. Lynch, D. Bazin
and E. Elkaim, “Hydrotalcite Decomposition Mechanism:
A Clue to the Structure and Reactivity of Spinel-Like
Mixed Oxides,” The Journal of Physical Chemistry, Vol.
100, No. 20, 1996, pp. 8535-8542. doi:10.1021/jp960040i
[4] F. Cavani, F. Trifiro and A. Vaccari, “Hydrotalcite-Type
Anionic Clays: Preparation, Properties and Applications,”
Catalysis Today, Vol. 11, No. 2, 1991, pp. 173-301.
doi:10.1016/0920-5861(91)80068-K
[5] A. De Roy and J. P. Besse, “Conductivité Ionique de
Composés de Type Hydrotalcite,” Solid State Ionics, Vol.
35, No. 1-2, 1989, pp. 35-43.
doi:10.1016/0167-2738(89)90009-X
[6] J. E. Moneyron, A. De Roy and J. P. Besse, “Realization
of a Humidity Sensor,” Sensors and Actuators B: Chemi-
cal, Vol. 4, No. 1-2, 1991, pp. 189-194.
doi:10.1016/0925-4005(91)80197-R
[7] G. Carja and G. Delahay, “Mesoporous Mixed Oxides
Derived from Pillared Oxovanadates Layered Double hy-
Droxides as New Catalysts for the Selective Catalytic
Reduction of NO by NH3,” Applied Catalysis B: Envi-
ronmental, Vol. 47, No. 1, 2004, pp. 59-66.
doi:10.1016/j.apcatb.2003.07.004
[8] H. Schaper, J. J. Berg-Slot and W. H. J. Stork, “Stabilized
Magnesia: A Novel Catalyst (Support) Material,” Applied
Catalysis, Vol. 54, No. 1, 1989, pp. 79-90.
doi:10.1016/S0166-9834(00)82356-8
[9] P. C. Pavan, G. D. Gomes and J. B. Valim, “Adsorption
of Sodium Dodecyl Sulfate on Layered Double Hydrox-
ides,” Microporous and Mesoporous Materials, Vol. 21,
No. 4-6, 1998, pp. 659-665.
doi:10.1016/S1387-1811(98)00054-7
[10] P. C. Pavan, E. L. Crepaldi, G. D. Gomes and J. B. Valim,
“Adsorption of Sodium Dodecylsulfate on a Hydro-
tal-Cite-Like Compound. Effect of Temperature, pH and
Ionic Strength,” Colloids and Surfaces A, Vol. 154, No. 3,
1999, pp. 399-410. doi:10.1016/S0927-7757(98)00847-4
[11] M. Meyn, K. Beneke and G. Lagaly, “Anion-Exchange
Reactions of Layered Double Hydroxides,” Inorganic
Chemistry, Vol. 29, No. 26, 1990, pp. 5201-5207.
doi:10.1021/ic00351a013
[12] J. H. Choy, S. Y. Kwak, J. S. Park, Y. J. Jeong and J.
Portier, “Intercalative Nanohybrids of Nucleoside Mono-
phos-Phates and DNA in Layered Metal Hydroxide,”
Journal of the American Chemical Society, Vol. 121, No.
6, 1999, pp. 1399-1400. doi:10.1021/ja981823f
[13] J. H. Choy, J. S. Jung, J. M. Oh, M. Park, J. Jeong, Y. K.
Kang and O. J. Han, “Layered Double Hydroxide as an
Efficient Drug Reservoir for Folate Derivatives,” Bio-
materals, Vol. 25, No. 15, 2004, pp. 3059-3064.
doi:10.1016/j.biomaterials.2003.09.083
[14] M. Valcheva-Traykova, V. Davidova and A. Weiss,
“Thermal Decomposition of Mg, Al-Hydrotalcite Mate-
Copyright © 2011 SciRes. MSA