Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 605-608 doi:10.4236/msa.2011.26081 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 605 Microscopic Structure of NaCd Compound Forming Binary Molten Alloys Sujeet Kumar Chatterjee1, Lokesh Chandra Prasad2, Ajaya Bhattarai1 1Department of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar, Nepal; 2 Department of Chemistry, Tilka Manjhi Bhagalpur University, Bhagalpur, India. Email: sujeetkumarchatterjee@yahoo.com Received March 14th, 2011; revised April 15th, 2011; accepted May 5th, 2011. ABSTRACT A simple statistical mechanical theory based on compound formation model (compound Aμ Bν formed by the preferen- tial arrangement of A and B constituent atoms of the alloy AB, μA + νB ⇌ Aμ Bν) has been used to investigate the phe- nomena of compound formation in NaCd liquid alloys through the study of concentration fluctuation in the long wave- length region [SCC(0)] and chemical short range order parameter (α1). The study explains the observed asymmetry suc- cessfully and suggest the existence of Cd2Na complex in the melt. The tendency of compound has been found to be weak. A tendency of inversion from segregation to order although very small, has been observed, in NaCd liquid alloys. Keywords: A Simple Statistical Mechanical Theory, Compound Formation Model, Concentration Fluctuation in the Long Wave Length Region [SCC(0)] and Chemical Short Range Order Parameter (α1) 1. Introduction Although the value of ex cess free energy of mixing XS M G 0.66 for NaCd liquid alloys is small (–4.49 KJ at cd C ), still it shows asymmetry around equiato mic composition. This interesting aspect led the authors to undertake a theoretical investigation of NaCd liquid alloys. Theoreti- cians have worked with various theoretical models [1-10], to understand the alloying behavior of compound form- ing binary molten alloys. All the theoretical model led to the fact that interatomic interaction plays an important role in comound formation. Due to the presence of strong interatomic interaction, these alloys form intermetallic compounds at one or more stoichiometric composition, which is also manifested in their phase diagram. The formation of compound in solid state led many theoreticians to believe in the existence of chemical complexes, pesudomolecules and privileged group of atoms near the melting temperature in binary liquid al- loys. Many theoretical models based on the above assump- tions have been used to investigate the alloying behavior of such type of binary molten alloys. The microscopic properties such as [SCC(0), α1] show maximum deviation from ideal behavior around compound forming concen- tration and α1 has large negative values indicating chem- ical order essential for compound formation. The phase diagram of NaCd alloy shows the existence of Cd2Na compound in the solid state. Hence, in the present investigation, a simple theoreti- cal model [2,7-11] of compound formation has been used to investigate the asymmetry in the property of mixing [Scc(o), α1] of Cd2Na compound forming liquid alloys. 2. Theoretical Consideration Let the binary solution contains in all NA = NC and NB = N(1 – C) gm moles of A and B atoms, respectively, C being the atomic fraction of A atoms. Following Bhatia and Hargrovem [1] and Bhatia et al. [3], only on e type of complex Aμ Bν (μ and ν are small integers) is assumed to be formed. If, in the solution n1 g mole A atoms and n2 g mole of B atoms and n3 g mole of Aμ Bν are formed, then from the law of conservation of atoms, 13 23 123 1 1 nNC n nN Cn nn nnNn 3 (1) where n is the total number of atoms in the case of com- pound formation. Under the frame work of above model, the free energy of mixing GM can be expressed as 3M Gng G (2) where (–n3g) is lowering of free energy due to the forma-  Microscopic Structure of NaCd Compound Forming Binary Molten Alloys 606 tion of the complex and “g” is the free energy of mixing of ternary mixture of fixed n1, n2 and n3 whose constitu- ents A, B an d Aμ Bν are supposed to interact weakly with one another. Flory [12] gave an expression of G taking into account the difference of size between the unassoci- ated species given as 3 33 1ln ln ii i ij ij GRTn nNnnN nn V NRT (3) where Vij(i, j = 1, 2, 3) are the interaction energies through which the left over A atom, B atom and the com- plex Aμ Bν interact with one another. The energies Vij are independent of concentration but may depend upon tem- perature and pressure. It is convenient to write the ex- pression for GM from Equations (2) and (3): 3 33 1ln ln Mii i ij ij GngRTnnNn nN nn V NRT 3 (4) The assignment of the value n3 for the evaluation of GM, through Equation (4) is the main problem which can be solved by using equilibrium relation given as 3,,, 0 M TPnC G n (5) Equations (4) and (5) give 1 12 3exp 1nnnNgRT Z (6) where 1 13 132323 2112 Z NRTnnVnnV nnV (7) Once the expression for GM obtained, other thermody- namic and microscopic functions, which are related to GM through standard thermodynamic relation follows readily as, ,, M MM TPN G HGT T (8a) MMM SHGT (8b) 22 1 ,, 0 1 CC M AA TPN SNRTGC Caa C (8c) 22 1 ,, 0 1 CC M BB TPN SNRTGC CaaC (8d) where aA, aB are the activities of A and B components respectively. When Equation (4) is used in Equations (8c) and (8d), the expression for Scc(0) is obtained as 1 2 311 2 1 2 0i CCij ij ii n SN nnv nNRT (9) The prime denotes differentiation with respect to con- centration i.e. 1 1 1 23 1 1 nn nn 3 (10) Differentiation of Equation (6) gives 1 3 n The determination of partial structure factor at q = 0, i.e. Scc(0) poses much more difficult experimental prob- lem and has never been accomplished successfully. Till now Scc(0) can not be evaluated experimentally by dif- fraction experiment. It can be determined experimentally from the measured activity data following two equalities of Equation (8d). This is usually considered as experi- mental data. The chemical short range order parameter (α1) quanti- fies the degree of order. It provides the immediate insight into local arrangement of atoms in the alloy. Preference for unlike atoms pairing or heterocoordination is indi- cated by α1 < 1, whereas like atoms pairing or self coor- dination is indicated by α1 > 1, α1 = 0 indicates complete disorder of the atom in the alloys. From probability approach one can show the limiting range of α1 easily as 1 1 1,1 2 1 11,1 2 cc c cc c (11) The minimum possible value of , gives complete order, whereas , gives complete segregation of atoms in the alloy. max 11 max 11 The experimental data of α1 is also very difficult be- cause it requires the knowledge of SCC(q) and SNN(q) which are concentration-concentration structure factor and number - number structure factor, respectively. However, in practice it is difficult to determine SNN(q) and SCC(q) for all kinds of binary alloys. The theoretical relation between α1 and SCC(0) for the first neighbour shell exists as 1 1 1 0 CC S SZS (12) Copyright © 2011 SciRes. MSA 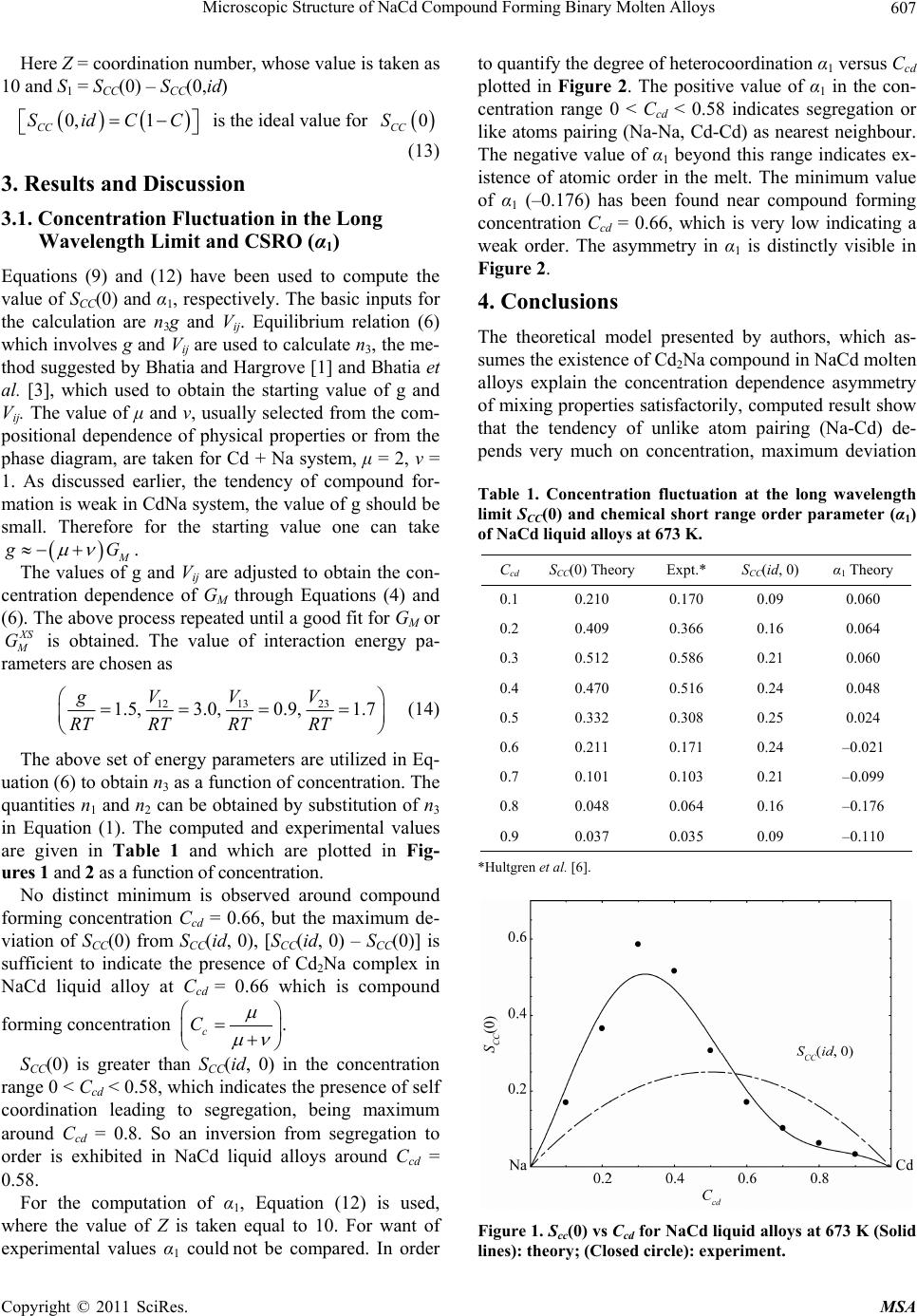 Microscopic Structure of NaCd Compound Forming Binary Molten Alloys607 Here Z = coordination number, whose value is taken as 10 and S1 = SCC(0) – SCC(0,id) 0, 1 CC SidCC is the ideal value for 0 CC S (13) 3. Results and Discussion 3.1. Concentration Fluctuation in the Long Wavelength Limit and CSRO (α1) Equations (9) and (12) have been used to compute the value of SCC(0 ) and α1, respectively. The basic inputs for the calculation are n3g and Vij. Equilibrium relation (6) which involves g and Vij are used to calculate n3, the me- thod suggested by Bhatia and Hargrove [1] and Bhatia et al. [3], which used to obtain the starting value of g and Vij. The value of μ and ν, usually selected from the com- positional dependence of physical properties or from the phase diagram, are taken for Cd + Na system, μ = 2, ν = 1. As discussed earlier, the tendency of compound for- mation is weak in CdNa system, the value of g should be small. Therefore for the starting value one can take M g G . The values of g and Vij are adjusted to obtain the con- centration dependence of GM through Equations (4) and (6). The above process repeated until a good fit for GM or XS M G is obtained. The value of interaction energy pa- rameters are chosen as 13 23 12 1.5,3.0,0.9, 1.7 VV V g RT RTRTRT (14) The above set of energy parameters are utilized in Eq- uation (6) to obtain n3 as a function of concentration. The quantities n1 and n2 can be obtained by substitution of n3 in Equation (1). The computed and experimental values are given in Table 1 and which are plotted in Fig- ures 1 and 2 as a function of concentration. No distinct minimum is observed around compound forming concentration Ccd = 0.66, but the maximum de- viation of SCC(0) from SCC(id, 0), [SCC(id, 0) – SCC(0)] is sufficient to indicate the presence of Cd2Na complex in NaCd liquid alloy at Ccd = 0.66 which is compound forming concentration c C . SCC(0) is greater than SCC(id, 0) in the concentration range 0 < Ccd < 0.58, which indicates the presence of self coordination leading to segregation, being maximum around Ccd = 0.8. So an inversion from segregation to order is exhibited in NaCd liquid alloys around Ccd = 0.58. For the computation of α1, Equation (12) is used, where the value of Z is taken equal to 10. For want of experimental values α1 could not be compared. In order to quantify the degree of heterocoordination α1 versus Ccd plotted in Figure 2. The positive value of α1 in the con- centration range 0 < Ccd < 0.58 indicates segregation or like atoms pairing (Na-Na, Cd-Cd) as nearest neighbour. The negative value of α1 beyond this range indicates ex- istence of atomic order in the melt. The minimum value of α1 (–0.176) has been found near compound forming concentration Ccd = 0.66, which is very low indicating a weak order. The asymmetry in α1 is distinctly visible in Figure 2. 4. Conclusions The theoretical model presented by authors, which as- sumes the existence of Cd2Na compound in NaCd molten alloys explain the concentration dependence asymmetry of mixing properties satisfactorily, computed result show that the tendency of unlike atom pairing (Na-Cd) de- pends very much on concentration, maximum deviation Table 1. Concentration fluctuation at the long wavelength limit SCC(0) and chemical short range order parameter (α1) of NaCd liquid alloys at 673 K. Ccd S CC(0) TheoryExpt.* SCC(id, 0) α1 Theory 0.1 0.210 0.170 0.09 0.060 0.2 0.409 0.366 0.16 0.064 0.3 0.512 0.586 0.21 0.060 0.4 0.470 0.516 0.24 0.048 0.5 0.332 0.308 0.25 0.024 0.6 0.211 0.171 0.24 –0.021 0.7 0.101 0.103 0.21 –0.099 0.8 0.048 0.064 0.16 –0.176 0.9 0.037 0.035 0.09 –0.110 *Hultgren et al. [6]. Figure 1. Scc(0) vs Ccd for NaCd liquid alloys at 673 K (Solid lines): theory; (Closed circle): experiment. Copyright © 2011 SciRes. MSA  Microscopic Structure of NaCd Compound Forming Binary Molten Alloys Copyright © 2011 SciRes. MSA 608 [3] A. B. Bhatia, W. H. Hargrove and D. E. Thorntron, “Con- centration Fluctuations and Partial Structure Factors of Compound-Forming Binary Molten Alloys,” Physical Review B, Vol. 9, No. 2, 1974, pp. 435-444. doi:10.1103/PhysRevB.9.435 [4] L. C. Prasad, R. N. Singh, V. N. Singh and S. K. Chatter- jee, “Compound Formation in Sn-Based Liquid Alloys,” Physica B: Physics of Condensed Matter, Vol. 215, No. 2-3, 1995, pp. 225-232. doi:10.1016/0921-4526(95)00393-N [5] L. C. Prasad, S. K. Chatterjee and V. N. Singh, “Inter- metallic Associations in AlMg Liquid Alloys,” Physica B: Physics of Condensed Matter, Vol. 217, No. 3-4, 1996, pp. 285-291. doi:10.1016/0921-4526(95)00885-3 [6] R. Hultgren, et al., “Selected Values of the Thermody- namic Properties of Binary Alloys,” The American Soci- ety of Metals, Ohio, 1973. Figure 2. α1 vs Ccd for NaCd liquid alloys at 673 K (Solid lines). [7] L. C. Prasad and R. N. Singh, “A Quasi-Lattice Model for the Thermodynamic Properties of Au-Zn Liquid Alloys,” Physics and Chemistry of Liquids, Vol. 22, No. 1-2, 1990, pp. 1-9. doi:10.1080/00319109008036406 is observed around Ccd = 0.66, which is compound form- ing concentration, indicates existence of Cd2 Na complex in the melt. The low negative value of α1 is sufficient to indicate the tendency of compound formation is very weak. An inversion from segregation to order is also clearly visible in NaCd liquid alloys. [8] K. Hoshino and W. H. Young, “Entropy of Mixing of Compound Forming Liquid Binary Alloys,” Journal of Physics F: Metal Physics, Vol. 10, No. 7, 1980, p. 1365. doi:10.1088/0305-4608/10/7/006 [9] N. K. P Singh, R. N. Singh and R. B. Choudhary, “Ther- modynamic Investigation of Atomic Order in AlMg Liq- uid Alloys,” Journal of Physics: Condensed Matter, Vol. 3, No. 20, 1991, p. 3635. doi:10.1088/0953-8984/3/20/024 5. Acknowledgements The authors are grateful to Prof. R. N. Singh, presently at the department of Physics, Sultan Quaboos University, Muscat, OMAN for his valuable suggestions and discus- sions. [10] R. N. Singh and F. Sommer, “Temperature Dependence of the Thermodynamic Functions of Strongly Interacting Liquid Alloys,” Journal of Physics: Condensed Matter, Vol. 4, No. 24, 1992, p. 5345. doi:10.1088/0953-8984/4/24/004 REFERENCES [1] A. B. Bhatia and W. H. Hargrove, “Concentration Fluc- tuations and Thermodynamic Properties of Some Com- pound Forming Binary Molten Systems,” Physical Re- view B, Vol. 10, No. 8, 1974, pp. 3186-3196. doi:10.1103/PhysRevB.10.3186 [11] S. K. Chatterjee and L. C. Prasad, “Microscopic Structure of CuSn Compound Forming Binary Molten Alloys,” In- dian Journal of Pure and Applied Physics, Vol. 42, 2004, pp. 283-287. [12] P. J. Flory, “Thermodynamics of High Polymer Solu- tions,” Journal of Chemical Physics, Vol. 10, 1942, pp. 51-61. doi:10.1063/1.1723621 [2] R. N. Singh, “Short-Range Order and Concentration Fluctuations in Binary Molten Alloys,” Candian Journal of Physics, Vol. 65, No. 3, 1987, pp. 309-325. doi:10.1139/p87-038 |

