Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 564-571 doi:10.4236/msa.2011.26075 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA TiO2/CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application Leinig Perazolli1*, Luciana Nuñez1, Milady Renata Apolinário da Silva2*, Guilherme Francisco Pegler1, Ademir Geraldo Cavalarri Costalonga1, Rossano Gimenes2, Márcia Matiko Kondo2, Maria Aparecida Zaghete Bertochi1 1Microwave Sintering and Photocatalysis Laboratory, Instituto de Química de Araraquara, Univ Estadual de São Paulo, Araraquara, Brazil; 2UNIFEI-Federal University of Itajubá, Itajubá, Brazil. Email: leinigp@iq.unesp.br, milady@unifei.edu.br Received February 5th, 2011; revised March 20th, 2011; accepted April 12th, 2011. ABSTRACT In the present work, the hybrid catalyst films of TiO2/CuO containing up to 10% in mol of copper were deposited onto glass surface. Precursor solutions were obtained by citrate precursor method. Films were porous and the average par- ticle size was 20 nm determined by FEG-SEM analysis. The photocatalytic activities of these films were studied using Rhodamine B as a target compound in a fixed bed reactor developed in our laboratory and UV lamp. It was observed that the addition of copper to TiO2 increased significantly its photocatalytic activity during the oxidation of Rhodamine B. The degradation exceeded 90% within 48 hours of irradiation compared to 38% when pure TiO2 was used. More- over, there was a reduction in the particles band gap energy when compared to that of pure TiO2. These results indicate that the TiO2/CuO films are promising catalysts for the development of fixed bed reactors to be used to treat effluents containing azo dyes. Keywords: TiO2/CuO, Citrate Precursor Method, Thin Film, Rhodamine B, Photocatalytic Oxidation 1. Introduction The contamination of potable water supplies is one of the major problems that has drawn global attention. As a consequence of the growing global industrialization the water quality of rivers and reservoirs is compro- mised, mainly as a result of the complex nature of the industrial pollutants that are being released to the envi- ronment, in addition to the deficiency of domestic sew- age treatment [1]. Because of the great amount of organic contaminated effluent generated and disposed without treatment, the textile industry can have a negative impact to the envi- ronment [2]. The problem continues even with the inap- propriate disposal of dyes and pigments considered non toxic. These compounds when discharged in superficial water tend to inhibit the passage of solar light, lead ing to the reduction of the local biodiversity. Approximately 60% of the dyes used worldwide belong to the family of azo dyes (azo group, –N=N–), which in combination with other chromophoric groups promote their colors [3]. Nonetheless, the main problem is the presence of the groups of toxic chemical substances in the composition of these dyes, which are mostly carcinogenic and muta- genic [4,5]. The majority of azo dyes are toxic and non biodegradable, which inhibit or make it difficult to use biological treatment processes [6-8]. For this reason, there has been a constant search for more efficient, inno- vative, and less costly technologies for the treatment of industrial effluents. Several researchers have investigated the mineraliza- tion of organics using Advanced Oxidative Processes (AOPs), in which the majority of these contaminants can be degraded into CO2, H2O and inorganic anions. These degradations are possible due to the reactions that in- clude transitory oxidative species, mainly hydroxyl radi- cals [9]. These radicals present a oxidation potential of 2.8 V, that is only lower than that of fluorine which is 3.03 V [10]. Among AOPs, TiO2 heterogenous photocatalysis has been successfully employed to destroy numerous classes of organic compounds [11], and also as a biocide agent [12-14]. This process was used initially by Pruden and Ollis [15,16] to degrade chloroform and trichloro ethylen e, which were totally mineralized by ultraviolet rad iation in  TiO /CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application565 2 the presence of TiO2. The semiconductors, such as TiO2, in their fundamen- tal state do not present a continuous level of energy, and therefore they do not present electrical conductivity. However, when irradiated with photons with the same or higher energy of the band gap (3.2 eV), an electronic excitation occurs and the electron can be promoted from the valence band to the conduction band, thereby gener- ating the electron/hole pair (e–/h+) [17]. The photogener- ated e–/h+ pair can either recombine to release heat, or make their separate ways to the surface of TiO2, where they can react with the adsorbed species on the catalyst, inducing the degradation of these substances. The anatase form of TiO2 is widely used, mainly due to its properties that include higher photosensibility, non-toxic nature, band gap value considered ideal for use with ultraviolet radiation in the germicidal range of 254 nm to solar light, elevated chemical stability, relatively lower cost, and it can also be optimized using dopants [18,19]. The TiO2 can be employed in suspension or with some kind of support, normally prepared by the sol-gel process [20,21]. However, the Pechini method [22] can also be used. This method uses polymeric precursors of citrate as well as ethylene glycol, which can facilitate the homogeneous distribution of cations in the crystalline structure and the acquisition of the thin films. In despite of the wide use of TiO2, several modifica- tions have been proposed in the surface and/or structure trying to increase the photocatalytic efficiency of this semiconductor. Its combination with other semiconduc- tors such as CdS, WO3, SnO2, including its doping with different metals have been largely studied [23-28]. Moreover, there have also been reports related to the combination of CuO with TiO2 [29-31] and the main reason for such combination was the possibility to obtain a potential increase in the photocatalytic efficiency, es- pecially when visible light is used [32-35]. In order to evaluate the pho tocatalytic activ ity of TiO2/ CuO binary system films, obtained by citrate precursor method, the present work studied the Rhodamine B deg- radation as the central parameter. Rhodamine B, which belongs to the dyes family known as fluorides, is an or- ganic molecule considered to be very stable that does not suffer degradation with visible light, and therefore can be used in pulsed dye lasers. 2. Experimental 2.1. Chemicals The reagents used in this study were 97% titanium iso- propoxide; citric acid P.A., titanium citrate P.A. and copper nitrate P.A. purchased from Aldrich. Extran, polyethylene glycol (PEG 20000) P.A. from Merck. Rhodamine B and ethylene glycol P.A. were purchase from Synth. 2.2. Catalyst and Film Preparation Titanium isopropoxide, citric acid and ethylene glycol were used in the molar rations of 1:4:16. Ethylene glycol was heated to 60˚C under magnetic stirred and titanium isopropoxide was added the agitation until the solution become clear. Then, the citric acid was added and the temperature was raised to 90˚C under magnetic stirred for 120 minutes. Copper nitrate was dissolved in deion- ized water and added drop wise into the aqueous solution of titanium citrate. TiO2/CuO catalyst with molar rations of 90/10 and 95/05 were obtained by the citrate precursor method. The precursor solution was magnetically stirred and heated at 110˚C. The pH was around 5 - 6 and in th is pH the precipitation of titanium and copper salts was avoid. Ethylene glycol was added to start the polysterifi- cation reaction and the solution was stirred and heated for two hours. After cooling the solution 1 mL of Ex- tran and polyethylene glycol (PEG 20000) were added in a proportion of 80% w/w in relation to the expected mass of oxide. These solutions was deposited onto boro- silicate glass surface using a spin coating technique (NS-400B-6NPP/LITE/10K model, Laurell Technologies) and it was thermally treated at 600˚C during 90 min, us- ing a heating ration of 1˚C·min –1. In order to compare the results and evaluated the influence of Cu, solutions of TiO2 without Cu was prepared and deposited in the same way of TiO2/CuO. Crystalline phases of films were analyzed by X-Ray diffraction (Rigaku 2000 X-ray Diffractometer, 2θ = 20 to 80 degrees, step scan at 0.2 s–1). Thickness and mor- phology of films were observed by scanning electron microscopy of field emission FEG-SEM (ZEISS™ FEG-UP Supra 35) and ultraviolet-visible region absorp- tion on UV-vis spectrometer (Varian 5G). 2.3. Photocatalytic Reactor The photocatalytic reactor was developed in our labora- tory according to illustration of Figure 1. The system, i.e. beaker, catalyst supported film, silicone tubes, and pump were assembled into a closed box in order to avoid the entrance of external light during the degradation experi- ment. The beaker containing 50 mL solution of 0.01 mM Rhodamine B was placed inside the box. The solution was forced to flow from the top to the bottom of the ox- ide film by the pump and recycled at 20 mL·min–1 flow rate. The system was irradiated by germicidal ultraviolet light of 9 W, with maximum wavelength of 254 nm and a film exposition area of 12 cm2. Samples of 2 mL solution were withdrawn taking into account the irradiation ex- posure time. The absorbance spectrum, in the range of Copyright © 2011 SciRes. MSA  TiO2/CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application Copyright © 2011 SciRes. MSA 566 Figure 1. Reactor illustration for the study of Rhodamine B decomposition using TiO2-CuO where a glass plate was used as a support. The recycle flow was 20 mL·min –1. 300 to 700 nm, was obtained using a spectrophotometer UV-vis. Control experiments were also carried out irra- diating the Rhodamine B solution using glass plate with films of pure TiO2 as well as without the catalyst, placed under similar conditions to that of the TiO2 film doped with Cu. 3. Results and Discussion 3.1. Films Characterization 3.1.1. X-Ray The X-ray diffractograms of the pure TiO2 and the films with 5 and 10 mol% of CuO are presented in Figure 2. The diffractograms allow only the identification of the anatase phase of TiO2 by the presence of the peak (101) in 2θ = 25.4˚, which was observed for all the three com- positions under study. The presence of CuO seems to favor the crystallization of anatase phase, since the FWHM of (101) peak narrows, as observed in Figure 2, for both TiO2/CuO films. 3.1.2. Field Emission Scanning Electron Microscope (FEG-SEM) Based on the analysis of the micrographic images of the pure TiO2 and the films doped with 5% and 10% mol of CuO (Figure 3), it was possible to verify the morphology and its th ickness, as well as th e average si ze of their par- ticles. The morphology of the films was homogeneous and appears almost spherical particles, with presence of porous forming a high porosity surface, which can en- hance the catalytic activity of the films. Analyzing the results showed on the Table 1 it is clear that the pure TiO2 film is thicker than TiO2/CuO films. This charac- teristic is due to the higher viscosity of precu rso r solution of the Ti than that of the Ti-CuO, since for the last ones, the solutions were diluted in order to guarantee the coo- per ions stability. Zhang [36] suggested that the op timum particle size is within the range of 11 - 21 nm to b e a good photocatalyst. Wh en the particle size is below the range of 5 - 10 nm, the surface recombination of electron-hole pairs was found to be significant, thus, leading to low pho tocatalytic effi- ciency. The present work showed particle size of 15 nm to the pure TiO2 and 20 nm to the TiO2/CuO. Those val- ues are similar to the particle size of the P-25 Degussa, 20 nm, as calculated by Tseng [29]. These results suggest that it is possible to synthesize powders and films from the citrate precursor method, obtaining average sizes of ideal particles for the application in photocatalysis. 3.2. Rhodamine B Degradation The UV-vis absorption spectra of the degradation of the Rhodamine B solutions using pure TiO2 films are pre- sented in Figure 4. It was observed that the Rhodamine B presented a maximum absorp tion peak within the region of 550 nm. This band was used as an indication of Rho- damine B degradation comparing the decrease of its ab- sorption during irradiation with the initial absorption in each system. The reduction occurred due to the catalytic activity of the pure TiO2 film. Figure 5 illustrates the absorption decrease, at 550 nm of the Rhodamine B solutions, during exposition time Figure 2. X-ray diffractograms of TiO2-films doped with u. C  TiO /CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application 567 2 (a) (b) (c) Figure 3. Micrographs of FEG-SEM of TiO2-films obtained by citrate precursor method: (a) Pure TiO2 films; (b) TiO2-CuO 5 mol%; (c) TiO2-CuO 10 mol%. Table 1. Thickness and particle size of TiO2 films obtained by citrate precursor method. Material Thickness (nm) Average Particle Size (nm) TiO2 269 ± 7 15 ± 2 TiO2 CuO 5% 180 ± 7 20 ± 2 TiO2 CuO 10% 183 ± 7 20 ± 2 using different photocatalytic systems. It can be observed that after 48 hours, the decrease in the absorbance of the irradiated solutions using pure TiO2 films reached about 38%. This result confirms the film activity, considering that the direct photolysis of Rhodamine B reached only 17% within the same irradiation time (data not shown). When TiO2/CuO films were employed as catalysts, it was observed a decrease of nearly 50% of Rhodamine B concentration after 12 and 10 hours of irradiation, using 5 and 10% of copper on TiO2 films, respectively. In ad- dition, after 48 hours of irradiation, there was a decrease in absorption of 90% and 94% in 550 nm for TiO2 films containing 5 and 10% of copper, respectively. However, the increase of CuO from 5 to 10% in the TiO2 film did not lead to any significant increase in the film photoac- tivity to the degradation of Rhodamine B. Nonetheless, analyzing the obtained results in the ab- sorption spectrum and takin into account the low flow g Copyright © 2011 SciRes. MSA 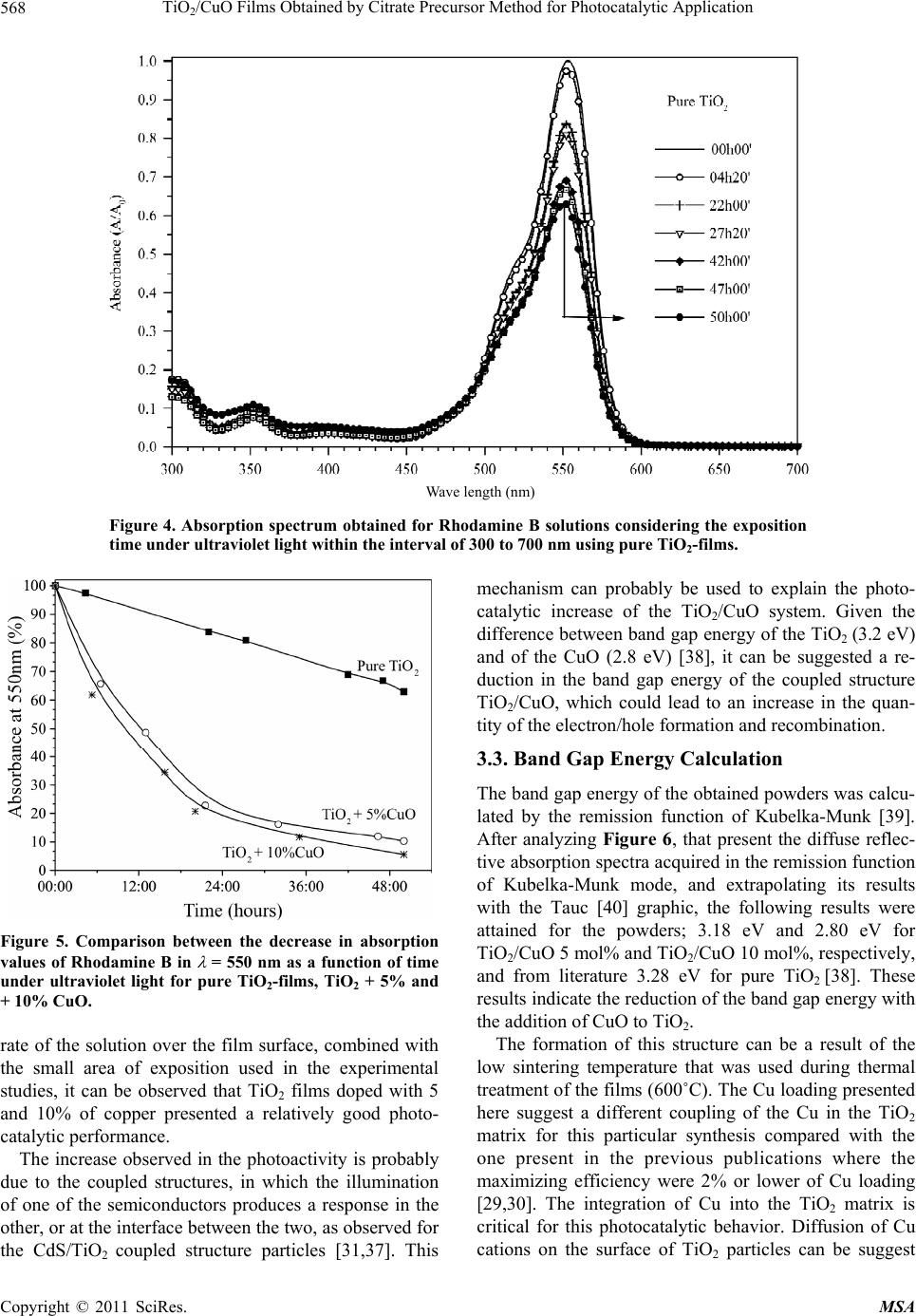 TiO /CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application 568 2 Wave length (nm) Figure 4. Absorption spectrum obtained for Rhodamine B solutions considering the exposition time under ultraviolet light within the interval of 300 to 700 nm using pure TiO2-films. Figure 5. Comparison between the decrease in absorption values of Rhodamine B in = 550 nm as a function of time under ultraviolet light for pure TiO2-films, TiO2 + 5% and + 10% CuO. rate of the solution over the film surface, combined with the small area of exposition used in the experimental studies, it can be observed that TiO2 films doped with 5 and 10% of copper presented a relatively good photo- catalytic performance. The increase observed in the photoactivity is probably due to the coupled structures, in which the illumination of one of the semiconductors produces a response in the other, or at the interface between the two, as observed for the CdS/TiO2 coupled structure particles [31,37]. This mechanism can probably be used to explain the photo- catalytic increase of the TiO2/CuO system. Given the difference between band gap energy of the TiO2 (3.2 eV) and of the CuO (2.8 eV) [38], it can be suggested a re- duction in the band gap energy of the coupled structure TiO2/CuO, which could lead to an increase in the quan- tity of the electron/hole formation and recombination. 3.3. Band Gap Energy Calculation The band gap energy of the obtain ed powders was calcu- lated by the remission function of Kubelka-Munk [39]. After analyzing Figure 6, that present the diffuse reflec- tive absorption spectra acquired in the remission function of Kubelka-Munk mode, and extrapolating its results with the Tauc [40] graphic, the following results were attained for the powders; 3.18 eV and 2.80 eV for TiO2/CuO 5 mol% and TiO2/CuO 10 mol%, respectively, and from literature 3.28 eV for pure TiO2 [38]. These results indicate the reduction of the band gap energy with the addition of CuO to TiO2. The formation of this structure can be a result of the low sintering temperature that was used during thermal treatment of the films (600˚C). The Cu loading presented here suggest a different coupling of the Cu in the TiO2 matrix for this particular synthesis compared with the one present in the previous publications where the maximizing efficiency were 2% or lower of Cu loading [29,30]. The integration of Cu into the TiO2 matrix is critical for this photocatalytic behavior. Diffusion of Cu cations on the surface of TiO2 particles can be suggest Copyright © 2011 SciRes. MSA 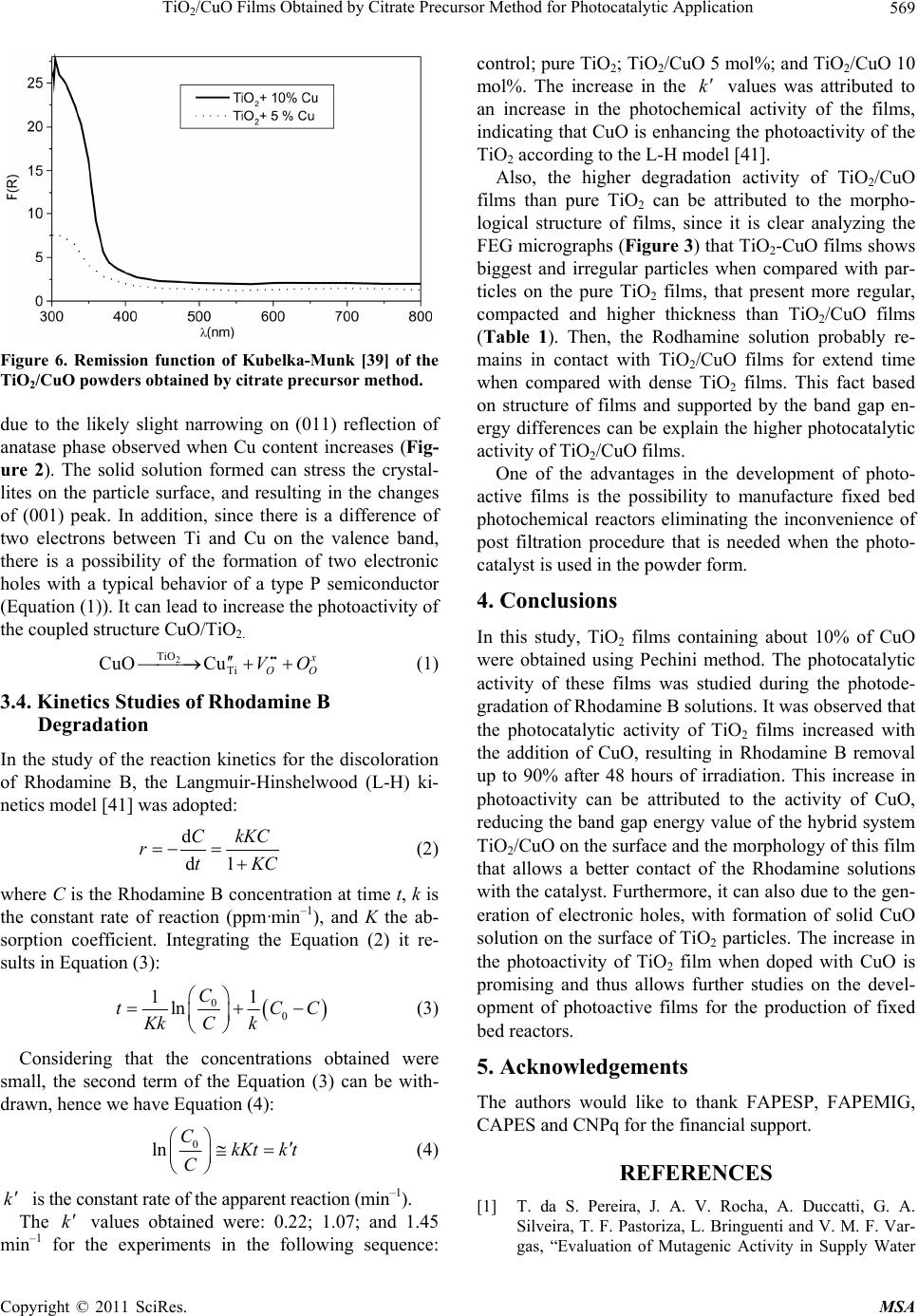 TiO /CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application569 2 Figure 6. Remission function of Kubelka-Munk [39] of the TiO2/CuO powders obtained by citr ate precursor method. due to the likely slight narrowing on (011) reflection of anatase phase observed when Cu content increases (Fig- ure 2). The solid solution formed can stress the crystal- lites on the particle surface, and resulting in the changes of (001) peak. In addition, since there is a difference of two electrons between Ti and Cu on the valence band, there is a possibility of the formation of two electronic holes with a typical behavior of a type P semiconductor (Equation (1)). It can lead to increase the pho toactiv ity of the coupled structure CuO/TiO2. 2 TiO Ti CuO Cu x O VO O (1) 3.4. Kinetics Studies of Rhodamine B Degradation In the study of the reaction kinetics for the discoloration of Rhodamine B, the Langmuir-Hinshelwood (L-H) ki- netics model [41] was adopted: d d1 CkKC rtK C (2) where C is the Rhodamine B concentration at time t, k is the constant rate of reaction (ppm·min–1), and K the ab- sorption coefficient. Integrating the Equation (2) it re- sults in Equation (3): 00 11 ln C t KkC k C C (3) Considering that the concentrations obtained were small, the second term of the Equation (3) can be with- drawn, h ence we have E q ua tion (4): 0 ln CkKtk t C (4) k i s the constant rate of the apparent reaction (m in–1). The values obtained were: 0.22; 1.07; and 1.45 min–1 for the experiments in the following sequence: control; pure TiO2; TiO2/CuO 5 mol%; and TiO2/CuO 10 mol%. The increase in the values was attributed to an increase in the photochemical activity of the films, indicating that CuO is enhancing th e photoactivity of the TiO2 according to the L-H model [41]. k k Also, the higher degradation activity of TiO2/CuO films than pure TiO2 can be attributed to the morpho- logical structure of films, since it is clear analyzing the FEG micrographs (Figure 3) that TiO2-CuO films shows biggest and irregular particles when compared with par- ticles on the pure TiO2 films, that present more regular, compacted and higher thickness than TiO2/CuO films (Table 1). Then, the Rodhamine solution probably re- mains in contact with TiO2/CuO films for extend time when compared with dense TiO2 films. This fact based on structure of films and supported by the band gap en- ergy differences can be explain the higher photocatalytic activity of TiO2/CuO films. One of the advantages in the development of photo- active films is the possibility to manufacture fixed bed photochemical reactors eliminating the inconvenience of post filtration procedure that is needed when the photo- catalyst is used in the powder form. 4. Conclusions In this study, TiO2 films containing about 10% of CuO were obtained using Pechini method. The photocatalytic activity of these films was studied during the photode- gradation of Rhodamine B solutions. It was observed that the photocatalytic activity of TiO2 films increased with the addition of CuO, resulting in Rhodamine B removal up to 90% after 48 hours of irradiation. This increase in photoactivity can be attributed to the activity of CuO, reducing the band gap en ergy value of the hybrid system TiO2/CuO on the surface and the morphology of this f ilm that allows a better contact of the Rhodamine solutions with the catalyst. Furthermore, it can also due to the gen- eration of electronic holes, with formation of solid CuO solution on the surface of TiO2 particles. The increase in the photoactivity of TiO2 film when doped with CuO is promising and thus allows further studies on the devel- opment of photoactive films for the production of fixed bed reactors. 5. Acknowledgements The authors would like to thank FAPESP, FAPEMIG, CAPES and CNPq for the financial support. REFERENCES [1] T. da S. Pereira, J. A. V. Rocha, A. Duccatti, G. A. Silveira, T. F. Pastoriza, L. Bringuenti and V. M. F. Var- gas, “Evaluation of Mutagenic Activity in Supply Water Copyright © 2011 SciRes. MSA  TiO /CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application 570 2 at Three Sites in the State of Rio Grande do Sul, Brazil,” Mutation Research, Vol. 629, No. 2, 2007, pp. 71-80. doi:10.1016/j.mrgentox.2006.12.008 [2] I. Arslan, A. I. Balcioglu and D. W. Bahneman, “Ad- vanced Chemical Oxidation of Reactive Dyes in Simu- lated Dyehouse Effluents by Ferrioxalate-Fenton/UV-A and TiO2/ UV-A Processes,” Dyes and Pigments, Vol. 47, No. 3, 2000, pp. 207-218. doi:10.1016/S0143-7208(00)00082-6 [3] P. V. Vandeviver, R. Bianch and W. Verstraete, “Review: Treatment and Reuse of Wastewater from the Textile Wet-Processing Industry: Review of Emerging Technolo- gies,” Journal of Chemical Technology and Biotechnol- ogy, Vol. 72, No. 4, 1998, pp. 289-302. doi:10.1002/(SICI)1097-4660(199808)72:4<289::AID-JC TB905>3.0.CO;2-# [4] V. S. Houk, “The Genotoxicity of Industrial-Wastes and Effluents: A Review,” Mutation Research, Vol. 277, No. 2, 1992, pp. 91-138. doi:10.1016/0165-1110(92)90001-P [5] M. A. Brown and S. C. Devito, “Predicting Azo Dye Toxicity,” Critical Reviews in Environmeantal Science and Technology, Vol. 23, No. 3, 1993, pp. 249-324. doi:10.1080/10643389309388453 [6] N. Daneshvar, D. Salari and A. R. Khataee, “Photocata- lytic Degradation of Azo Dye Acid Red 14 in Water: In- vestigation of the Effect of Operational Parameters,” Journal of Photochemistry and Photobiology A, Vol. 157, No. 1, 2003, pp. 111-116. doi:10.1016/S1010-6030(03)00015-7 [7] F. P. van der Zee and S. Villaverde, “Combined Anaero- bic-Aerobic Treatment of Azo Dyes—A Short Review of Bioreactor Studies,” Water Research, Vol. 39, No. 8, 2005, pp. 1425-1440. doi:10.1016/j.watres.2005.03.007 [8] B. Y. Chen, “Understanding Decolorization Characteris- tics of Reactive Azo Dyes by Pseudomonas Luteola: Toxicity and Kinetics,” Process Biochemistry, Vol. 38, No. 3, 2002, pp. 437-446. doi:10.1016/S0032-9592(02)00151-6 [9] R. Andreozzi, V. Caprio, A. Insola and R. Marotta, “Ad- vanced Oxidation Process (AOP) for Water Purification and Recovery,” Catalysis Today, Vol. 53, No. 1, 1999, pp. 51-59. doi:10.1016/S0920-5861(99)00102-9 [10] P. Wardman, “Reduction Potentials of One Electron Couples involving Free Radicals in Aqueous Solution,” Journal of Physical Chemical Reference Data, Vol. 18, No. 4, 1989, pp. 1637-1723. doi:10.1063/1.555843 [11] R. F. P. Nogueira and W. G. Jardim, “Heterogeneous Photocatalysis and Its Environmental Applications,” Química Nova, Vol. 21, No. 1, 1998, pp. 69-72. doi:10.1590/S0100-40421998000100011 [12] A. Mills, R. H. Davies and D.Worsley, “Water Purifica- tion by Semiconductor Photocatalysis,” Chemical Society Reviews, Vol. 22, No. 6, 1993, pp. 417-425. doi:10.1039/cs9932200417 [13] K. Rajeshwara, M. E. Osugi, W. Chanmanee, C. R. Chenthamarakshana, M. V. B. Zanoni, P. Kajitvichyanu- kul and R. Krishnan-Ayera, “Heterogeneous Photocata- lytic Treatment of Organic Dyes in Air and Aqueous Me- dia,” Journal of Photochemistry and Photobiology C, Vol. 9, No. 4, 2008, pp. 171-192. doi:10.1016/j.jphotochemrev.2008.09.001 [14] W. A. Jacoby , P. C. Maness, E. J. Wolfrum, D. M. Blake and J. A. Fennel, “Mineralization of Bacterial Cell Mass on a Photocatalytic Surface in Air,” Environmental Sci- ence and Technology, Vol. 32, No. 17, 1998, pp. 2650- 2653. doi:10.1021/es980036f [15] A. L. Pruden and D. F. Ollis, “Photoassisted heterogene- ous Catalysis—The Degradation of Trichloroethylene in Water,” Journal of Catalysis, Vol. 82, No. 2, 1983, pp. 404-417. doi:10.1016/0021-9517(83)90207-5 [16] A. L. Pruden and D. F. Ollis, “Degradation of Chloroform by Photoassisted Heterogeneous Catalysis in dilute Aqueous Suspensions of Titanium-Dioxide,” Environ- mental Science and Technology, Vol. 17, No. 10, 1983, pp. 628-631. doi:10.1021/es00116a013 [17] A. Fujishima and K. Honda, “Electrochemical Photolysis of Water at a Semiconductor Electrode,” Nature, Vol. 238, 1972, pp. 37-38. doi:10.1038/238037a0 [18] A. Fujishima, X. Zhang and D. A. Tryk, “TiO2 Photo- catalysis and Related Surface Phenomena,” Surface Sci- ence Reports, Vol. 63, No. 12, 2008, pp. 515-582. doi:10.1016/j.surfrep.2008.10.001 [19] S. Sakthivel, M. V. Shankar, M. Palanichamy, B. Ara- bindoo, D. W. Bahnemann and V. Murugesan, “En- hancement of Photocatalytic Activity by Metal Deposi- tion: Characterization and Photonic Efficiency of Pt, Au and Pd Deposited on TiO2 Catalyst,” Water Research, Vol. 38, No. 13, 2004, pp. 3001-3008. doi:10.1016/j.watres.2004.04.046 [20] C. Guillard, B. Beaugiraud, J. Herrmann, H. Jaffrezic, N. Jaffrezic-Reault and M. Lacroix, “Physicochemical Prop- erties and Photocatalytic Activities of TiO2-Films Pre- pared by Sol-Gel Methods,” Applied Catalysis B, Vol. 39, No. 4, 2002, pp. 331-342. doi:10.1016/S0926-3373(02)00120-0 [21] I. Kazuya; R. Sun, M. Toki, K. Hirota and O. Yamaguchi, “Sol-Gel-Derived TiO2/Poly(Dimethylsiloxane) Hybrid Films and Their Photocatalytic Activities,” Journal of Physics and Chemistry of Solids, Vol. 64, No. 3, 2003, pp. 507-513. doi:10.1016/S0022-3697(02)00357-8 [22] M. P. Pechini, US Patent, No. 3330697, 1967. [23] N. Serpone, P. Maruthamuthu, E. Pelizzetti and H. Hi- daka, “Exploiting the Interparticle Electron Transfer Process in the Photocatalysed Oxidation of Phenol, 2-Chlorophenol and Pentachlorophenol: Chemical Evi- dence for Electron and Hole Transfer between Coupled Semiconductors,” Journal of Photochemistry and Photo- biology A, Vol. 85, No. 3, 1995, pp. 247-255. doi:10.1016/1010-6030(94)03906-B [24] K. Y. Song, M. K. Park, Y. T. Kwon, H. W. Lee, W. J. Chung and W. I. Lee, “Preparation of Transparent Par- ticulate MoO3/TiO2 and WO3/TiO2 films and Their Photocatalytic Properties,” Chemistry of Materials, Vol. 13, No. 7, 2001, pp. 2349-2355. doi:10.1021/cm000858n Copyright © 2011 SciRes. MSA  TiO2/CuO Films Obtained by Citrate Precursor Method for Photocatalytic Application Copyright © 2011 SciRes. MSA 571 [25] A. A. Ismail, I. A. Ibrahim, R. M. Mohamed and H. El-Shall, “Sol-Gel Synthesis of Titania-Silica Photocata- lyst for Cyanide Photodegradation,” Journal of Photo- chemistry and Photobiology A, Vol. 63, No. 3, 2004, pp. 445-451. doi:10.1016/j.jphotochem.2004.01.017 [26] B. Pal, T. Hata, K. Goto and G. Nogami, “Photocatalytic Degradation of O-Cresol Sensitized by Iron-Titania Bi- nary Photocatalysts,” Journal of Molecular Catalysis A: Chemical, Vol. 169, No. 1-2, 2001, pp. 147-155. doi:10.1016/S1381-1169(00)00549-5 [27] A. A. Ismail, “Synthesis and Characterization of Y2O3/ Fe2O3/TiO2 Nanoparticles by Sol-Gel Method,” Applied Catalysis B: Environmental, Vol. 58, No. 1-2, 2005, pp. 115-121. doi:10.1016/j.apcatb.2004.11.022 [28] Y. Wang, H. Cheng, L. Zhang, Y. Hao, J. Ma, B. Xu and W. Li, “The Preparation, Characterization, Photoelectro- chemical and Photocatalytic Properties of Lanthanide Metal-Ion-Doped TiO2 Nanoparticles,” Journal of Mo- lecular Catalysis A: Chemical, Vol. 151, No. 1-2, 2000, pp. 205-216. doi:10.1016/S1381-1169(99)00245-9 [29] I.-H. Tseng, W. C. Chang and J. C. S. Wu, “Photoreduc- tion of CO2 Using Sol-Gel Derived Titania and Tita- nia-Supported Copper Catalysts,” Applied Catalysis B: Environmental, Vol. 37, No. 1, 2002, pp. 37-48. doi:10.1016/S0926-3373(01)00322-8 [30] I.-H. Tseng and J. C.-S. Wu, “Chemical States of Metal-Loaded Titania in the Photoreduction of CO2,” Catalysis Today, Vol. 97, No. 2-3, 2004, pp. 113-119. doi:10.1016/j.cattod.2004.03.063 [31] G. H. Li, N. M. Dimitrijevic, L. Chen, T. Rajh and K. A. Gray, “Role of Surface/Interfacial Cu2 Sites in the Photo- catalytic Activity of Coupled CuO TiO2 Nanocompo- sites,” The Journal of Physical Chemistry C, Vol. 112, No, 48, 2008, pp. 19040-19044. [32] H. Irie, K. Kamiya, T. Shibanuma, S. Miura, D. A. Tryk, T. Yokoyama and K. Hashimoto, “Visible Light-Sensitive Cu(II)-Grafted TiO2 Photocatalysts: Activities and X-ray Absorption Fine Structure Analyses,” The Journal of Physical Chemistry C, Vol. 113, No. 24, 2009, pp. 10761-10766. doi:10.1021/jp903063z [33] H. Irie, S. Miura, K. Kamiya, K. Hashimoto and K. Hashimo, “Efficient Visible Light-Sensitive Photocata- lysts: Grafting Cu(II) Ions onto TiO2 and WO3 Photo- catalysts,” Chemical Physics Letters, Vol. 457, No. 1-3, 2008, pp. 202-205. doi:10.1016/j.cplett.2008.04.006 [34] E. Vigil, F. A. Fernández-Lima, J. A. Ayllón, I. Zumeta, B. González, L. Curbelo, H. D. Fonseca Filho, M. E. H. M. da Costa, C. Domingo, M. Behar and F. C. Zawislak, “TiO2-CuO Three-Dimensional Heterostructure Obtained Using Short Time Photochemical Deposition of Copper Oxide Inside a Porous Nanocrystalline TiO2 Layer,” Mi- croporous and Mesoporous Materials, Vol. 109, No. 1-3, 2008, pp. 560-566. doi:10.1016/j.micromeso.2007.06.004 [35] E. Vigil, B. González, I. Zumeta, C. Domingo, X. Domé- nech and J. A. Ayllón, “Preparation of Photoelectrodes with Spectral Response in the Visible without Applied Bias Based on Photochemically Deposited Copper Oxide Inside a Porous Titanium Dioxide Film,” Thin Solid Films, Vol. 489, No. 1-2, 2005, pp. 50-55. doi:10.1016/j.tsf.2005.04.098 [36] Z. Zhang, C.-C. Wang, R. Zakaria and J. Y. Ying, “Role of Particle Size in Nanocrystalline TiO2-Based Photo- catalysts,” The Journal of Physical Chemistry B, Vol. 102, No. 52, 1998, pp. 10871-10878. doi:10.1021/jp982948+ [37] A. Mills and S. Le Hunte, “An Overview of semiconduc- tor Photocatalysis,” Journal of Photochemistry and Pho- tobiology A: Chemistry, Vol. 108, No. 1, 1997, pp. 1-35. doi:10.1016/S1010-6030(97)00118-4 [38] A. R. West, “Solid State Chemistry and Its Applications,” Wiley, New Jersey, 1987. [39] P. kubelka and F. Munk, “An Article on Optics of Paint Layers,” Zeitschrift für technische Physik, Vol. 12, 1931, pp. 593-609. [40] J. Tauc and D. L. Wod, “Weak Absorption Tails in Amor- phous Semiconductors,” Physical Review B, Vol. 5, No. 8, 1972, pp. 3144-3151. doi:10.1103/PhysRevB.5.3144 [41] I. K. Konstantinou and T. A. Albanis, “TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous So- lution: Kinetic and Mechanistic Investigations: A Re- view,” Applied Catalysis B, Vol. 49, No. 1, 2004, pp. 1-14. doi:10.1016/j.apcatb.2003.11.010 |

