Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 555-563 doi:10.4236/msa.2011.26074 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 555 Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures Jie Leng1, Qiulin Liao1, Yong Gao1, Huaming Li1,2 1College of Chemistry, Xiangtan University, Xiangtan, China; 2Key Laboratory of Polymeric Materials & Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymeric Materials of College of Hunan Province, and Key Lab of Envi- ronment-friendly Chemistry and Application in Ministry of Education, Xiangtan University, Xiangtan, China. Email: lihuaming@xtu.edu.cn Received March 27th, 2011; revised April 12th, 2011; accepted April 30th, 2011. ABSTRACT The self-assembly of organic 1-(2-pyridylazo)-2-naphthol (PAN) into hierarchical architectures, such as microfibers, mi- crorods, and sheaflike structures, in solution was successfully achieved by reprecipitation method with the assistance of thermoresponsive diblock copolymer poly(N,N-dimethylacrylamide)-b-poly(N-isopropylacrylamide) (PDMA-b-PNIPAM). It was found that the morphology modification can be readily controlled by varying the polymer concentrations. The optical absorption and fluorescence emission properties of the as-prepared PAN architectures were investigated. Time-dependent spectra of the precipitating solution for sheaflike structures formation were measured to monitor the self-assembly process of PAN molecules. The results showed that the PAN microstructures exhibited intense fluores- cence emission, indicating an unusual aggregation-induced emission enhancement (AIEE) phenomenon for PAN, which has great potential for future use in optoelectronic microdevices. Keywords: Organic Microstructures, Block Copolymer, Self-Assembly, Reprecipitation 1. Introduction Nano/microstructures based on small organic molecules have attracted considerable attention during the past few years due to the potential applications in diverse fields such as color-tunable display, field-effect transistors, chemical sensors, and optical waveguides [1-7]. There- fore, the fabrication of organic nano/microstructures with controlled sizes and shapes have been an intense and rapidly developing field of research since the properties are intimately related to its morphology [8-18]. Recently, extensive efforts have been devoted to the development of synthetic strategies for preparing organic nano/micro- structures such as reprecipitation [19-21], solvent evapo- ration [22], physical vapor deposition (PVD) [23], and template-directed method [24,25]. For example, tris(8- hydroxyquinoline)aluminum nanowires were success- fully synthesized by adsorbent-assisted PVD [23]. Addi- tionally, 1,4-bis(2-(5-phenyloxazolyl))benzene nanowires were also prepared using anodic aluminum oxide (AAO) membrane as a template [25]. However, it is still a big challenge to develop a simple and easy-tuned route for the fabrication of organic hierarchical architectures be- cause of the uncontrollability of intermolecular interac- tions and complexities of self-assembly process [18,26]. In recent articles dealing with the preparation of or- ganic nano/microstructures, the reprecipitation method has become one of the most popular methods because of its easy and versatile operation [27,28]. This facile method is based on solvent displacement by pouring mi- croamounts of organic compound solution into ma- croamounts of poor solvent. The sudden changes of en- vironment then induce the self-assembly of organic molecules. It has been reported that surfactant [24,29] and polymer micelles [19,30] can be employed as a addi- tive or template for the preparation of organic nano/mi- crostructures. For instance, Qi [19] and co-worker pre- pared uniform dye Sudan microrods with the assistance of Pluronic F127. In our previous paper [31], we have reported the syn- thesis of diblock copolymer PDMA-b-PNIPAM via re- versible addition fragmentation chain transfer (RAFT) polymerization. The expected transition from molecu- larly dissolved unimers at low temperature to aggregated  Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures 556 micelles above its critical micelle temperature was ob- served upon heating the copolymer aqueous solution. From the viewpoint of applications, it would be greatly beneficial if the micelles self-assembled in aqueous me- dia may respond to the external changes. This copolymer is well suited to act as a template for the fabrication of organic architectures. The present work is designed to fabricate 1-(2-pyridylazo)-2-naphthol (PAN) hierarchical architectures by reprecipitation method. Although PAN for analytical purposes has been investigated and widely applied in liquid-liquid extraction separation [32-33] and spectrometric determination of metal ions [34-36], there is little work on the synthesis of morphology-controlled PAN superstructures. In this work, hierarchical architec- tures of organic PAN were prepared through a tem- plate-assisted reprecipitation method. By tuning the tem- plate concentrations in the reaction system, the mor- phologies of PAN structure can be manipulated from fibers to rods, and then to sheaflike structures. The opti- cal properties of the prepared products were investigated by absorption and fluorescence spectroscopy. 2. Experimental Section 2.1. Materials PAN was recrystallized twice from EtOH. Block co- polymer PDMA-b-PNIPAM and homo-PDMA were synthesized by RAFT polymerization as described else- where [31,37]. Copolymer with a molecular weight of 54,000 g/mol (denoted as DMA268-NIPAM243) was used in this study. 2.2. Characterization Scanning electron microscope (SEM) images were re- corded using a field emission scanning electron micro- scope (JEOL, JSM-6360LV), and the samples were loaded on the mica surface, previously sputter-coated with a homogeneous gold layer for charge dissipation during the SEM imaging. The powder X-ray diffraction (XRD) patterns of the samples were measured on a Japan Rigaku D/max-2500 diffractometer with CuKa radiation (λ = 1.5418 Å). FTIR spectra in KBr pellets were re- corded on a PE Spectrum One FTIR spectrophotometer. UV-Vis spectra were measured using PE Lamada 25 spectrometer. Fluorescence spectra were recorded on a PE LS-55 luminescence spectrometer. Dynamic light scattering (DLS) was performed on a Brookhaven In- struments BI-200 SM equipped with a BI-APD correlator and a crystalaser GCL-100-L operating at 532 nm with a fixed scattering angle of 90˚, and the temperature was controlled by a PolyScience 9102 digital temperature controller. To ensure that DLS measurements were not affected by dust, all solution samples were filtered through 200 nm membrane filters. 2.3. Synthesis of PAN Hierarchical Architectures The organic hierarchical architectures of PAN were pre- pared through reprecipitation method. In a typical prepa- ration, a solution of PAN in ethanol (2 mM, 400 mL) was injected into 5 mL of aqueous DMA268-NIPAM243 solu- tion (10 mg/mL) with vigorous stirring at 50˚C. After stirring for 5 min, the sample was left undisturbed for about 8 days. The resultant precipitate was centrifuged, washed thoroughly with deionized water, and dried under vacuum at room temperature for characterization. Repre- cipitation was also carried out at varied DMA268-NI- PAM243 concentrations and temperatures. For compari- son purposes, copolymer DMA268-NIPAM243 was re- placed by homo-PDMA for the reprecipitation of PAN. 3. Results and Discussion 3.1. Preparation of Polymeric Micelles Polymer micelles were employed to assist the fabrication of PAN hierarchical architectures in this study. To obtain PDMA-b-PNIPAM micelles, aqueous solution of the diblock copolymer DMA268-NIPAM243 with different concentrations was raised from room temperature to their micellization temperature. The expected transition from molecularly dissolved unimers at low temperature to ag- gregated micelles above their critical micelle temperature (CMT) was observed by DLS measurements. Above the CMT, the NIPAM segment became dehydrated due to an entropy gain resulting from the release of water mole- cules upon association of the isopropyl groups [38]. The diameter of micelles was also determined by DLS. It is found that the CMT and the micelle diameter are sensi- tive to the concentration of the diblock copolymer. For example, the CMTs of the DMA268-NIPAM243 aqueous solution at concentrations of 0.5, 2.0, 5.0 and 10.0 mg/mL are approximately 37˚C, 36˚C, 34˚C and 33˚C, respectively, while the hydrodynamic diameters of the formed micelles are approximately 63, 58, 54 and 48 nm, respectively (Figure 1). It is important to note that the temperature induced association/dissociation process was reversible over numerous heating and cooling cycles, and micelle sizes remained approximately constant. These observations are consistent with a recent report by McCormick and coworkers [37]. In the following study, the DMA268-NIPAM243 micelles were used to assist the reprecipitation of PAN hierarchical architectures. 3.2. Reprecipitation of PAN from Aqueous Copolymer Solution Due to the insolubility of PAN in water, we turn to ther- moresponsive amphiphilic PDMA-b-PNIPAM polymer Copyright © 2011 SciRes. MSA 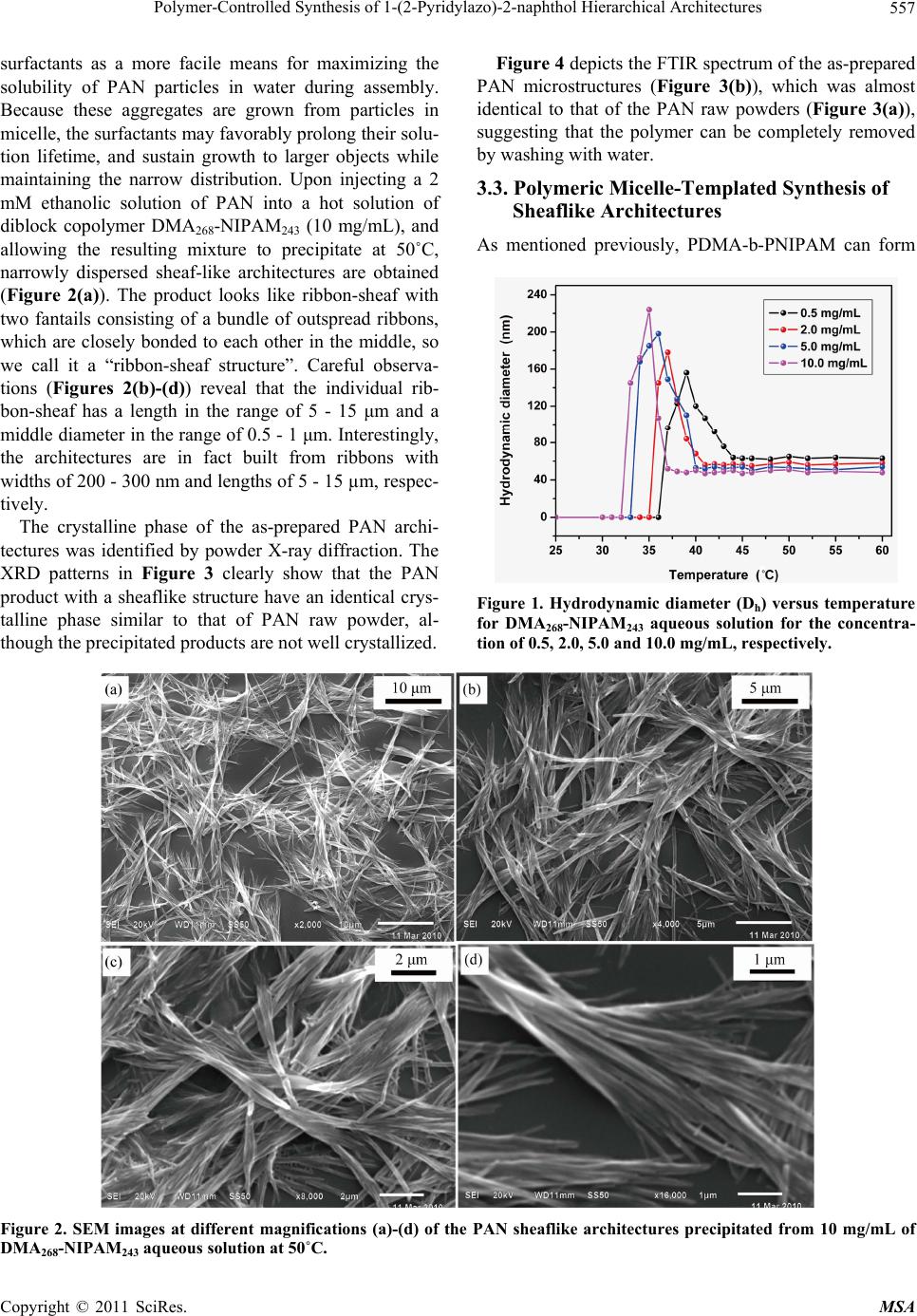 Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures Copyright © 2011 SciRes. MSA 557 Figure 4 depicts the FTIR spectrum of the as-prepared PAN microstructures (Figure 3(b)), which was almost identical to that of the PAN raw powders (Figure 3(a)), suggesting that the polymer can be completely removed by washing with water. surfactants as a more facile means for maximizing the solubility of PAN particles in water during assembly. Because these aggregates are grown from particles in micelle, the surfactants may favorably prolong their solu- tion lifetime, and sustain growth to larger objects while maintaining the narrow distribution. Upon injecting a 2 mM ethanolic solution of PAN into a hot solution of diblock copolymer DMA268-NIPAM243 (10 mg/mL), and allowing the resulting mixture to precipitate at 50˚C, narrowly dispersed sheaf-like architectures are obtained (Figure 2(a)). The product looks like ribbon-sheaf with two fantails consisting of a bundle of outspread ribbons, which are closely bonded to each other in the middle, so we call it a “ribbon-sheaf structure”. Careful observa- tions (Figures 2(b)-(d)) reveal that the individual rib- bon-sheaf has a length in the range of 5 - 15 μm and a middle diameter in the range of 0.5 - 1 μm. Interestingly, the architectures are in fact built from ribbons with widths of 200 - 300 nm and lengths of 5 - 15 µm, respec- tively. 3.3. Polymeric Micelle-Templated Synthesis of Sheaflike Architectures As mentioned previously, PDMA-b-PNIPAM can form The crystalline phase of the as-prepared PAN archi- tectures was identified by powder X-ray diffraction. The XRD patterns in Figure 3 clearly show that the PAN product with a sheaflike structure have an identical crys- talline phase similar to that of PAN raw powder, al- though the precipitated products are not well crystallized. Figure 1. Hydrodynamic diameter (Dh) versus temperature for DMA268-NIPAM243 aqueous solution for the concentra- tion of 0.5, 2.0, 5.0 and 10.0 mg/mL, respectively. Figure 2. SEM images at different magnifications (a)-(d) of the PAN sheaflike architectures precipitated from 10 mg/mL of MA268-NIPAM243 aqueous solution at 50˚C. D 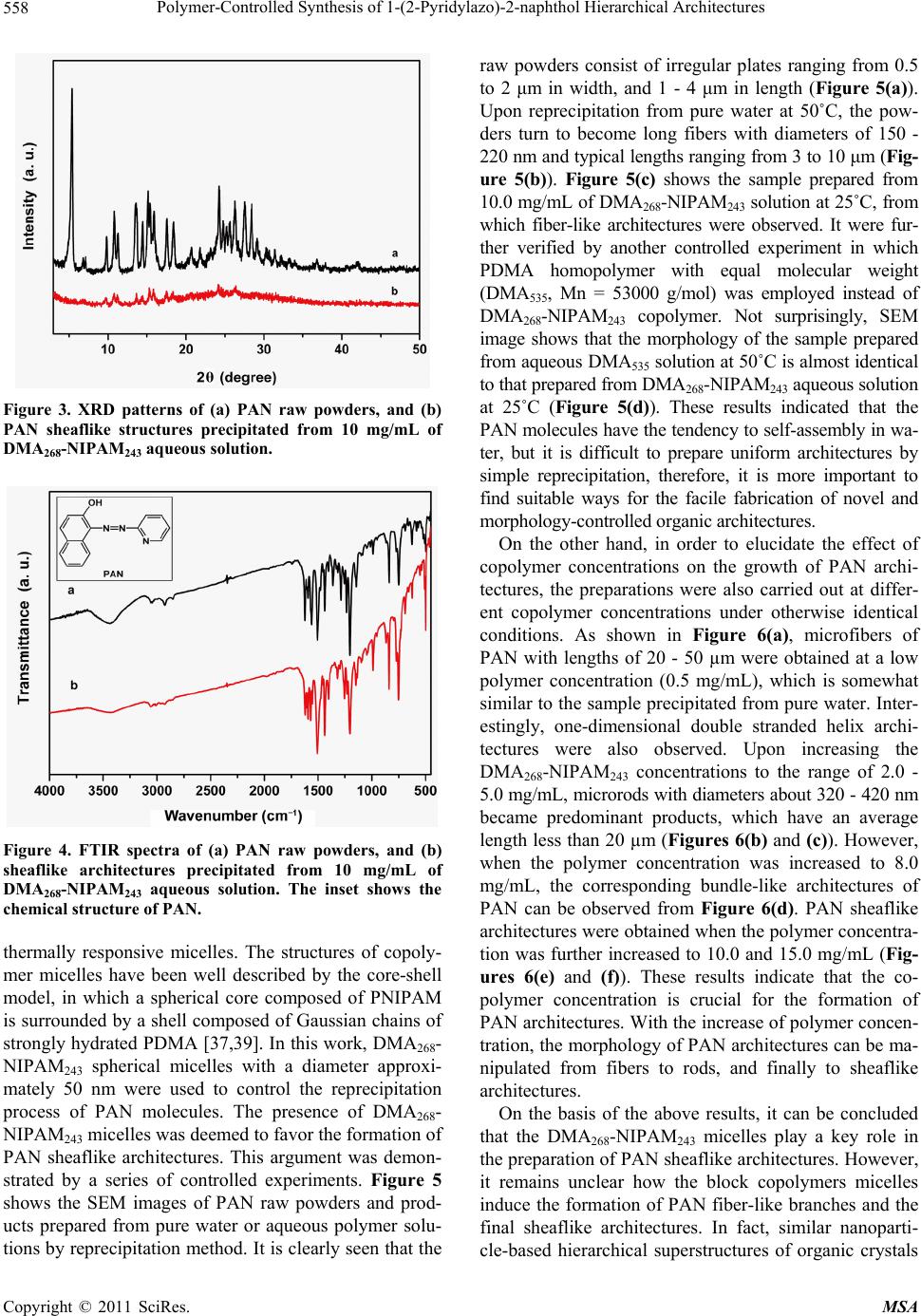 Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures 558 Figure 3. XRD patterns of (a) PAN raw powders, and (b) PAN sheaflike structures precipitated from 10 mg/mL of DMA268-NIPAM243 aqueous solution. Figure 4. FTIR spectra of (a) PAN raw powders, and (b) sheaflike architectures precipitated from 10 mg/mL of DMA268-NIPAM243 aqueous solution. The inset shows the chemical structure of PAN. thermally responsive micelles. The structures of copoly- mer micelles have been well described by the core-shell model, in which a spherical core composed of PNIPAM is surrounded by a shell composed of Gaussian chains of strongly hydrated PDMA [37,39]. In this work, DMA268- NIPAM243 spherical micelles with a diameter approxi- mately 50 nm were used to control the reprecipitation process of PAN molecules. The presence of DMA268- NIPAM243 micelles was deemed to favor the formation of PAN sheaflike architectures. This argument was demon- strated by a series of controlled experiments. Figure 5 shows the SEM images of PAN raw powders and prod- ucts prepared from pure water or aqueous polymer solu- tions by reprecipitation method. It is clearly seen that the raw powders consist of irregular plates ranging from 0.5 to 2 μm in width, and 1 - 4 μm in length (Figure 5(a)). Upon reprecipitation from pure water at 50˚C, the pow- ders turn to become long fibers with diameters of 150 - 220 nm and typical lengths ranging from 3 to 10 μm (Fig- ure 5(b)). Figure 5(c) shows the sample prepared from 10.0 mg/mL of DMA268-NIPAM243 solution at 25˚C, from which fiber-like architectures were observed. It were fur- ther verified by another controlled experiment in which PDMA homopolymer with equal molecular weight (DMA535, Mn = 53000 g/mol) was employed instead of DMA268-NIPAM243 copolymer. Not surprisingly, SEM image shows that the morphology of the sample prepared from aqueous DMA535 solution at 50˚C is almost identical to that prepared from DMA268-NIPAM243 aqueous solution at 25˚C (Figure 5(d)). These results indicated that the PAN molecules have the tendency to self-assembly in wa- ter, but it is difficult to prepare uniform architectures by simple reprecipitation, therefore, it is more important to find suitable ways for the facile fabrication of novel and morphology-controlled organic architectures. On the other hand, in order to elucidate the effect of copolymer concentrations on the growth of PAN archi- tectures, the preparations were also carried out at differ- ent copolymer concentrations under otherwise identical conditions. As shown in Figure 6(a), microfibers of PAN with lengths of 20 - 50 µm were obtained at a low polymer concentration (0.5 mg/mL), which is somewhat similar to the sample precipitated from pure water. Inter- estingly, one-dimensional double stranded helix archi- tectures were also observed. Upon increasing the DMA268-NIPAM243 concentrations to the range of 2.0 - 5.0 mg/mL, microrods with diameters about 320 - 420 nm became predominant products, which have an average length less than 20 µm (Figures 6(b) and (c)). However, when the polymer concentration was increased to 8.0 mg/mL, the corresponding bundle-like architectures of PAN can be observed from Figure 6(d). PAN sheaflike architectures were obtained when the polymer concentra- tion was further increased to 10.0 and 15.0 mg/mL (Fig- ures 6(e) and (f)). These results indicate that the co- polymer concentration is crucial for the formation of PAN architectures. With the increase of polymer concen- tration, the morphology of PAN architectures can be ma- nipulated from fibers to rods, and finally to sheaflike architectures. On the basis of the above results, it can be concluded that the DMA268-NIPAM243 micelles play a key role in the preparation of PAN sheaflike architectures. However, it remains unclear how the block copolymers micelles induce the formation of PAN fiber-like branches and the final sheaflike architectures. In fact, similar nanoparti- cle-based hierarchical superstructures of organic crystals Copyright © 2011 SciRes. MSA 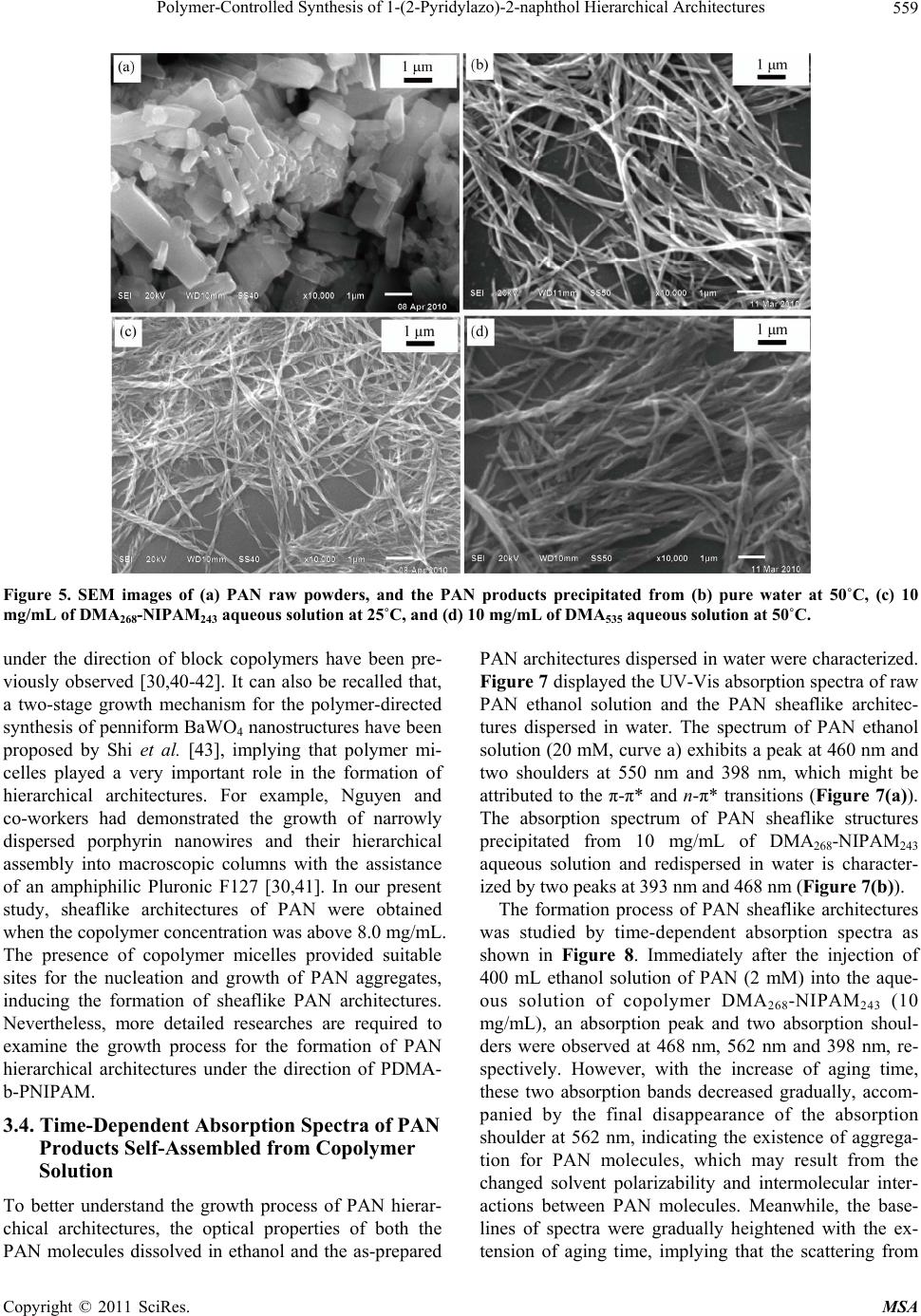 Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures559 Figure 5. SEM images of (a) PAN raw powders, and the PAN products precipitated from (b) pure water at 50˚C, (c) 10 mg/mL of DMA268-NIPAM243 aqueous solution at 25˚C, and (d) 10 mg/mL of DMA535 aqueous solution at 50˚C. under the direction of block copolymers have been pre- viously observed [30,40-42]. It can also be recalled that, a two-stage growth mechanism for the polymer-directed synthesis of penniform BaWO4 nanostructures have been proposed by Shi et al. [43], implying that polymer mi- celles played a very important role in the formation of hierarchical architectures. For example, Nguyen and co-workers had demonstrated the growth of narrowly dispersed porphyrin nanowires and their hierarchical assembly into macroscopic columns with the assistance of an amphiphilic Pluronic F127 [30,41]. In our present study, sheaflike architectures of PAN were obtained when the copolymer concentration was above 8.0 mg/mL. The presence of copolymer micelles provided suitable sites for the nucleation and growth of PAN aggregates, inducing the formation of sheaflike PAN architectures. Nevertheless, more detailed researches are required to examine the growth process for the formation of PAN hierarchical architectures under the direction of PDMA- b-PNIPAM. 3.4. Time-Dependent Absorption Spectra of PAN Products Self-Assembled from Copolymer Solution To better understand the growth process of PAN hierar- chical architectures, the optical properties of both the PAN molecules dissolved in ethanol and the as-prepared PAN architectures dispersed in water were characterized. Figure 7 displayed the UV-Vis absorption spectra of raw PAN ethanol solution and the PAN sheaflike architec- tures dispersed in water. The spectrum of PAN ethanol solution (20 mM, curve a) exhibits a peak at 460 nm and two shoulders at 550 nm and 398 nm, which might be attributed to the π-π* and n-π* transitions (Figure 7(a)). The absorption spectrum of PAN sheaflike structures precipitated from 10 mg/mL of DMA268-NIPAM243 aqueous solution and redispersed in water is character- ized by two peaks at 393 nm and 468 nm (Figure 7(b)). The formation process of PAN sheaflike architectures was studied by time-dependent absorption spectra as shown in Figure 8. Immediately after the injection of 400 mL ethanol solution of PAN (2 mM) into the aque- ous solution of copolymer DMA268-NIPAM243 (10 mg/mL), an absorption peak and two absorption shoul- ders were observed at 468 nm, 562 nm and 398 nm, re- spectively. However, with the increase of aging time, these two absorption bands decreased gradually, accom- panied by the final disappearance of the absorption shoulder at 562 nm, indicating the existence of aggrega- tion for PAN molecules, which may result from the changed solvent polarizability and intermolecular inter- actions between PAN molecules. Meanwhile, the base- lines of spectra were gradually heightened with the ex- tension of aging time, implying that the scattering from Copyright © 2011 SciRes. MSA  Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures 560 Figure 6. SEM images of PAN products precipitated from DMA268-NIPAM243 aqueous solutions with different polymer con- centrations: (a) 0.5; (b) 2; (c) 5; (d) 8; (e) 10; and (f) 15 mg/mL. larger particles could also be detected for longer aging times. Upon further aging of the precipitating solution for 8 days, PAN sheaflike architectures were obtained (Fig- ure 7(b)), which exhibit two absorption peaks at 393 nm and 468 nm. The fluorescence emission properties of both the PAN ethanol solution and PAN sheaflike architectures redis- persed in water were also characterized as shown in Fig- ure 9. The PAN ethanol solution shows two emission peaks around 330 and 350 nm when excited at 273 nm (Figure 9(a)). Different from that of PAN ethanol solu- tion, two main emission peaks at 332 and 423 nm are observed in the spectrum of PAN sheaflike architectures when excited at 273 nm (Figure 9(b)). It is important to note that there is a strong red shift of emission peak from 350 to 423 nm. To gain insight into the above mentioned phenomenon, we investigated the time-dependent fluorescence emis- sion spectra obtained after injecting 400 mL ethanol solu- tion of PAN (2 mL) into an aqueous solution of DMA268-NIPAM243 (10 mg/mL), which is shown in Fig- ure 10. Immediately after the injection, three very weak emission peaks at around 332, 423, and 484 nm were observed. With increasing aging time, the emission in- tensities of spectra increase gradually but the peak posi- tions are immovable, suggesting an unusual aggregation induced emission enhancement (AIEE) process, which is consistent with the previous observation [44-46]. Both of the decreasing rotations of rigid molecular planes and the change of solvent polarity are considered to be beneficial Copyright © 2011 SciRes. MSA 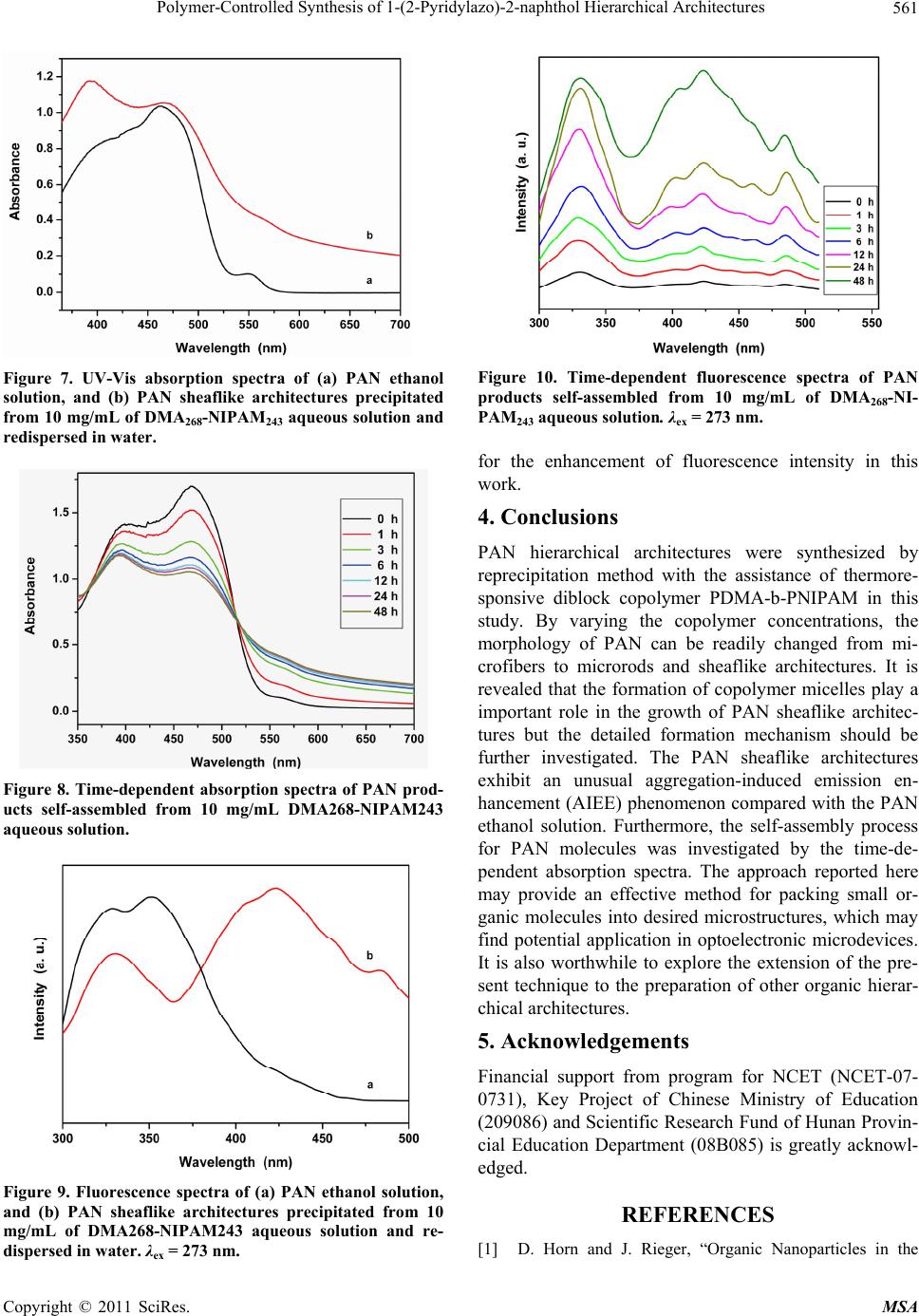 Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures561 Figure 7. UV-Vis absorption spectra of (a) PAN ethanol solution, and (b) PAN sheaflike architectures precipitated from 10 mg/mL of DMA268-NIPAM243 aqueous solution and redispersed in water. Figure 8. Time-dependent absorption spectra of PAN prod- ucts self-assembled from 10 mg/mL DMA268-NIPAM243 aqueous solution. Figure 9. Fluorescence spectra of (a) PAN ethanol solution, and (b) PAN sheaflike architectures precipitated from 10 mg/mL of DMA268-NIPAM243 aqueous solution and re- dispersed in water. λex = 273 nm. Figure 10. Time-dependent fluorescence spectra of PAN products self-assembled from 10 mg/mL of DMA268-NI- PAM243 aqueous solution. λex = 273 nm. for the enhancement of fluorescence intensity in this work. 4. Conclusions PAN hierarchical architectures were synthesized by reprecipitation method with the assistance of thermore- sponsive diblock copolymer PDMA-b-PNIPAM in this study. By varying the copolymer concentrations, the morphology of PAN can be readily changed from mi- crofibers to microrods and sheaflike architectures. It is revealed that the formation of copolymer micelles play a important role in the growth of PAN sheaflike architec- tures but the detailed formation mechanism should be further investigated. The PAN sheaflike architectures exhibit an unusual aggregation-induced emission en- hancement (AIEE) phenomenon compared with the PAN ethanol solution. Furthermore, the self-assembly process for PAN molecules was investigated by the time-de- pendent absorption spectra. The approach reported here may provide an effective method for packing small or- ganic molecules into desired microstructures, which may find potential application in optoelectronic microdevices. It is also worthwhile to explore the extension of the pre- sent technique to the preparation of other organic hierar- chical architectures. 5. Acknowledgements Financial support from program for NCET (NCET-07- 0731), Key Project of Chinese Ministry of Education (209086) and Scientific Research Fund of Hunan Provin- cial Education Department (08B085) is greatly acknowl- edged. REFERENCES [1] D. Horn and J. Rieger, “Organic Nanoparticles in the Copyright © 2011 SciRes. MSA  Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures 562 Aqueous Phase-Theory, Experiment, and Use,” Ange- wandte Chemie International Edition, Vol. 40, No. 23, 2001, pp. 4330-4361. doi:10.1002/1521-3773(20011203)40:23<4330::AID-ANI E4330>3.0.CO;2-W [2] F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, “About Supramolecular Assemblies of π-Conjugated Systems,” Chemical Reviews, Vol. 105, No. 4, 2005, pp. 1491-1546. doi:10.1021/cr030070z [3] Y. S. Zhao, H. Fu, A. Peng, Y. Ma, D. Xiao and J. Yao, “Low-Dimensional Nanomaterials Based on Small Or- ganic Molecules: Preparation and Optoelectronic Proper- ties,” Advanced Materials, Vol. 20, No. 15, 2008, pp. 2859-2876. doi:10.1002/adma.200800604 [4] Q. Tang, L. Jiang, Y. Tong, H. Li, Y. Liu, Z. Wang, W. Hu, Y. Liu and D. Zhu, “Micrometer- and Nanome- ter-Sized Organic Single-Crystalline Transistors,” Ad- vanced Materials, Vol. 20, No. 15, 2008, pp. 2947-2951. doi:10.1002/adma.200800669 [5] S. Cui, H. Liu, L. Gan, Y. Li and D. Zhu, “Fabrication of Low-Dimension Nanostructures Based on Organic Con- jugated Molecules,” Advanced Materials, Vol. 20, No. 15, 2008, pp. 2918-2925. doi:10.1002/adma.200800619 [6] F. Bertorelle, D. Lavabre and S. Fery-Forgues, “Den- drimer-Tuned Formation of Luminescent Organic Micro- crystals,” Journal of the American Chemical Society, Vol. 125, No. 20, 2003, pp. 6244-6253. doi:10.1021/ja029822h [7] Y. S. Zhao, H. Fu, A. Peng, Y. Ma, Q. Liao and J. Yao, “Construction and Optoelectronic Properties of Organic One-Dimensional Nanostructures,” Accounts of Chemical Research, Vol. 43, No. 3, 2009, pp. 409-418. doi:10.1021/ar900219n [8] M. Abyan, F. Bertorelle and S. Fery-Forgues, “Use of Linear Polymers to Control the Preparation of Lumines- cent Organic Microcrystals,” Langmuir, Vol. 21, No. 13, 2005, pp. 6030-6037. doi:10.1021/la046877j [9] L. Bîrlǎ, F. Bertorelle, F. Rodrigues, S. Badré, R. Pansu and S. Fery-Forgues, “Effects of DNA on the Growth and Optical Properties of Luminescent Organic Microcrys- tals,” Langmuir, Vol. 22, No. 14, 2006, pp. 6256-6265. doi:10.1021/la0532510 [10] H. B. Fu and J. N. Yao, “Size Effects on the Optical Prop- erties of Organic Nanoparticles,” Journal of the American Chemical Society, Vol. 123, No. 7, 2001, pp. 1434-1439. doi:10.1021/ja0026298 [11] D. Xiao, L. Xi, W. Yang, H. Fu, Z. Shuai, Y. Fang and J. Yao, “Size-Tunable Emission from 1,3-Diphenyl-5-(2- anthryl)-2-pyrazoline Nanoparticles,” Journal of the American Chemical Society, Vol. 125, No. 22, 2003, pp. 6740-6745. doi:10.1021/ja028674s [12] C. F. J. Faul and M. Antonietti, “Facile Synthesis of Op- tically Functional, Highly Organized Nanostructures: Dye-Surfactant Complexes,” Chemistry—A European Journal, Vol. 8, No. 12, 2002, pp. 2764-2768. doi:10.1002/1521-3765(20020617)8:12<2764::AID-CHE M2764>3.0.CO;2-X [13] A. Taden, K. Landfester and M. Antonietti, “Crystalliza- tion of Dyes by Directed Aggregation of Colloidal Inter- mediates: A Model Case,” Langmuir, Vol. 20, No. 3, 2004, pp. 957-961. doi:10.1021/la035723l [14] H. P. Cong and S. H. Yu, “Recrystallization and Shape Control of Crystals of the Organic Dye Acid Green 27 in a Mixed Solvent,” Chemistry—A European Journal, Vol. 13, No. 5, 2007, pp. 1533-1538. doi:10.1002/chem.200600881 [15] T. K. Zhang, J. H. Zhu, H. B. Yao and S. H. Yu, “Novel Fluorescein Hierarchical Structures Fabricated by Re- crystallization under Control of Polyelectrolytes,” Crystal Growth & Design, Vol. 7, No. 12, 2007, pp. 2419-2428. doi:10.1021/cg0703644 [16] Y. Wang, H. Fu, A. Peng, Y. S. Zhao, D. Xiao, J. Ma, Y. Ma and J. Yao, “Distinct Nanostructures from Isomeric Molecules of Bis(Iminopyrrole) Benzenes: Effects of Molecular Structures on Nanostructural Morphologies,” Chemical Communications, No. 16, 2007, pp. 1623-1625. doi:10.1039/b701327b [17] Y. Gao, X. Zhang, C. Ma, X. Li and J. Jiang, “Mor- phology-Controlled Self-Assembled Nanostructures of 5,15-Di[4-(5-Acetylsulfanylpentyloxy)Phenyl]Porphyrin Derivatives. Effect of Metal-Ligand Coordination Bond- ing on Tuning the Intermolecular Interaction,” Journal of the American Chemical Society, Vol. 130, No. 50, 2008, pp. 17044-17052. doi:10.1021/ja8067337 [18] X. J. Zhang, X. H. Zhang, K. Zou, C. S. Lee and S. T. Lee, “Single-Crystal Nanoribbons, Nanotubes, and Nanowires from Intramolecular Charge-Transfer Organic Mole- cules,” Journal of the American Chemical Society, Vol. 129, No. 12, 2007, pp. 3527-3532. doi:10.1021/ja0642109 [19] H. Yu and L. Qi, “Polymer-Assisted Crystallization and Optical Properties of Uniform Microrods of Organic Dye Sudan II,” Langmuir, Vol. 25, No. 12, 2009, pp. 6781-6786. doi:10.1021/la900296y [20] R. O. Al-Kaysi, A. M. Müller, T. S. Ahn, S. Lee and C. J. Bardeen, “Effects of Sonication on the Size and Crystal- linity of Stable Zwitterionic Organic Nanoparticles Formed by Reprecipitation in Water,” Langmuir, Vol. 21, No. 17, 2005, pp. 7990-7994. doi:10.1021/la051183b [21] H. Jiang, X. Sun, M. Huang, Y. Wang, D. Li and S. Dong, “Rapid Self-Assembly of Oligo(O-Phenylenediamine) into One-Dimensional Structures through a Facile Repre- cipitation Route,” Langmuir, Vol. 22, No. 7, 2006, pp. 3358-3361. doi:10.1021/la053091s [22] M. Kastler, W. Pisula, D. Wasserfallen, T. Pakula and K. Müllen, “Influence of Alkyl Substituents on the Solution- and Surface-Organization of Hexa-Peri-Hexabenzocoro- nenes,” Journal of the American Chemical Society, Vol. 127, No. 12, 2005, pp. 4286-4296. doi:10.1021/ja0430696 [23] Y. S. Zhao, C. Di, W. Yang, G. Yu, Y. Liu and J. Yao, “Photoluminescence and Electroluminescence from Tris (8-Hydroxyquinoline)Aluminum Nanowires Prepared by Adsorbent-Assisted Physical Vapor Deposition,” Ad- vanced Functional Materials, Vol. 16, No. 15, 2006, pp. 1985-1991. doi:10.1002/adfm.200600070 [24] H. Fu, D. Xiao, J. Yao and G. Yang, “Nanofibers of Copyright © 2011 SciRes. MSA  Polymer-Controlled Synthesis of 1-(2-Pyridylazo)-2-naphthol Hierarchical Architectures Copyright © 2011 SciRes. MSA 563 1,3-Diphenyl-2-Pyrazoline Induced by Cetyltrimethyl- ammonium Bromide Micelles,” Angewandte Chemie In- ternational Edition, Vol. 42, No. 25, 2003, pp. 2883-2886. doi:10.1002/anie.200350961 [25] J. K. Lee, W. K. Koh, W. S. Chae and Y. R. Kim, “Novel Synthesis of Organic Nanowires and Their Optical Prop- erties,” Chemical Communications, 2002, pp. 138-139. doi:10.1039/b109881k [26] L. Jiang, Y. Fu, H. Li and W. Hu, “Single-Crystalline, Size, and Orientation Controllable Nanowires and Ul- tralong Microwires of Organic Semiconductor with Strong Photoswitching Property,” Journal of the Ameri- can Chemical Society, Vol. 130, No. 12, 2008, pp. 3937-3941. doi:10.1021/ja077600j [27] E. V. Keuren, E. Georgieva and J. Adrian, “Kinetics of the Formation of Organic Molecular Nanocrystals,” Nano Letters, Vol. 1, No. 3, 2001, pp. 141-144. doi:10.1021/nl005523j [28] H. Kasai, H. S. Nalwa, H. Oikawa, S. Okada, H. Matsuda, N. Minami, A. Kakuta, K. Ono, A. Mukoh and H. Naka- nishi, “A Novel Preparation Method of Organic Micro- crystals,” Japanese Journal of Applied Physics, Vol. 31, 1992, pp. 1132-1134. doi:10.1143/JJAP.31.L1132 [29] X. Zhang, X. Zhang, W. Shi, X. Meng, C. Lee and S. Lee, “Morphology-Controllable Synthesis of Pyrene Nanos- tructures and Its Morphology Dependence of Optical Properties,” Journal of Physical Chemistry B, Vol. 109, No. 40, 2005, pp. 18777-18780. doi:10.1021/jp052385j [30] S. J. Lee, J. T. Hupp and S. T. Nguyen, “Growth of Nar- rowly Dispersed Porphyrin Nanowires and Their Hierar- chical Assembly into Macroscopic Columns,” Journal of the American Chemical Society, Vol. 130, No. 30, 2008, pp. 9632-9633. doi:10.1021/ja801733t [31] J. Liu, Z. Nie, Y Gao, A. Adronov and H. Li, “ ‘Click’ Coupling between Alkyne-Decorated Multiwalled Carbon Nanotubes and Reactive PDMA-PNIPAM Micelles,” Journal of Polymer Science A, Vol. 46, No. 21, 2008, pp. 7187-7199. doi:10.1002/pola.23026 [32] R. G. Anderson and G. Nickless, “Heterocyclic Azo Dye- Stuffs in Analytical Chemistry. A Review,” Analyst, Vol. 92, No. 1093, 1967, pp. 207-238. doi:10.1039/an9679200207 [33] J. Gao, B. Peng, H. Fan, J. Kang and X. Wang, “Spectro- photometric Determination of Palladium after Solid-Liq- uid Extraction with 1-(2-Pyridylazo)-2-Naphthol at 90˚C,” Talanta, Vol. 44, No. 5, 1997, pp. 837-842. doi:10.1016/S0039-9140(96)02122-4 [34] A. K. De, R. A. Chalmers, and S. M. Khopkar, “Solvent Extraction of Metals,” Van Nostrand Reinhold, London, 1970. [35] H. Faghihian, A. Hajishabani, S. Dadfarnia and H. Zamani, “Use of Clinoptilolite Loaded with 1-(2-Pyridy- lazo)-2-Naphthol as a Sorbent for Preconcentration of Pb(II), Ni(II), Cd(II) and Cu(II) Prior to Their Determina- tion by Flame Atomic Absorption Spectroscopy,” Inter- national Journal of Environmental Analytical Chemistry, Vol. 89, No. 4, 2009, pp. 223-231. doi:10.1080/03067310802262656 [36] S. Shibata, “Solvent Extraction and Spectrophotometric Determination of Metals with 1-(2-Pyridylazo)-2-naph- thol,” Analytical Chimica Acta, Vol. 25, No. 4, 1961, pp. 348-359. doi:10.1016/0003-2670(61)80213-4 [37] A. J. Convertine, B. S. Lokitz, Y. Vasileva, L. J. Myrick, C. W. Scales, A. B. Lowe and C. L. McCormick, “Direct Synthesis of Thermally Responsive DMA/NIPAM Diblock and DMA/NIPAM/DMA Triblock Copolymers via Aqueous, Room Temperature RAFT Polymerization,” Macromolecules, Vol. 39, No. 5, 2006, pp. 1724-1730. doi:10.1021/ma0523419 [38] E. C. Cho, J. Lee and K. Cho, “Role of Bound Water and Hydrophobic Interaction in Phase Transition of Poly (N-Isopropylacrylamide) Aqueous Solution,” Macro- molecules, Vol. 36, No. 26, 2003, pp. 9929-9934. doi:10.1021/ma034851d [39] M. Heskins and J. E. Guillet, “Solution Properties of Poly(N-Isopropylacrylamide),” Journal of Macromolecu- lar Science A, Vol. 2, No. 8, 1968, pp. 1441-1455. doi:10.1080/10601326808051910 [40] Z. Wang, K. J. Ho, C. J. Medforth and J. A. Shelnutt, “Porphyrin Nanofiber Bundles from Phase-Transfer Ionic Self-Assembly and Their Photocatalytic Self-Metalliza- tion,” Advanced Materials, Vol. 18, No. 19, 2006, pp. 2557-2560. doi:10.1002/adma.200600539 [41] S. J. Lee, C. D. Malliakas, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, “Amphiphilic Porphyrin Nanocrystals: Morphology Tuning and Hierarchical Assembly,” Ad- vanced Materials, Vol. 20, No. 18, 2008, pp. 3543-3549. doi:10.1002/adma.200800003 [42] L. Kang, Z. Wang, Z. Cao, Y. Ma, H. Fu and J. Yao, “Colloid Chemical Reaction Route to the Preparation of Nearly Monodispersed Perylene Nanoparticles: Size-Tun- able Synthesis and Three-Dimensional Self-Organiza- tion,” Journal of the American Chemical Society, Vol. 129, No. 23, 2007, pp. 7305-7312. doi:10.1021/ja068710d [43] H. Shi, L. Qi, J. Ma and H. Cheng, “Polymer-Directed Synthesis of Penniform BaWO4 Nanostructures in Re- verse Micelles,” Journal of the American Chemical Soci- ety, Vol. 125, No. 12, 2003, pp. 3450-3451. doi:10.1021/ja029958f [44] J. Xu, X. Liu, J. Lv, M. Zhu, C. Huang, W. Zhou, X. Yin, H. Liu, Y. Li and J. Ye, “Morphology Transition and Ag- gregation-Induced Emission of an Intramolecular Charge- transfer Compound,” Langmuir, Vol. 24, No. 8, 2008, pp. 4231-4237. doi:10.1021/la703662w [45] Y. Dong, J. W. Y. Lam, A. Qin, J. X. Sun, J. Z. Liu, Z. Li, J. Z. Sun, H. H. Y. Sung, I. D. Williams and H. S. Kwok, “Aggregation-Induced and Crystallization-Enhanced Emissions of 1,2-Diphenyl-3,4-bis(Diphenylmethylene)- 1-cyclobutene,” Chemical Communications, 2007, pp. 3255-3257. doi:10.1039/b704794k [46] J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, “Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5- pentaphenylsilole,” Chemical Communications, 2001, pp. 1740-1741. doi:10.1039/b105159h |

