Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio ns, 2011, 2, 521-525 doi:10.4236/msa.2011.26070 Published Online June 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 521 Kinetics of Indium Extraction from Mechanically Activated ITO Scrap Xuanhai Li1, Yanjuan Zhang1, Jinhuan Yao2, Xiumin Li1, Liuping Pan1 1School of Chemistry and Chemical Engineering, Guangxi University, Nanning, China; 2Department of Material and Chemical En- gineering, Guilin University of Technology, Guilin, China. Email: xuanhli@gxu.edu.cn; xianquan919@163.com Received February 23rd, 2011; revised March 15th, 2011; accepted April 6th, 2011. ABSTRACT The indium tin oxide scrap (ITOS) was mechanically activated by a stirring ball mill and subsequently studied for the leaching behavior and kinetics of extracting indium from ITOS in hydrochloric acid solution. The X-ray diffraction (XRD) analysis showed that MA caused the decrease in crysta lline phase and increase in lattice distortion. The effects of reaction temperature and hydrochloric acid concentration on the leaching rate of indium were also investigated, which showed that the ind ium extraction from ITOS had significan t depend ency on tempera ture and HCl concen tration. The equal-recovery method was used for kinetics analysis. When ITOS was mechanically activated for 15 and 30 min, the apparent activation energy decreased from 90.6 kJ/mol to 70.3 and 53.0 kJ/mol, respectively, which indicated that MA could enhance the reactivity of ITOS and accelerated the reaction. The reaction orders of extracting indium from the nonactivated, milled for 15 and 30 min ITOS with respect to HCl concentration were 2.30, 1.44, and 1.31, respec- tively. Keywords: ITO, Mechanical Activation, Indium, Leachin g Kinet i cs 1. Introduction As a rare metal, indium is widely used in many fields due to its quite unique physical and chemical properties [1,2]. In recent years, Most of the indium (about 70%) is used to produce indium tin oxide (ITO), which plays an im- portant role in optoelectronic devices, transparent elec- trodes for liquid crystal display and solar cells [3-6]. With the rapid increase in demand of liquid crystal dis- play, the production of ITO becomes blooming. In the manufacture of transparent conduction oxide (TCO), only about 15% of ITO is consumed in making TCO, and the rest (85%) becomes scrap [7]. In addition, some left- over material, smear metal and wasters are also produced during the production of ITO targets. Due to the increas- ing demand and over-exploitation, the resource of indium is gradually exhausted. So, it is very important to recover indium from indium-contained wastes. Current indium sources estimate that 65% of the indium from used ITO targets is recovered via reclaim processing. Therefore, it is of great importance to study the techniques of recy- cling indium from these wastes. In the production of ITO targets, different proportion of In2O3 and SnO2 forms different crystal structure phases under the high tem- perature and high pressure conditions. The difference in crystal structure leads to the difference in recycling in- dium from ITO scraps (ITOS). For some crystal phases, it is very difficult to be dissolved in acid solution. Therefore, the investigation of enhancing indium extrac- tion from ITOS has great significance both in theory and practice. Mechanical activation (MA) may increase the internal energy and reaction activity due to the formation of lat- tice distortion, the defect in crystal lattice, and amorphi- zation in solid material via the mechanical force by high-energy milling [8-10]. In recent years, many re- searchers have investigated the effect of MA on hydro- metallurgical extraction of valuable metals from minerals by using different methods and activation equipment, such as vibrating mill, planetary mill, and drum ball mill, showing that MA plays a positive role in the dissolution of minerals [11-13]. In this study, MA was used for the pretreatment of ITOS. The purpose of this work was to investigate the effects of MA on the change in crystal structure of ITOS and the kinetics of extracting indium from ITOS in hydrochloric acid. 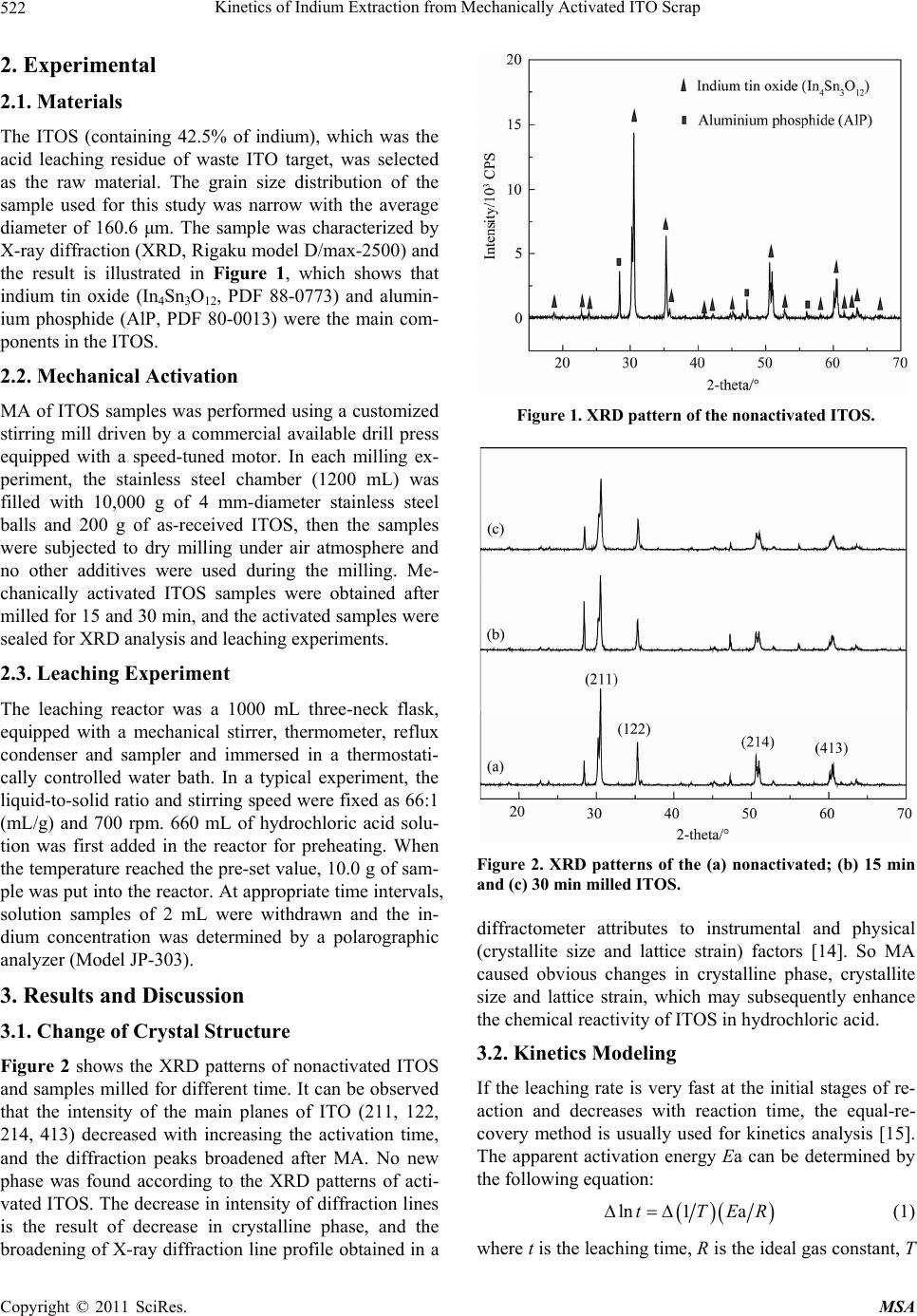 Kinetics of Indium Extraction from Mechanically Activated ITO Scrap 522 2. Experimental 2.1. Materials The ITOS (containing 42.5% of indium), which was the acid leaching residue of waste ITO target, was selected as the raw material. The grain size distribution of the sample used for this study was narrow with the average diameter of 160.6 μm. The sample was characterized by X-ray diffraction (XRD, Rigaku model D/max-2500) and the result is illustrated in Figure 1, which shows that indium tin oxide (In4Sn3O12, PDF 88-0773) and alumin- ium phosphide (AlP, PDF 80-0013) were the main com- ponents in the ITOS. 2.2. Mechanical Activation MA of ITOS samples was performed using a customized stirring mill driven by a commercial available drill press equipped with a speed-tuned motor. In each milling ex- periment, the stainless steel chamber (1200 mL) was filled with 10,000 g of 4 mm-diameter stainless steel balls and 200 g of as-received ITOS, then the samples were subjected to dry milling under air atmosphere and no other additives were used during the milling. Me- chanically activated ITOS samples were obtained after milled for 15 and 30 min, and the activated samples were sealed for XRD analysis and leaching experiments. 2.3. Leaching Experiment The leaching reactor was a 1000 mL three-neck flask, equipped with a mechanical stirrer, thermometer, reflux condenser and sampler and immersed in a thermostati- cally controlled water bath. In a typical experiment, the liquid-to-solid ratio and stirring speed were fixed as 66:1 (mL/g) and 700 rpm. 660 mL of hydrochloric acid solu- tion was first added in the reactor for preheating. When the temperature reached the pre-set value, 10.0 g of sam- ple was put into the reactor. At appropriate time intervals, solution samples of 2 mL were withdrawn and the in- dium concentration was determined by a polarographic analyzer (Model JP-303). 3. Results and Discussion 3.1. Change of Crystal Structure Figure 2 shows the XRD patterns of nonactivated ITOS and samples milled for different time. It can be observed that the intensity of the main planes of ITO (211, 122, 214, 413) decreased with increasing the activation time, and the diffraction peaks broadened after MA. No new phase was found according to the XRD patterns of acti- vated ITOS. The decrease in intensity of diffraction lines is the result of decrease in crystalline phase, and the broadening of X-ray diffraction line profile obtained in a Figure 1. XRD pattern of the nonactivated ITOS. Figure 2. XRD patterns of the (a) nonactivated; (b) 15 min and (c) 30 min milled ITOS. diffractometer attributes to instrumental and physical (crystallite size and lattice strain) factors [14]. So MA caused obvious changes in crystalline phase, crystallite size and lattice strain, which may subsequently enhance the chemical reactivity of ITOS in hydrochloric acid. 3.2. Kinetics Modeling If the leaching rate is very fast at the initial stages of re- action and decreases with reaction time, the equal-re- covery method is usually used for kinetics analysis [15]. The apparent activation energy Ea can be determined by the following equation: ln1 atTE R (1) where t is the leaching time, R is the ideal gas constant, T Copyright © 2011 SciRes. MSA 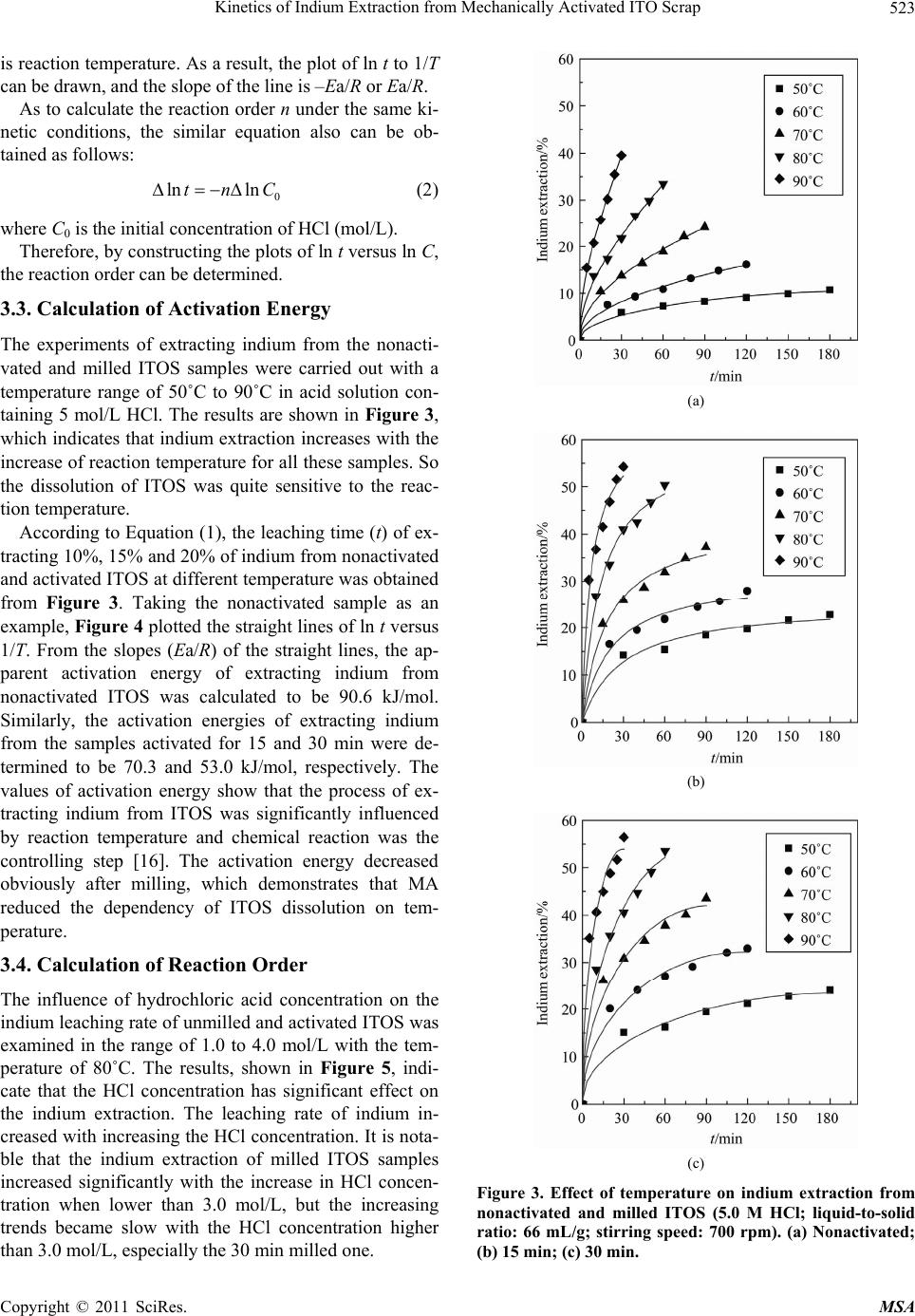 Kinetics of Indium Extraction from Mechanically Activated ITO Scrap523 is reaction temperature. As a result, the plot of ln t to 1/T can be drawn, and the slope of the line is –Ea/R or Ea/R. As to calculate the reaction order n under the same ki- netic conditions, the similar equation also can be ob- tained as follows: 0 ln lntnC (2) where C0 is the initial concentration of HCl (mol/L). Therefore, by constructing the plots of ln t versus ln C, the reaction order can be determined. 3.3. Calculation of Activation Energy The experiments of extracting indium from the nonacti- vated and milled ITOS samples were carried out with a temperature range of 50˚C to 90˚C in acid solution con- taining 5 mol/L HCl. The results are shown in Figure 3, which indicates that indium extraction increases with the increase of reaction temperature for all these samples. So the dissolution of ITOS was quite sensitive to the reac- tion temperature. According to Equation (1), the leaching time (t) of ex- tracting 10%, 15% and 20% of indium from nonactivated and activated ITOS at different temperature was obtained from Figure 3. Taking the nonactivated sample as an example, Figure 4 plotted the straight lines of ln t versus 1/T. From the slopes (Ea/R) of the straight lines, the ap- parent activation energy of extracting indium from nonactivated ITOS was calculated to be 90.6 kJ/mol. Similarly, the activation energies of extracting indium from the samples activated for 15 and 30 min were de- termined to be 70.3 and 53.0 kJ/mol, respectively. The values of activation energy show that the process of ex- tracting indium from ITOS was significantly influenced by reaction temperature and chemical reaction was the controlling step [16]. The activation energy decreased obviously after milling, which demonstrates that MA reduced the dependency of ITOS dissolution on tem- perature. 3.4. Calculation of Reaction Order The influence of hydrochloric acid concentration on the indium leaching rate of unmilled and activated ITOS was examined in the range of 1.0 to 4.0 mol/L with the tem- perature of 80˚C. The results, shown in Figure 5, indi- cate that the HCl concentration has significant effect on the indium extraction. The leaching rate of indium in- creased with increasing the HCl concentration. It is nota- ble that the indium extraction of milled ITOS samples increased significantly with the increase in HCl concen- tration when lower than 3.0 mol/L, but the increasing trends became slow with the HCl concentration higher than 3.0 mol/L, especially the 30 min milled one. (a) (b) (c) Figure 3. Effect of temperature on indium extraction from nonactivated and milled ITOS (5.0 M HCl; liquid-to-solid ratio: 66 mL/g; stirring speed: 700 rpm). (a) Nonactivated; (b) 15 min; (c) 30 min. Copyright © 2011 SciRes. MSA 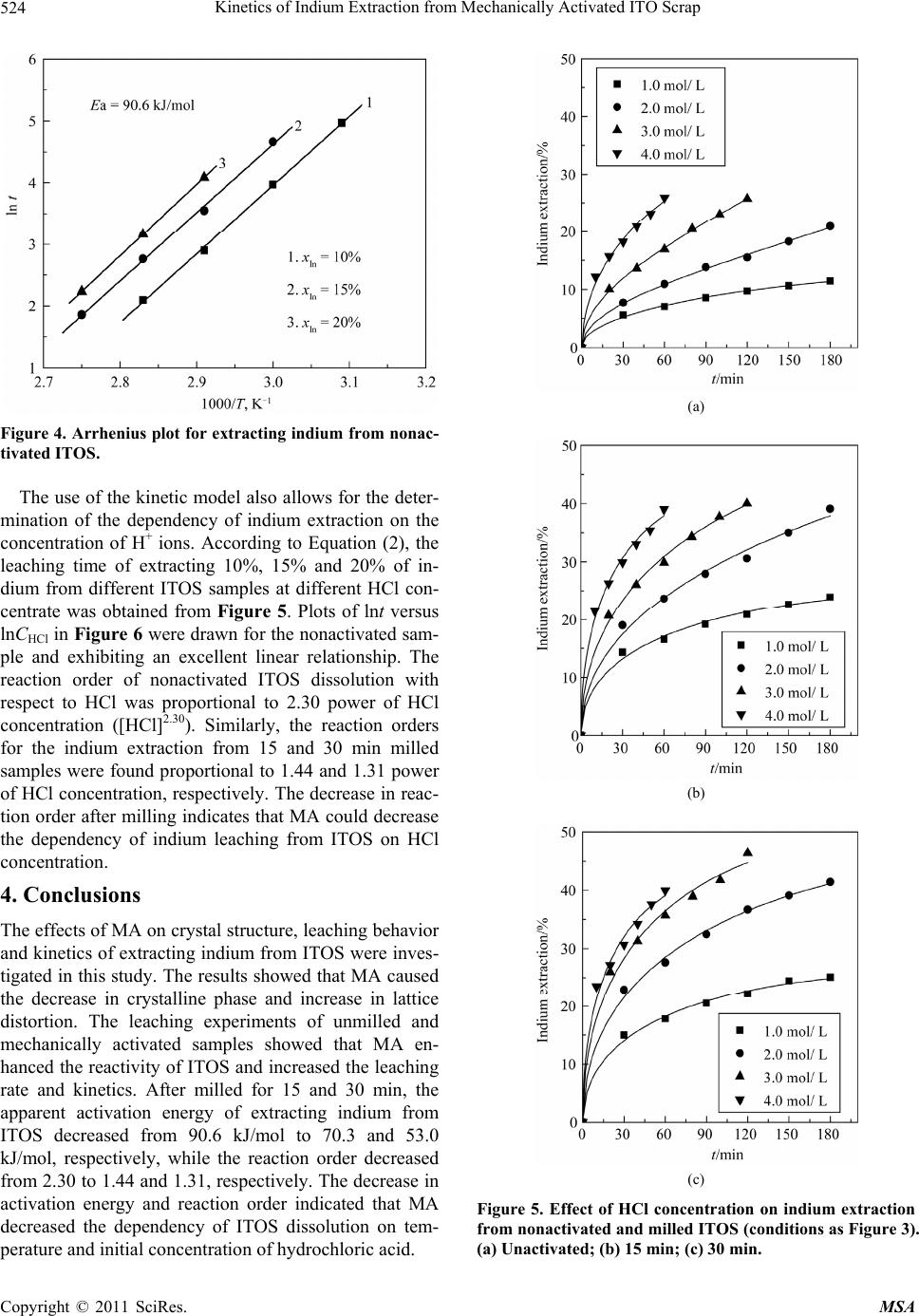 Kinetics of Indium Extraction from Mechanically Activated ITO Scrap 524 Figure 4. Arrhenius plot for extracting indium from nonac- tivated ITOS. The use of the kinetic model also allows for the deter- mination of the dependency of indium extraction on the concentration of H+ ions. According to Equation (2), the leaching time of extracting 10%, 15% and 20% of in- dium from different ITOS samples at different HCl con- centrate was obtained from Figure 5. Plots of lnt versus lnCHCl in Figure 6 were drawn for the nonactivated sam- ple and exhibiting an excellent linear relationship. The reaction order of nonactivated ITOS dissolution with respect to HCl was proportional to 2.30 power of HCl concentration ([HCl]2.30). Similarly, the reaction orders for the indium extraction from 15 and 30 min milled samples were found proportional to 1.44 and 1.31 power of HCl concentration, respectively. The decrease in reac- tion order after milling indicates that MA could decrease the dependency of indium leaching from ITOS on HCl concentration. 4. Conclusions The effects of MA on crystal structure, leaching behavior and kinetics of extracting indium from ITOS were inves- tigated in this study. The results showed that MA caused the decrease in crystalline phase and increase in lattice distortion. The leaching experiments of unmilled and mechanically activated samples showed that MA en- hanced the reactivity of ITOS and increased the leaching rate and kinetics. After milled for 15 and 30 min, the apparent activation energy of extracting indium from ITOS decreased from 90.6 kJ/mol to 70.3 and 53.0 kJ/mol, respectively, while the reaction order decreased from 2.30 to 1.44 and 1.31, respectively. The decrease in activation energy and reaction order indicated that MA decreased the dependency of ITOS dissolution on tem- perature and initial concentration of hydrochloric acid. (a) (b) (c) Figure 5. Effect of HCl concentration on indium extraction from nonactivated and milled ITOS (conditions as Figure 3). (a) Unactivated; (b) 15 min; (c) 30 min. Copyright © 2011 SciRes. MSA  Kinetics of Indium Extraction from Mechanically Activated ITO Scrap Copyright © 2011 SciRes. MSA 525 Figure 6. Determination of reaction order for extracting indium from nonactivated ITOS. 5. Acknowledgements This work was financially supported by National Natu- ral Science Foundation of China (No. 51064002) and Natural Science Foundation of Guangxi, China (No. 0728238). REFERENCES [1] A. M. Alfantazi and R. R. Moskalyk, “Processing of In- dium: A Review,” Minerals Engineering, Vol. 16, No. 8, 2003, pp. 687-694. doi:10.1016/S0892-6875(03)00168-7 [2] X. Tong, S. X. Song, J. He and A. Lopez-Valdivieso, “Flotation of Indium-Beard Marmatite from Multi-Me- tallic Ore,” Rare Metals, Vol. 27, No. 2, 2008, pp. 107-111. doi:10.1016/S1001-0521(08)60096-0 [3] S. Calnan, H. M. Upadhyaya, M. J. Thwaites and A. N. Tiwari, “Properties of Indium Tin Oxide Films Deposited Using High Target Utilisation Sputtering,” Thin Solid Films, Vol. 515, No. 15, 2007, pp. 6045-6050. doi:10.1016/j.tsf.2006.12.063 [4] A. B. Chebotareva, G. G. Untila, T. N. Kost, S. Jorgensen and A. G. Ulyashinc, “ITO Deposited by Pyrosol for Photovoltaic Applications,” Thin Solid Films, Vol. 515, No. 24, 2007, pp. 8505-8510. doi:10.1016/j.tsf.2007.03.097 [5] J. Liu, D. Wu, N. Zhang and Y. Wang, “Effect of Surfac- tants on the Structure and Photoelectric Properties of ITO Films by Sol-Gel Method,” Rare Metals, Vol. 29, No. 2, 2010, pp. 143-148. doi:10.1007/s12598-010-0025-3 [6] W. S. Jung, S. G. Yoon, S. M. Kang, S. Kim and D. H. Yoon, “Electrical and Optical Properties of ITO: Ca Composite Thin Films for TEOLED Cathode,” Thin Solid Films, Vol. 516, No. 16, 2008, pp. 5445-5448. doi:10.1016/j.tsf.2007.07.070 [7] S. Hsieh, C. Chen and W. C. Say, “Process for Recovery of Indium from ITO Scraps and Metallurgic Microstruc- tures,” Materials Science and Engineering B, Vol. 158, No. 1-3, 2009, pp. 82-87. doi:10.1016/j.mseb.2009.01.019 [8] C. Sasikumar, D. S. Rao, S. Srikanth, B. Ravikumar, N. K. Mukhopadhyay and S. P. Mehrotra, “Effect of Mechani- cal Activation on the Kinetics of Sulfuric Acid Leaching of Beach Sand Ilmenite from Orissa, India,” Hydrometal- lurgy, Vol. 75, No. 1-4, 2004, pp. 189-204. doi:10.1016/j.hydromet.2004.08.001 [9] M. Achimovičová and P. Baláž, “Influence of Mechanical Activation on Selectivity of Acid Leaching of Arsenopy- rite,” Hydrometallurgy, Vol. 77, No. 1-2, 2005, pp. 3-7. doi:10.1016/j.hydromet.2004.09.008 [10] E. Godočíková, P. Baláž and E. Boldižárová, “Structural and Temperature Sensitivity of the Chloride Leaching of Copper, Lead and Zinc from a Mechanically Activated Complex Sulphide,” Hydrometallurgy, Vol. 65, No. 1, 2002, pp. 83-93. doi:10.1016/S0304-386X(02)00094-4 [11] A. M. Amer, “Investigation of the Direct Hydrometallur- gical Processing of Mechanically Activated Low-Grade Wolframite Concentrate,” Hydrometallurgy, Vol. 58, No. 3, 2000, pp. 251-259. doi:10.1016/S0304-386X(00)00134-1 [12] P. Baláž, “Mechanical Activation in Hydrometallurgy,” International Journal of Mineral Processing, Vol. 72, No. 1-4, 2003, pp. 341-354. doi:10.1016/S0301-7516(03)00109-1 [13] Y. J. Zhang, X. H. Li, L. P. Pan, Y. S. Wei and X. Y. Liang, “Effect of Mechanical Activation on the Kinetics of Extracting Indium from Indium-Bearing Zinc Ferrite,” Hydrometallurgy, Vol. 102, No. 1-4, 2010, pp. 95-100. doi:10.1016/j.hydromet.2010.02.003 [14] R. Ebrahimi-Kahrizsangi, M. H. Abbasi and A. Saidi, “Mechanochemical Effects on the Molybdenite Roasting Kinetics,” Chemical Engineering Journal, Vol. 121, No. 2-3, 2006, pp. 65-71. doi:10.1016/j.cej.2006.04.011 [15] Z. W. Zhao, G. Zhang, G. S. Huo and H. G. Li, “Kinetics of Atmospheric Leaching Molybdenum from Metallifer- ous Black Shales by Air Oxidation in Alkali Solution,” Hydrometallurgy, Vol. 97, No. 3-4, 2009, pp. 233-236. doi:10.1016/j.hydromet.2009.02.004 [16] M. Ashraf, Z. I. Zafar and T. M. Ansari, “Selective Leaching Kinetics and Upgrading of Low-Grade Cal- careous Phosphate Rock in Succinic Acid,” Hydrometal- lurgy, Vol. 80, No. 4, 2005, pp. 286-292. doi:10.1016/j.hydromet.2005.09.001 |

