 Journal of Biosciences and Medicines, 2014, 2, 1-7 Published Online December 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.210001 How to cite this paper: Gnimpieba, E.Z., Bousserouel, S. and Chango, A. (2014) Mathematical Modeling of a Metabolic Network to Study the Impact of Food Contaminants on Genomic Methylation and DNA Instability. Journal of Biosciences and Medicines, 2, 1-7. http://dx.doi.org/10.4236/jbm.2014.210001 Mathematical Modeling of a Metabolic Network to Study the Impact of Food Contaminants on Genomic Methylation and DNA Instability Etienne Z. Gnimpieba1*, Souad Bousserouel2, Abalo Chango1 1Department of Nutritional Sciences and Health, EGEAL UPSP: 2007.05.137-Institut Polytechnique Lasalle Beauvais, Beauvais, Fra nce 2Laboratoire de Prévention Nutritionnelle du Ca ncer, Inserm U682-IR CAD, Strasbourg, France Email: souadbousserouel@gmail.com Received Sep te mber 20 14 Abstract Environmental contamination of food is a worldwide public health problem. Folate mediated one- carbon metabolism plays an important role in epigenetic regulation of gene expression and mu- tagenesis. Many contaminants in food cause cancer through epigenetic mechanisms and/or DNA instability i.e. default methylation of uracil to thymine, subsequent to the decrease of 5-meth yl te- trahydrofolate (5 mTHF ) pool in the one-carbon metabolism network. Evaluating consequences of an exposure to food contaminants based on systems biology approaches is a promising alternative field of investigation. This report presents a dynamic mathematical modeling for the study of the alteration in the one-carbon metabolism network by environmental factors. It provides a model for predicting “the impact of arbitrary contaminants that can induce the 5 mTHF deficiency. The model allows for a given experimental condition, the analysis of DNA methylation activity and dumping methylation in the de novo pathway of DNA synthesis. Keywords DNA-Methylation, DNA Instability, Mathematical Modeling, Logic Programming, Metabolic Network, Food Contaminant 1. Introduction An inadequate methyl group donor enhances the risk of cancer because the one-carbon unit (CH3) has critical functions in biological methylation reactions such as DNA-cytosine methylation, and in DNA synthesis or repair [1]-[3]. The methylation of DNA is a fundamental mechanism for epigenetic control of gene expression and the maintenance of genomic integrity, as well as uracil methylation into thymidine for the maintenance of DNA sta- * Current address: Computer Science Department, University of South Dakota, USA. 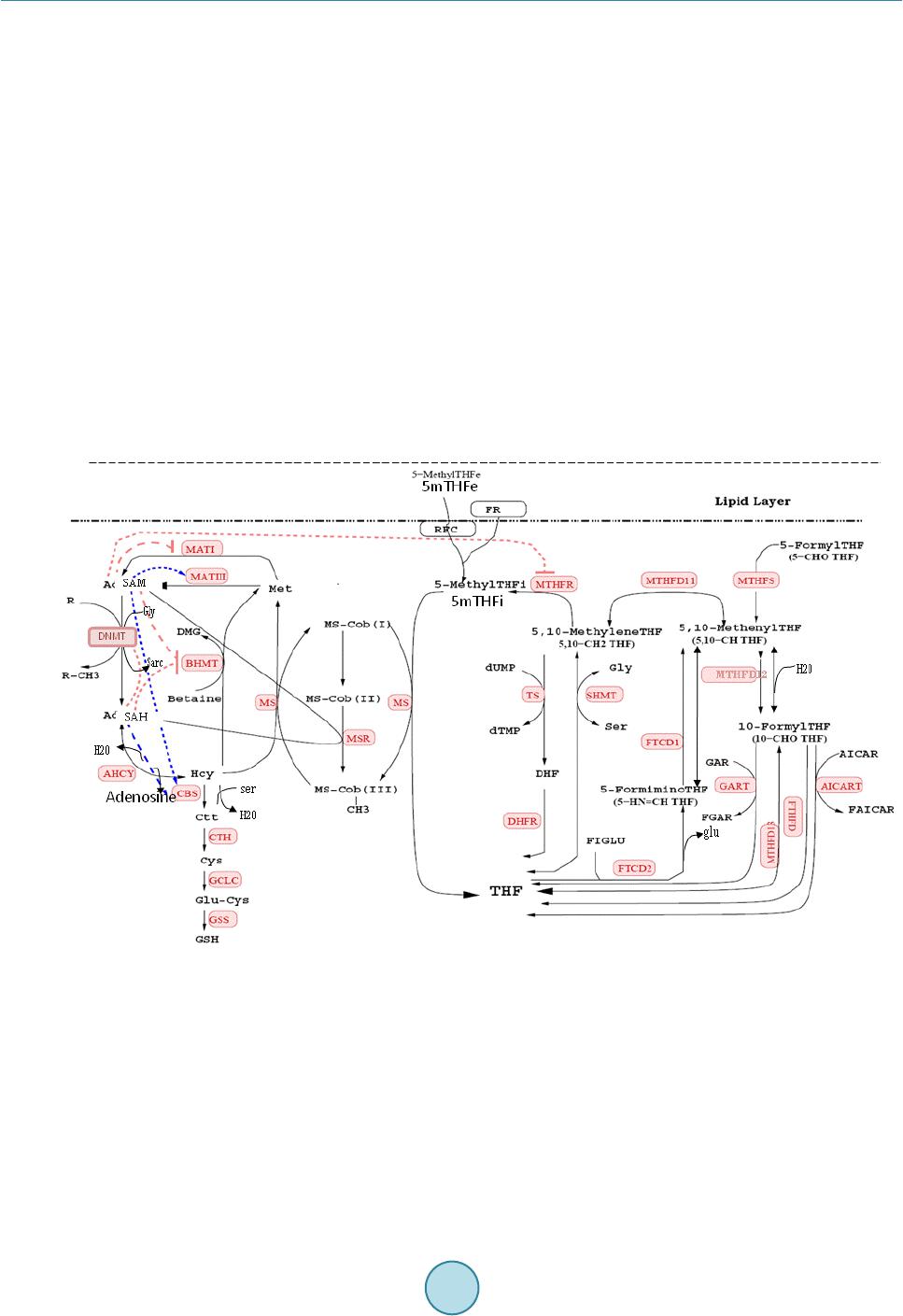 E. Z. Gnimpieba et al. bility to ensure chromosomal integrity during replication process [4 ] . Low 5-methytetrahydrofolate (5 mTHF) (Figure 1) reduces DNA methylation, and thymidylate synthase-mediated methylation of deoxyuridylate (dU) to thymidylate (dT). This results in a higher dU/dT ratio, increase in uracil misincorporation into DNA, inefficient DNA repair, and chromosomal breakage [5]-[7]. The role of dietary factors in DNA methylation processes diseases following folate repletion, and the conse- quences of genetic defect has been underlined in 2001 during the Trans-HHS Workshop in the USA [ 8 ]. Ab- normal DNA methylation has been reported in disease prevention such as cancer, hepatotoxicity, pancreatic toxicity, diabetes, atherosclerosis, birth defects, and neurological disturbances. Poirier et al., [9] have suggested three major causes of diseases related to methyl group insufficiency: dietary deficiency, genetic polymorphism, and a third prospective cause, chemicals. Evidence now suggest that some environmental contaminants, includ- ing exogenous chemicals such as polyhalogenated compounds, metals, some mycotoxins, and others, can pro- duce abnormal methylation processes or abnormal folate uptake [10] linked to the etiology of diseases. Recently, Lawley et al. (2011) [11] demonstrates the application of a mathematical model to arsenic methylation in human studies in Bangladesh. However, in their model, various biochemical aspect of arsenic metabolism have been greatly simplified. DNA hypomethylation and uracil misincorporation/repair are not exclusive mechanisms and both could be important in diagnostics. Since both aspects of DNA modification are strongly associated with 5 mTHF metabo- lism and carcinogenesis, development of methods for analyzing DNA-cytosine methylation and DNA-uracil in the aetiology of diseases is of great interest. Figure 1. The Folate-mediated one carbon metabolism network [12]. Légende: Black arrow: normal reaction; Red arrow: in- hibition (negative regulation); Blue arrow: activation (positive regulation). Red box: Enzyme catalyzes reaction; Black no box text: metabolites. Abbreviation: AHCY: S-adenosylhomocysteine hydrolase. AICART: Aminoimidazole carboxamide ribonucléotide (AICAR) formyltran sférase; BHMT: betaine homocysteine methyltransferase. CBS: cystathionine beta-syn - thase; CTH: cystathionine gamma-lyase; DHFR: dihydrofolate reductase. FR: folate receptor; DNA MéthylTransférase; FTCD: formiminotransferase cyclodeaminase. GART: Phosporibosylglycinamide formyltransferas, phosphoribosylglycinamide syn- thase, phosphoribosylaminoimidazole synthase; GLCL: gammaglutamylcysteine synthase; GSS: glutathion synthase. (MATI/ MATIII): adénosylmethionine transferase gene I/III. MS: methionine synthase. MSR: methionine synthase reductase; MTs: Methyltransferase MTHFD: methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase, formylte- trahydrofolate synthase. MTHFR: 5,10-methylenetetrahydrofolate reductase. MTHFS: 5,10-methylenetetrahydrofolate syn- thase 5-formyltetrahydrofolate cycloligase. RFC: reduced folate; AdoMet: S-adénosylmethionine; AdoHcy: S-adénosy- lhomocystéine; SHMT: serine hydroxymethyltransferase; TS: tymidylate synthase. 5mTHF: 5-methyltetrah ydrofolate.  E. Z. Gnimpieba et al. 2. Motivation One of the major difficulties when working at the cellular level is describing the dynamic regulation of metabo- lites accurately to explain how subcellular processes such as DNA methylation or uracil methylation, are driven by environmental conditions such as 5 mTHF deficiency. Besides featuring complex networks in a living cell, wit h interconnecting pathways that consist of hundreds of reactions, there is need to take into account that the metabolism is subject to control and regulatory mechanisms. During the last ten years, there were many attempts to develop mathematical models for the study of cellular functions or cellular processes. We recently, built a mathematical model that integrates experimental conditions using logic programming for the study of the fo- late-mediated one-carbon metabolism regulation (FOCM) [12]. Here, we present the model, simulating the im- pact of environmental conditions such as the presence of a specific food contaminant on the network. 3. Contaminant Agents and the Alteration of Methylation Processes Among mechanisms by which environmental agents can induce tumor formation are DNA hypomethylation as- sociated with gene expression modification and uracil misincorporation into DNA leading to chromosomal breaks, micronucleus formation. Studies have showed that several classes of environmental chemicals that mod- ify epigenetic marks, including metals (cadmium, arsenic, nickel, chromium, methylmercury), peroxisome pro- liferators (trichloroethylene, dichloroacetic acid, trichloroacetic acid), air pollutants (particulate matter, black carbon, benzene), and endocrine-disrupting/reproductive toxicants (diethylstilbestrol, bisphenol A, persistent organic pollutants, dioxin). Most studies conducted so far have been centered on DNA methylation [9] [13]. There is a need to explore the causes of many other environmental effects on methylation processes. Several paths of interaction of these contaminants on the methylation process: alteration of 5 mTHF uptake, of methyl- tranferase activity, or other key enzyme of the network. In this report we are interested to the interaction im- pinging on the availability of 5 mTHF in the cell. This is the case for instance of arsenic which may alter the ex- pression of folate carrier and reducing the 5 mTHF uptake by cells [14]. 4. Principle and Interest of Mathematical Modeling Models describing biological systems such as the one-carbon metabolism network are too complex to be ana- lyzed manually and therefore typically are solved numerically, using computers to solve the mathematical equa- tions. With appropriated mathematical tools and the availability of computer-based techniques for solving equa- tions, it is possible to predict and analyze the responses of a biological system to different conditions. In many cases the computer simulations called “dry experiments” require much lower investment and much less time compared with the typically more time-consuming and expensive biological experiments (“wet experiments”). Among benefits offer by mathematical models there are the fact that discrepancies between systems behaviors predicted by a mathematical model and actual behaviors measured in experiments can point to components that still are missing from the mathematical model, thereby assisting in developing a more comprehensive picture of a biological process. Even if it is not clear which components are missing from the system under investigation, the results obtained with the mathematical model may help to guide the design of additional experiments to cla- rify the issue (Systems: http :/ /pubs.niaaa. ni h.gov /publicatio ns/arh311/49-59.htm). The rationale of modeling is that it assists investigators in the analysis of the system in various possible experimental conditions. Predictions (or modeling results) are then compared to experimental measurements. 5. The One-Carbon Metabolism Network Folate-mediated one-carbon metabolism is fundamental for cell growth and differentiation. In cells, folate up- take in the 5 mTHF form is converted to THF by transfer of the methyl group to homocysteine forming methio- nine and THF (F ig ur e 1) [15]. Methionine can then be converted to S-adenosyl-methionine (AdoMet). AdoMet is involved in more than 100 reactions, and at least 80 AdoMet-dependent enzymes have been identified [16]. S-Adenosyl-homocysteine (AdoHcy), the by-product of methyl transfer reactions, is hydrolyzed, thus regene- rating homocysteine, which then becomes available to start a new cycle of methyl-group transfer. In most tissues, homocysteine is re-methylated back to methionine through two pathways: the methionine synthase/methionine synthase reductase (MS/MSR) pathway. In the folate cycle, the THF reacts with serine synthetizing N5,10-me thy - leneTHF (5,10-CH2-THF) in a reaction catalyzed by serine hydroxymethyltransferase (SHMT). The 5,10-CH2- THF is reduced into 5-CH3-THF by the enzyme methylenetetrahydrofolate reductase (MTHFR), or oxidized into  E. Z. Gnimpieba et al. 5,10-methenylTHF by a reversible reaction catalyzed by methylenetetrahydrofolate dehydrogenase (MTHFD). 5,10-methenyl-THF can be converted to 10-formyl-THF by 5,10-methenyl-THF cyclohydrolase. Folate serves for DNA methylation in the transmethylation pathways [17 ], for synthesis of purines and a pyrimidine nucleo- side (thymidine). It provides carbon units for de novo purine and thymidylate biosynthesis. Purine biosynthesis requires 10-formyl -THF for the C2 and C8 carbons of the purine ring catalyzed by glycinamide ribonucleotide transformylase (GART). Thymidylate biosynthesis requires CH2-THF for the reductive methylation of deoxyu- ridylate catalyzed by the enzyme thymidylate synthase (TS). The OCM system, presented in the Figure 1, considers an extracellular 5 mTHF uptake and a set of enzyme reactions. We considered a “generic” mammalian cell OCM including 26 enzymes reactions (i.e. 30 variables and 27 reactions involved). The model emphasises three functional units: one for the folate uptake and inter- nalisation, one for the folate cycle, and one for methionine/homocysteine cycle. Each unit consists of a pool of interconnected metabolites by enzymatic reactions. The set of these reactions produces a system that we simu- late using a mathematical framework. Mathematical model and reaction kinetic laws involved in the OCM model were reported in our previous study [12]. We modeled the kinetics of reactions of the OCM system using Ordinary Differentials Equations (ODE). Metabolites represent the system variables and variations of their con- centration indicate the difference between their synthesis and catabolism levels. 6. Experimental Conditions and Logic Programming We have proposed the use of the logic programming that represents an automatic way to study the possible im- pact of exogenous contaminant on the OCM network [12 ] . It consists of an automatic generation of a model based on data and knowledge available. This approach, developed in the laboratory takes into account several experimental conditions, which are considered as a set of constraints, in addition to the kinetic descriptions and as an original contribution to the standard mathematical modeling. In the logic programming we encoded the set of data as a set of rules or facts. Each rule is encoded in the form “IF condition THEN conclusion”. The first approach allows to study the structure of the biological system of interest, whereas the second proposes an analysis of the qualitative properties. As a complementary approach, we propose herein to apply such a theoretical framework for estimating the values of parameters in accuracy with the available experimental knowledge. The kinetic rates and known parameters (P’) were inferred by using the logic programming inference as follow. All existing data (including experiments and literature based knowledge) are gather in a knowledge base (KB). We proceed to an inference by a back-tracking technique us- ing the logic programming tool. In the KB, data are spread into a fact base (FB), that represents encoded bio- logical knowledge (extracted from the literature) of our model; and the rule base (RB) that sums up the biologi- cal conditions monitoring the system behaviours. Formally, a given biological knowledge BKi consists of sets of facts Fi = {Fij} and rules Ri = {Rij}, where Fij and Rij are respectively atomics fact and rules obtained from BKi. At first, the KB is empty and noted KB = { } (null). For each biological knowledge BKi, KB is updated using a simple unification principle when a novel biological knowledge is added. The Fact Based (FB) is then com- pleted by the new Fact set Fi and the Rules Base (RB) by the Ri ones. The constraints are either the biological referential in which the OCM is observed (i.e. tissue, cell, etc...), the clinical conditions (i.e . healthy or pathological), and if needed, experimental parameters (i.e. temperature and pH of in vitro enzyme reactions). Each constraint represents a condition in which the model must behave accu- rately with given known experimental conditions. Logic programming is a well-investigated domain of ma- chines learning artificial intelligence. Logical constraints are used to verify biological model behaviors during environmental perturbations via model-checking methods. The set of data is encoded as a set of rules or facts which gives rise of the opportunity to automatically learn novel rules by induction or deduction. Each rule is encoded in the form “IF condition THEN conclus ion”. The first approach allows studying the structure of the biological system of interest, whereas the second proposes an analysis of the qualitative properties. As a com- plementary approach, we propose herein to apply such a theoretical framework for estimating the values of pa- rameters accurately with the available experimental knowledge. The parameters identification, inference of known parameters and the model validation are reported elsewhere [12]. 7. Simulating the Impact of Contaminants by Reducing 5 mTHF Level in the Network Food contaminants such as arsenic, fumonisin B1 and other, decreases intracellular 5 mTHF pool by reducing 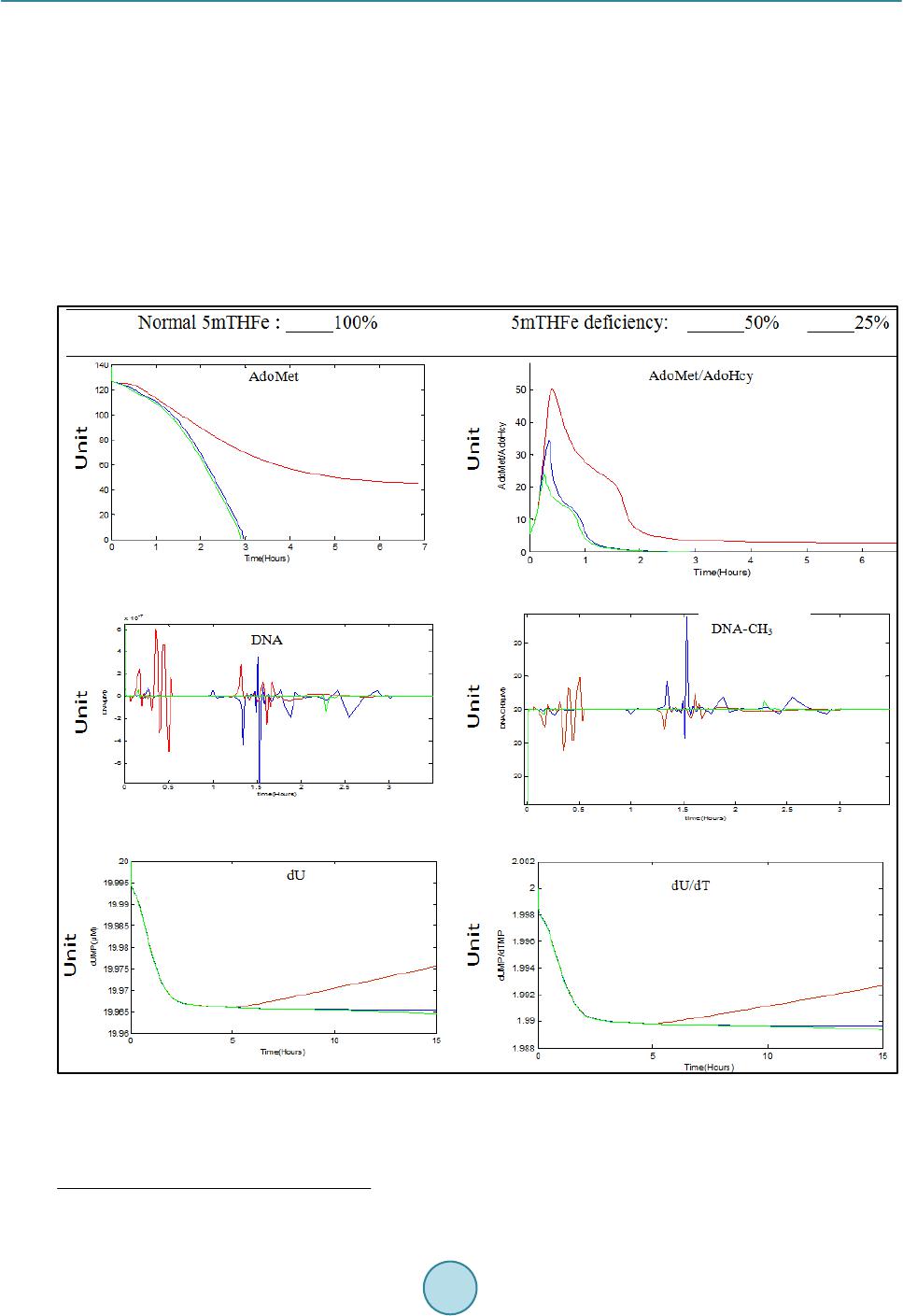 E. Z. Gnimpieba et al. the activity of 5 mTHF transporter [10] [14]. In a previous in vitro study using HepG2 cell line grown either in experimental complete medium or in folate-depleted medium, we shown global hypomethylation of genomic DNA induced by the absence of folic acid in folate-depleted medium and significant increase of uracil residues in the same DNA sample [15]. We simulate the impact of arbitrary contaminants that can induce the 5 mTHF deficiency. 5 mTHF was com- puted by testing 50% and 25% of extra cellular 5 mTHF (5 mTHFe) input compared to 100% (10 nM1) (Figure 2). With 25% of 5 mTHFe major metabolites were quite insensitive while other metabolites show differential behavior. About AdoHcy, the methyl group donor, we observe an inverse tendency. For 50% and 100%, there was decreasing from 22.87 Units to 5.21 Units at 0.27 h and rapid increase to 25.12 Units at 1h of running, then a second level of rapid increasing to 88.97 Units at 1.5h was observed. With 25%, AdoHcy shows a different behavior from 2.5 Units increasing slowly to reach 18.94 Units at 2.6 h to remain constant. Finally, the curve of AdoMet/AdoHcy ratio increased at the beginning of running, reaching the highest level at 0.42 h with a ratio of Figure 2. Result of simulating 5-methyltetrahydrofolate (5mTHF) deficiency [12]. Figure shows the variations relate to the transmethylation pathway, and the uracil methylation pathway. Disruptions of these subsystems are represented here by the behavior of state variables (metabolite concentrations over time). Thus, we observe an overall weak disturbance between the state without deficit (100% green) and the state with 50% deficit (blue). But the disruption is greater with 25% (red). Ab- breviation AdoMet: S-adenosylmethionine; AdoHcy: S-adénosylhomocystéine: dU: deoxyuridylate, dT: thymidylate. 1 This value represents also the simulation units (Units) that was at the nanomolar scale for 5mTH and other folate derived metabolites.  E. Z. Gnimpieba et al. 22; 34 and 50 respectively for 100%; 50% and 25% of 5 mTHFe condition respectively. In regard of the trans methylation reaction, results of time course DNA methylation process did not show characteristic differential behaviours with the three 5 mTHFe conditions. However, at steady state there are two oscillations of variable amplitude. These oscillations show lower amplitudes on methylated DNA (DNA-CH 3). The observed oscilla- tions are not clear. However it is interesting to note that physiologically folate deficiency produce both global genomic DNA hypomethylation and gene specific hyper methylation. For dU, after a rapid decreasing with the three 5 mTHFe conditions, we observed a progressive increasing with 25% condition from 19.96 Units at 5h of running to 19.97 Units at 15 h of running. Finally with 100% of 5 mTHFe, dU/dT ratio reaches the steady-state 1.95 after 2.5 h compared to 1.99 after 2 h with 25% of 5 mTHF. Finally, the model demonstrates that dependent of the impact of a contaminant on the decrease of 5 mTHF pool, there are different profiles of the dynamical behavior of the network. The questions emerging from such a study is how the stability of the critical reaction in the cycle is maintained when large localized changes occur either within the system or the input, and what is the significant of observed oscillations for DNA and DNA-CH3 curves for cell. Otherwise a recent study from Nijhout et al. [18] have shown the inhibition of GNMT by 5 mTH F and consequently the existing long-range interactions stabilize DNA methylation. This report didn’t in- clude this particular finding that will need to include in the future. 8. Conclusion The model allowed successfully the simulation of many key regulatory processes in one-carbon metabolism network. However it may be refined and used as tool in predictive nutritional toxicology to provide novel hypo- theses for pathogenesis. It can be a predictive tool and could, therefore, be substituted in the future to experi- mental techniques in some cases. Acknowledgements This study has been supported by the Comité de l’Oise de la Ligue Contre le Cancer. References [1] Duthie, S.J., Narayanan, S., Brand, G.M. and Gra n t, G. (2000) DNA Stability and Genomic Methylation Status in Colonocytes Isolated from Methyl-Donor-Deficient Rats. European Journal of Nutrition, 39, 106-111. http://dx.doi.org/10.1007/s003940070026 [2] Friso , S. and Cho i, S.W. (2002) Gene-Nutrient Interactions and DNA Methylation. Journal of Nutrition, 132, 2382 S- 2387S. [3] Gabriel , H.E., Crott, J.W., Ghandour, H., Dallal, G.E., Choi, S.W., Keyes, M.K., Jang, H., Liu, Z., Nadeau, M., Johns- ton, A., Mager, D. and Mason , J.B. (2006) Chronic Cigarette Smoking Is Associated with Diminished Folate Status, Altered Folate Form Distribution, and Increased Genetic Damage in the Buccal Mucosa of Healthy Adults. The Ame- rican Journal of Clinical Nutrition, 83, 835-84 1. [4] Toyota , M. and Suzuki, H. (2010) Epigenetic Drivers of Genetic Alterations. Advances in Genetics, 70, 309-323. http://dx.doi.org/10.1016/B978-0-12-380866-0.60011-3 [5] Blount, B.C., Mack, M.M., Wehr, C.M., MacGregor, J.T., Hiatt, R.A., Wang, G., Wickramasinghe, S.N., Everson, R.B. and A mes, B.N. (1997) Folate Deficiency Causes Uracil Misincorporation into Human DNA and Chromosome Break- age: Implications for Cancer and Neuronal Damage. Proceedings of the National Academy of Sciences of the USA, 94, 3290-3295. http://dx.doi.org/10.1073/pnas.94.7.3290 [6] Chango, A., Abdel Nour, A.M., Niquet, C. and Tessier, F.J. (2009) Simultaneous Determination of Genomic DNA Methylation and Uracil Misincorporation. Medical Principles and Practice, 18, 81-84. http://dx.doi.org/10.1159/000189803 [7] J ame s, S.J., Pogribny, I.P., Pogribna, M., Miller, B.J., Jernigan, S. and Me l nyk , S. (2003) Mechanisms of DNA Dam- age, DNA Hypomethylation, and Tumor Progression in the Folate/Methyl-Deficient Rat Model of Hepatocarcinogene- sis. Journal of Nutrition, 133, 37 40S -3747 S . [8] Ross, S.A. and Poirier, L. (2002) Proceedings of the Trans-HHS Workshop: Diet, DNA Methylation Processes and Health. Journal of Nutrition, 132, 232 9S -2332 S. [9] Poirier, L.A. (2002) The Effects of Diet, Genetics and Chemicals on Toxicity and Aberrant DNA Methylation: An In- troduction. Journal of Nutrition, 132, 2336 S-233 9S . [10] Abdel Nour, A.M., Ringot, D., Gueant, J.L. and Chango, A. (2007) Folate Receptor and Human Reduced Folate Car-  E. Z. Gnimpieba et al. rier Expression in HepG2 Cell Line Exposed to Fumonisin B1 and Folate Deficiency. Carcinogenesis, 28, 2291 -2297 . http://dx.doi.org/10.1093/carcin/bgm149 [11] Lawley, S.D., Cinderella, M., Hal l , M.N., Gamble, M.V., Nijhout, H.F. and Reed, M.C. (2011) Mathematical Model Insights into Arsenic Detoxification. Theoretical Biology and Medical Modelling, 8, 31. http://dx.doi.org/10.1186/1742-4682-8-31 [12] Gnimpieba, E.Z., Eveillard, D., Gueant, J.L. and Chango, A. (2011) Using Logic Programming for Modeling the One- Carbon Metabolism Network to Study the Impact of Folate Deficiency on Methylation Processes. Molecular BioSys- tems, 7, 2508-2521. http://dx.doi.org/10.1039/c1mb05102d [13] Baccarell i, A. and Bollati, V. (2009) Epigenetics and Environmental Chemicals. Current Opinion in Pediatrics, 21, 243-251. http://dx.doi.org/10.1097/MOP.0b013e32832925cc [14] Chango, A., Bousserouel, S., Ge , Z., Abdel Nour, A.N.N. and Abdennebi-Najar, L. (2011) Effects of Arsenic Exposure and Folate Deficiency on Methyl Metabolism in Human Fibroblast Cell Lines. The FASEB Journal, 25, 592-598. [15] Chango, A., Nour, A.A., Bousserouel, S., Eveillard, D., Anton, P.M. and Gueant, J.L. (2009) Time Course Gene Ex- pression in the One-Carbon Metabolism Network Using HepG2 Cell Line Grown in Folate-Deficient Medium. The Journal of Nutritional Biochemistry, 20, 312-320. http://dx.doi.org/10.1016/j.jnutbio.2008.04.004 [16] Kagan, K.O., Avgidou, K., Molina, F.S., Gajewska, K. and Nico laides, K.H. (2006) Relation between Increased Fetal Nuchal Translucency Thickness and Chromosomal Defects. Obstetrics Gynecology, 107 , 6-10. http://dx.doi.org/10.1097/01.AOG.0000191301.63871.c6 [17] Mih ai, D., Niculescu, M.D. and Zeisel , S.H. (2002) Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. Journal of Nutrition, 132, 2333S-2335S. [18] Nijhout, H.F., Reed, M.C., Anderson, D.F., Mattingly, J.C., James, S.J. and Ulrich, C.M. (2006) Long-Range Allos- teric Interactions between the Folate and Methionine Cycles Stabilize DNA Methylation Reaction Rate. Epigenetics, 1, 81-87. http://dx.doi.org/10.4161/epi.1.2.2677
|