Paper Menu >>
Journal Menu >>
 Vol.2, No.2, 53-62 (2011) Journal of Biophysical Chemistry doi:10.4236/jbpc.2011.22008 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ Ionization and transfection activity of n-methyl-substituted carbamoyl-cholesterol derivatives Samuel Ach eampong, Michalak is Savva* Division of pharmaceuticals Sciences, Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, New York, USA; *Corresponding Author: micsavva@gmail.com Received 10 November 2010; revised 13 December 2010; accepted 29 December 2010. ABSTRACT Five novel cationic lipids, the polar head group of which was attached to the cholesterol back- bone via a tertiary carbamate linker, were syn- thesized and their physicochemical properties were compared to their transfection efficiencies. Transfection activity of the primary amine ana- log was highest among the series, while the qu- aternary ammonium iodide salt was essentially transfection incompetent. Contrary to DC-Chol, methyl and ethyl carbamoyl derivatives of DC- Chol mediated high levels of transfection in the absence of DOPE. Ionization of the cationic as- semblies in 40 mM Tris buffer pH 7.2 exactly correlated with the competitive nature of the inductive and steric effects of the methyl groups on the aliphatic nitrogen of the lipids’ polar moiety. Interestingly, the pH interaction zone of all lipid dispersions at 25˚C was extended by ±2 pH units from the pKa, while the pKa of the cati- onic lipids determined in mixed vesicles com- posed of 90% DOPC and cholesterol was ap- proximately 1.3 to 1.5 times higher than that of pure cationic assemblies. The interaction of ca- tionic lipids with plasmid DNA was correlated with pKa, but not the transfection activity. Keywords: Cationic Lipids; DC-Chol; Transfection; Pka; Plasmid DNA; Monolayer; Surface Pressure 1. INTRODUCTION Cholesterol-based cationic lipids are one of the most promising series of reagents for in vitro and in vivo gene delivery [1-4]. Over the years, these structures were sys- tematically varied in an effort to generate structure- activity relationships to rationalize their design and ef- fectively optimize their transfection efficiency [5-8]. Most of the approaches focus on attaching a hydrophilic basic moiety capable of binding to the negatively char- ged nucleic acid, to the membrane compatible choles- terol moiety via a chemical linker. Quite frequently, the polar head group of these lipids is composed of a pri- mary, secondary, tertiary or quaternary amine and almost always these lipids require the presence of the helper lipid DOPE in order to mediate transfection. DOPE is a zwitterionic amphiphil that forms reverse hexagonal phases (HII) at physiological pH and is widely believed to promote fusion with cell membranes, facilitate en- dosomal escape, reduce the toxicity and increase the transfection activity of lipoplexes [9-13]. In our recent report, the physicochemical properties of cholesterol-based cationic lipids carrying a secondary carbamate linker were correlated with their transfection activity in the absence of helper lipids. The study found that the tertiary and quaternary amine derivatives, DC- Chol and TC-Chol (herein termed DC and TC), failed to mediate transfection and further concluded that the high transfection activity mediated by the primary and secon- dary analogs AC and MC in the absence of DOPE, was due to their increased association with plasma membrane and endosomal escape [14]. The aim of this investigation was to examine how methylation on the carbamoyl ni- trogen affects the lipids’ ionization, interaction with plasmid DNA and transfection activity. 2. MATERIAL AND METHODS N-methylethylenediamine 95%, N,N’-dimethylethyle- nediamine 99%, N,N,N’-trimethylethylene-diamine 97%, N,N-dimethyl-N’-ethylethylenediamine 98%, Cholesteryl chloroformate 97%, iodomethane 99.5%, 2-hydroxy- ethylmercaptan, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphen- yltetrazolium bromide (MTT) and o-nitrophenyl-β-D- galactopyranoside (ONPG) were purchased from Sigma- Aldrich (St. Louis, MO). Tris-(hydroxymethyl)-amin- omethane (ultrapure grade) and edetate disodium di- hydrate (EDTA) were purchased from Spectrum Chemi- cal Manufacturing Company (New Brunswick, NJ). Murine melanoma cells B16F0 and Dulbecco’s modified 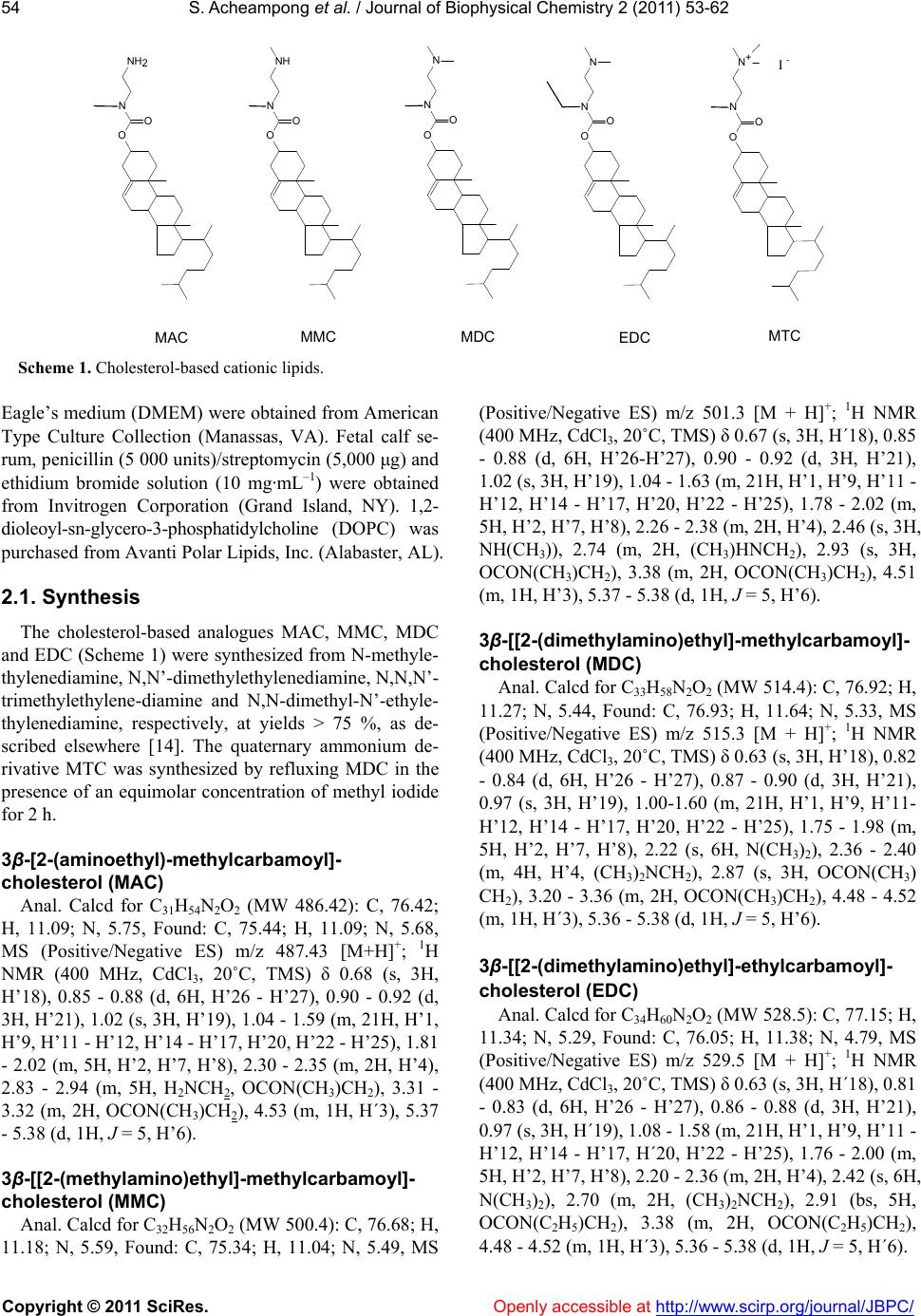 S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 54 I - MAC MMC MDCMTC O N O NH2 O N O NH O N O N O N O N+ EDC O N O N Scheme 1. Cholesterol-based cationic lipids. Eagle’s medium (DMEM) were obtained from American Type Culture Collection (Manassas, VA). Fetal calf se- rum, penicillin (5 000 units)/streptomycin (5,000 μg) and ethidium bromide solution (10 mg·mL–1) were obtained from Invitrogen Corporation (Grand Island, NY). 1,2- dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). 2.1. Synthesis The cholesterol-based analogues MAC, MMC, MDC and EDC (Scheme 1) were synthesized from N-methyle- thylenediamine, N,N’-dimethylethylenediamine, N,N,N’- trimethylethylene-diamine and N,N-dimethyl-N’-ethyle- thylenediamine, respectively, at yields > 75 %, as de- scribed elsewhere [14]. The quaternary ammonium de- rivative MTC was synthesized by refluxing MDC in the presence of an equimolar concentration of methyl iodide for 2 h. 3β-[2-(aminoethyl)-methylcarbamoyl]- cholesterol (MAC) Anal. Calcd for C31H54N2O2 (MW 486.42): C, 76.42; H, 11.09; N, 5.75, Found: C, 75.44; H, 11.09; N, 5.68, MS (Positive/Negative ES) m/z 487.43 [M+H]+; 1H NMR (400 MHz, CdCl3, 20˚C, TMS) δ 0.68 (s, 3H, H’18), 0.85 - 0.88 (d, 6H, H’26 - H’27), 0.90 - 0.92 (d, 3H, H’21), 1.02 (s, 3H, H’19), 1.04 - 1.59 (m, 21H, H’1, H’9, H’11 - H’12, H’14 - H’17, H’20, H’22 - H’25), 1.81 - 2.02 (m, 5H, H’2, H’7, H’8), 2.30 - 2.35 (m, 2H, H’4), 2.83 - 2.94 (m, 5H, H2NCH2, OCON(CH3)CH2), 3.31 - 3.32 (m, 2H, OCON(CH3)CH2), 4.53 (m, 1H, H´3), 5.37 - 5.38 (d, 1H, J = 5, H’6). 3β-[[2-(methylamino)ethyl]-methylcarbamoyl]- cholesterol (MMC) Anal. Calcd for C32H56N2O2 (MW 500.4): C, 76.68; H, 11.18; N, 5.59, Found: C, 75.34; H, 11.04; N, 5.49, MS (Positive/Negative ES) m/z 501.3 [M + H]+; 1H NMR (400 MHz, CdCl3, 20˚C, TMS) δ 0.67 (s, 3H, H´18), 0.85 - 0.88 (d, 6H, H’26-H’27), 0.90 - 0.92 (d, 3H, H’21), 1.02 (s, 3H, H’19), 1.04 - 1.63 (m, 21H, H’1, H’9, H’11 - H’12, H’14 - H’17, H’20, H’22 - H’25), 1.78 - 2.02 (m, 5H, H’2, H’7, H’8), 2.26 - 2.38 (m, 2H, H’4), 2.46 (s, 3H, NH(CH3)), 2.74 (m, 2H, (CH3)HNCH2), 2.93 (s, 3H, OCON(CH3)CH2), 3.38 (m, 2H, OCON(CH3)CH2), 4.51 (m, 1H, H’3), 5.37 - 5.38 (d, 1H, J = 5, H’6). 3β-[[2-(dimethylamino)ethyl]-methylcarbamoyl]- cholesterol (MDC) Anal. Calcd for C33H58N2O2 (MW 514.4): C, 76.92; H, 11.27; N, 5.44, Found: C, 76.93; H, 11.64; N, 5.33, MS (Positive/Negative ES) m/z 515.3 [M + H]+; 1H NMR (400 MHz, CdCl3, 20˚C, TMS) δ 0.63 (s, 3H, H’18), 0.82 - 0.84 (d, 6H, H’26 - H’27), 0.87 - 0.90 (d, 3H, H’21), 0.97 (s, 3H, H’19), 1.00-1.60 (m, 21H, H’1, H’9, H’11- H’12, H’14 - H’17, H’20, H’22 - H’25), 1.75 - 1.98 (m, 5H, H’2, H’7, H’8), 2.22 (s, 6H, N(CH3)2), 2.36 - 2.40 (m, 4H, H’4, (CH3)2NCH2), 2.87 (s, 3H, OCON(CH3) CH2), 3.20 - 3.36 (m, 2H, OCON(CH3)CH2), 4.48 - 4.52 (m, 1H, H´3), 5.36 - 5.38 (d, 1H, J = 5, H’6). 3β-[[2-(dimethylamino)ethyl]-ethylcarbamoyl]- cholesterol (EDC) Anal. Calcd for C34H60N2O2 (MW 528.5): C, 77.15; H, 11.34; N, 5.29, Found: C, 76.05; H, 11.38; N, 4.79, MS (Positive/Negative ES) m/z 529.5 [M + H]+; 1H NMR (400 MHz, CdCl3, 20˚C, TMS) δ 0.63 (s, 3H, H´18), 0.81 - 0.83 (d, 6H, H’26 - H’27), 0.86 - 0.88 (d, 3H, H’21), 0.97 (s, 3H, H´19), 1.08 - 1.58 (m, 21H, H’1, H’9, H’11 - H’12, H’14 - H’17, H´20, H’22 - H’25), 1.76 - 2.00 (m, 5H, H’2, H’7, H’8), 2.20 - 2.36 (m, 2H, H’4), 2.42 (s, 6H, N(CH3)2), 2.70 (m, 2H, (CH3)2NCH2), 2.91 (bs, 5H, OCON(C2H5)CH2), 3.38 (m, 2H, OCON(C2H5)CH2), 4.48 - 4.52 (m, 1H, H´3), 5.36 - 5.38 (d, 1H, J = 5, H´6).  S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 55 3β-[[2-(trimethylamino)ethyl]-methylcarbamoyl]- cholesterol iodide (MTC) Anal. Calcd for C34H61N2O2 I (MW 656.4): C, 62.16; H, 9.45; N, 4.27, Found: C, 58.19; H, 9.37; N, 4.05, MS (Positive/Negative ES) m/z 529.4 [cation of salt], 1H NMR (400 MHz, CdCl3, 20˚C, TMS) δ 0.68 (s, 3H, H’18), 0.85 - 1.74 (m, 33H, H’1, H’9, H’11 - H’12, H’14 - H’17, H’19 – H’27), 1.85 - 1.97 (m, 5H, H’2, H’7, H’8), 2.35 (m, 2H, H’4), 3.08 (bs, 3H, OCON(CH3)CH2), 3.50 (s, 9H, N(CH3)3), 3.80 - 4.04 (m, 4H, NCH2CH2N), 4.45 (m, 1H, H’3), 5.40 (d, 1H, J = 5, H’6). 2.2. Transfection and Cytotoxicity The studies were conducted as described elsewhere [14]. Briefly, the plasmid DNA pUC19-β-gal was propa- gated in DH5α-competent cells using standard protocols [15] and purified by gel permeation chromatography us- ing Sepharose 4B as the stationary phase and 2.5 M am- monium acetate as the mobile phase. Fractions containing plasmid DNA were pooled and precipitated with isopro- pyl alcohol. The precipitate was resuspended in a few mL of 40 mM TE buffer pH 8, dialyzed for 2 - 4 h at room temperature against 40 mM TE buffer to eliminate traces of ammonium acetate and quantified by its absorption at 260 nm (4.63 mg·mL–1). The integrity and purity of the pDNA was verified by agarose gel electrophoresis and a 260 nm/280 nm absorption ratio of 1.8. B16F0 cells were cultured to confluence using DMEM supplemented with 10% fetal bovine serum, 50 units·mL–1 penicillin, 50 g·mL–1 streptomycin in a 5% CO2 at 37˚C. Approxi- mately 50,000 cells in 0.5 mL growth media were added to each well of 48 well plates. After 12 to 14 hours later, the serum media were removed and the cells were trans- fected with 250 L lipoplexes per well. The amount of plasmid DNA was kept constant at 1 g per well, while the amount of cationic lipid was increased appropriately to achieve cationic lipid to DNA charge ratios of 1 : 1, 2 : 1 and 4 : 1. The cells were incubated for 4 hours, after which time the lipoplexes were removed and the serum free media were replaced with complete growth media. After an additional incubation of 44 h, the cells were washed with cold phosphate-buffered saline (PBS) pH 7.4 twice and lysed with 150 L lysis buffer (0.1 M Tris pH 7.2, 0.1% w/v Triton X) per well. Beta-galactosidase ac- tivity was quantified by an ONPG assay. The cytotoxicity of cationic lipids was evaluated by an MTT assay as de- scribed elsewhere [14]. 2.3. EtBr Displacement Studies A Cary Eclipse spectrofluorometer was used to assess the level of displacement of ethidium bromide (EtBr) from the pDNA (pUC19). Briefly, 0.8 g of EtBr and 22.5 g of pDNA were diluted to 3 mL in either Tris buffer pH 7.2 or in serum free media (SFM). Aliquots of 11.4 l of cationic lipid (1.2 mM) were added to the so- lution until there was either a complete quenching of the EtBr or no apparent change in the fluorescence intensity. All samples were scanned at an excitation wavelength of 515 nm and the emission collected at the wavelength between 550 - 605 nm (excitation and emission slit widths were set at 5 nm). All experiments were con- ducted at 22˚C. The EtBr emission intensity f, was plot- ted against the lipid to DNA charge ratio R, and the re- sults were fitted either linearly or parabolically within a 95% confidence level. The interaction of MTC was shown to be a special case of cooperative interaction and was simulated by Eq.1: max min min 1/2 1 n f f ff R R (1) where fmin and fmax are the lowest and highest normalized fluorescence intensities, respectively, R, is the +/– charge ratio, R½ is the charge ratio at which 50 % of plasmid DNA is neutralized or compacted by cationic lipid and the exponent n denotes the steepness of the curve. Data were fitted by the method of non-linear least-squares using the Microsoft Excel Solver function, through minimization of the square residuals with 3 adjustable parameters (fmin, fmax and R½) at 5% tolerance. 2.4. Gel Retardation Assay 0.8% Agarose solution was prepared in 40 mM TAE (Tris base, glacial acetic acid and EDTA) pH 7.4, fol- lowed by the addition of 10 L EtBr (10 mg mL–1). The lipoplexes were prepared by keeping the pDNA amount constant at 0.2 g while the amount of cationic lipid (0.6 mM) was varied to produce lipid to pDNA charge ratios of 0.5, 1, 2, 4, 6 and 8. Naked pDNA (0.2 g) was used as a control in the first and last lanes. To each sample 1 L of a 6X gel loading buffer (0.25% w/v bromophenol blue, 0.25% w/v Xylene cyanol FF, 30% v/v glycerol in water) was added and the volume was brought to 11 L with 40 mM TAE buffer pH 7.4. The samples were al- lowed to sit for 10 - 15 minutes before they were loaded onto the wells of the agarose gel. Afterwards, an appro- priate amount of running buffer (TAE) was used to sub- merge the gel and the gel was subjected to an electropho- retic field of 90 V for 35 min. The results of the gel elec- trophoresis were assessed using a Bio-Rad Mini Transil- luminator. 2.5. Langmuir Monolayer Studies Cationic lipid monolayers were studied at the air/water  S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 56 interface using the Langmuir film balance technique as described elsewhere [16]. Compression isotherms of the cationic lipids were obtained using a Langmuir film bal- ance (model KSV 1000, KSV instruments LTD, Finland) equipped with a Wilhelmy plate for measuring the surface pressure. The Teflon microtrough (24,225.0 mm2) is equipped with two barriers that allow for symmetric compression. Each run was followed by a thorough wash of both trough and barriers with ethanol and a final rinse with distilled water. The Wilhelmy plate was heated over a blue flame for about 30 seconds before each run. In a typical experiment, 15 μL (30.0 nmol) of cationic lipid (2 mM dissolved in chloroform) was spread onto the sub- phase (40 mM Tris buffer pH 7.2) maintained at 22˚C, with a Hamilton® micro syringe and allowed to sit for 15 min before the start of each run. The barriers were co- mpressed at a constant speed of 10 mm·min−1. All exper- iments were conducted at a temperature of 22˚C, while each run was repeated at least three times for reproduci- bility. Independent and dependent variables collected by the instrument were imported into an Excel spreadsheet and the compressibility modulus, K, was calculated from the first derivative of the monolayer surface pressure- trough Area isotherms using Eq.2: T KA (2) Equilibrium parameters at the onset of monolayer col- lapse were determined at the peak point of the K- iso- therm. 2.6. pKa Studies Buffer solutions (2 mL) made from 40 mM Tris and 40 mM MES having a pH from 1.2 to 13.2 were trans- ferred into plastic cuvettes together with 0.1 mL of lipid dispersions (0.6 mM for cationic lipid (CL) alone and 2 mM for CL/DOPC/Chol 0.2/0.9/0.9 molar ratio) and 6 L of TNS (0.1 mM) for CL alone and 20 L TNS (0.1 mM) for CL/DOPC/Chol 0.2/0.9/0.9 mol/mol/mol). The lipid to probe molar ratio was kept constant at 100 : 1. The static fluorescence intensity of these samples was registered at an excitation wavelength of 321 nm (slit width 5 nm) and an emission wavelength of 445 nm (slit width 5 nm) on a Cary Eclipse fluorescence spectropho- tometer at 25˚C. The emission intensity of TNS was plot- ted against the pH and the results were fitted using a modified version of the Henderson-Hasselbalch (Equa- tion 3), using the max min min c (pH) 110 p Ka ff ff (3) Microsoft Excel Solver through minimization of the sum of the squared residuals with four adjustable param- eters at a 5% tolerance level [17]. The parameter, f is the calculated TNS fluorescence, fmax and fmin are the maxi- mum and minimum fluorescence values of TNS and c is a constant that adjusts the slope of the curve. 2.7. Particle Size and Electrophoretic Mobility Studies A Malvern Zetasizer Nano Series instrument was used to determine the size and zeta potential of the pure cho- lesterol derivatives and corresponding lipoplexes at +/– charge ratios of 1 : 1, 2 : 1 and 4 : 1. Experiments were performed in both filtered Tris buffer (using 0.2 m membrane filter) and SFM at 22˚C. Lipoplexes were prepared by adding pDNA into 200 L of 0.6 mM lipid dispersion to produce the desired lipid to pDNA charge ratios and then diluted to 1.5 mL with Tris buffer (40 mM, pH 7.2) or SFM [17]. 3. RESULTS AND DIS CUSSION 3.1. In Vitro Transfection and Cytotoxicity Studies Regardless of charge ratio, the MAC mediated highest transfection activity while the quaternary ammonium salt, MTC, exhibited the lowest potency among its peers (Figure 1(a)). The invariable -gal expression levels by MAC could be due to decreased cell viability with in- creasing charge ratio. Impressively, unlike DC-Chol which is transfection incompetent in the absence of DOPE, the MDC and EDC that bear methyl and ethyl substitution at the carbamoyl nitrogen, respectively, me- diated significant transfection activity proportional to charge ratio. More specifically, at +/– charge ratio of 1, the activity mediated by the cationic lipids decreased with polar head methyl substitution, while at +/– charge ratio of 2 and 4 the transfection activity mediated by MMC, MDC and EDC was essentially indistinguishable. MDC exhibited the lowest toxicity maintaining greater than 70% cell viability, whereas MMC was quite cyto- toxic at all charge ratios (Figure 1(b) ). 3.2. pKa Studies The pKa of cationic lipids is conventionally determined in mixed lipid vesicles using the membrane potential indicator TNS [17-21]. In this investigation, an attempt was made to determine the apparent pKa of cationic lipids from pure assemblies in the absence of any co-lipids. As shown in Table 1, the pKa of the primary amine deriva- tive MAC is slightly lower than that of the secondary amine derivative MMC. Further methyl substitution on the amine polar head drastically decreases the pKa by one unit (MDC), while the apparent pKa of the ethyl carbam- oyl tertiary amine derivative EDC, is even lower reaching 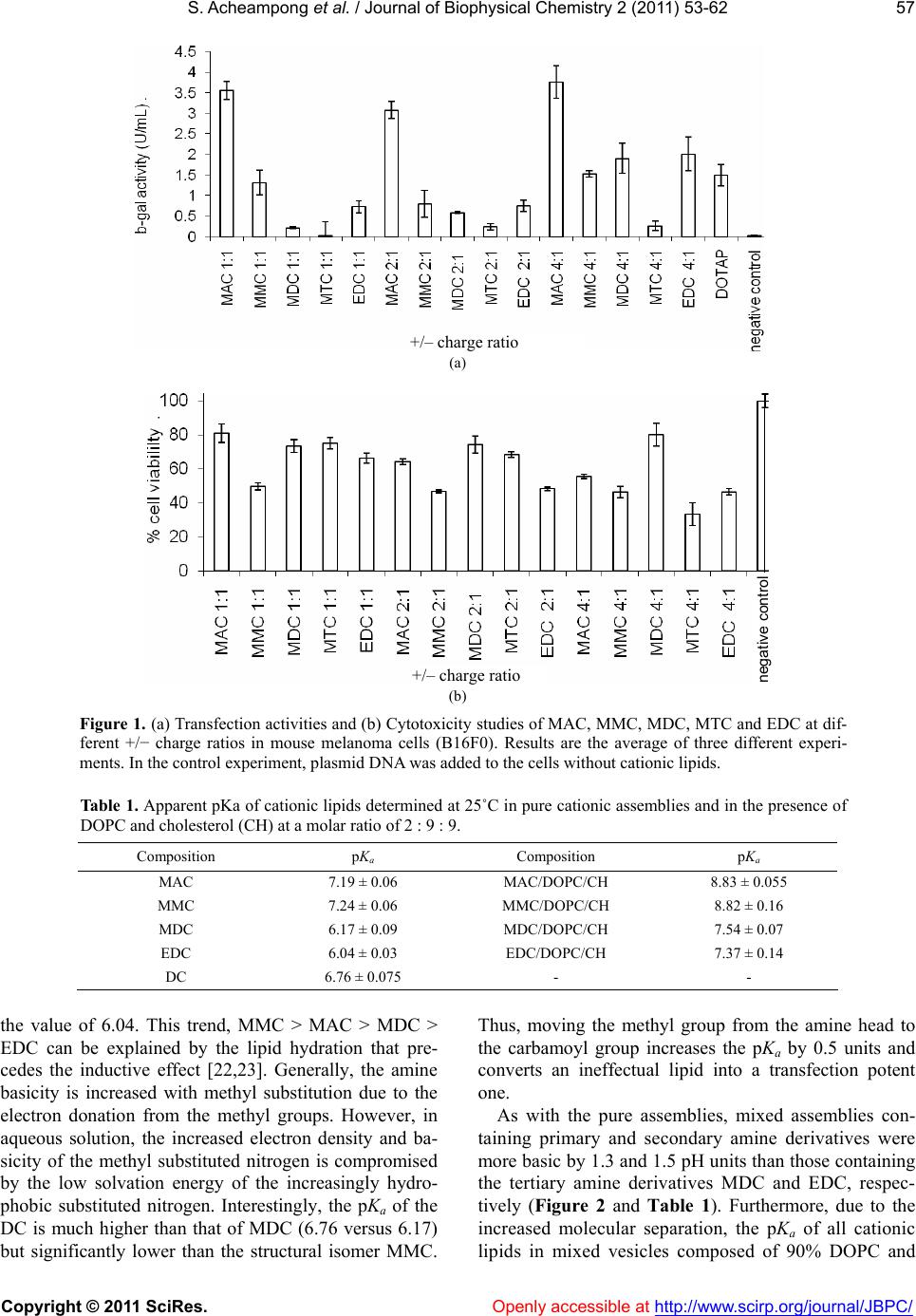 S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 57 (a) (b) Figure 1. (a) Transfection activities and (b) Cytotoxicity studies of MAC, MMC, MDC, MTC and EDC at dif- ferent +/− charge ratios in mouse melanoma cells (B16F0). Results are the average of three different experi- ments. In the control experiment, plasmid DNA was added to the cells without cationic lipids. Tab le 1. Apparent pKa of cationic lipids determined at 25˚C in pure cationic assemblies and in the presence of DOPC and cholesterol (CH) at a molar ratio of 2 : 9 : 9. Composition pKa Composition pKa MAC 7.19 ± 0.06 MAC/DOPC/CH 8.83 ± 0.055 MMC 7.24 ± 0.06 MMC/DOPC/CH 8.82 ± 0.16 MDC 6.17 ± 0.09 MDC/DOPC/CH 7.54 ± 0.07 EDC 6.04 ± 0.03 EDC/DOPC/CH 7.37 ± 0.14 DC 6.76 ± 0.075 - - the value of 6.04. This trend, MMC > MAC > MDC > EDC can be explained by the lipid hydration that pre- cedes the inductive effect [22,23]. Generally, the amine basicity is increased with methyl substitution due to the electron donation from the methyl groups. However, in aqueous solution, the increased electron density and ba- sicity of the methyl substituted nitrogen is compromised by the low solvation energy of the increasingly hydro- phobic substituted nitrogen. Interestingly, the pKa of the DC is much higher than that of MDC (6.76 versus 6.17) but significantly lower than the structural isomer MMC. Thus, moving the methyl group from the amine head to the carbamoyl group increases the pKa by 0.5 units and converts an ineffectual lipid into a transfection potent one. As with the pure assemblies, mixed assemblies con- taining primary and secondary amine derivatives were more basic by 1.3 and 1.5 pH units than those containing the tertiary amine derivatives MDC and EDC, respec- tively (Figure 2 and Table 1). Furthermore, due to the increased molecular separation, the pKa of all cationic lipids in mixed vesicles composed of 90% DOPC and + / – charge ratio +/– charge ratio negative control 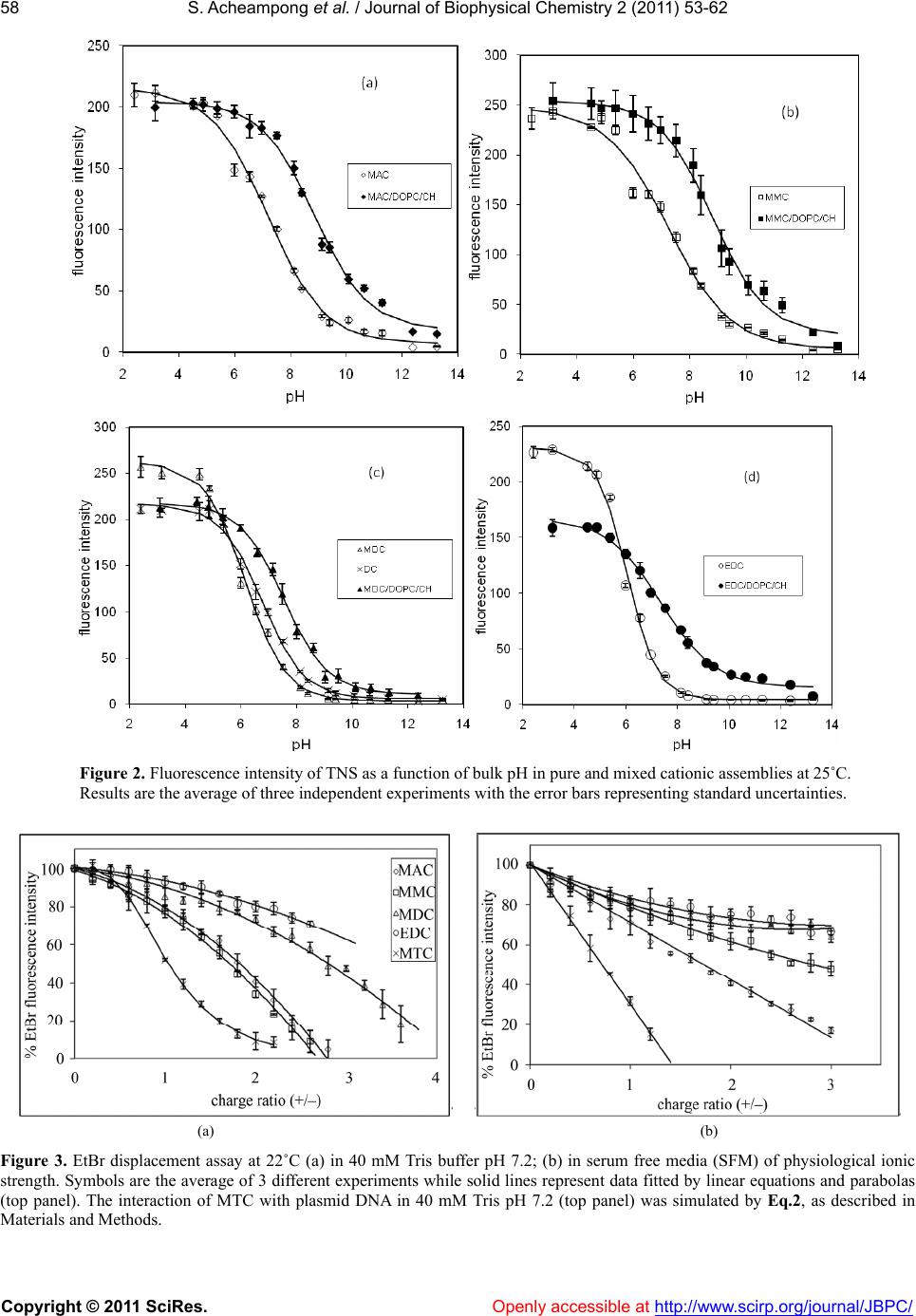 S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 58 Figure 2. Fluorescence intensity of TNS as a function of bulk pH in pure and mixed cationic assemblies at 25˚C. Results are the average of three independent experiments with the error bars representing standard uncertainties. (a) (b) Figure 3. EtBr displacement assay at 22˚C (a) in 40 mM Tris buffer pH 7.2; (b) in serum free media (SFM) of physiological ionic strength. Symbols are the average of 3 different experiments while solid lines represent data fitted by linear equations and parabolas (top panel). The interaction of MTC with plasmid DNA in 40 mM Tris pH 7.2 (top panel) was simulated by Eq.2, as described in Materials and Methods. 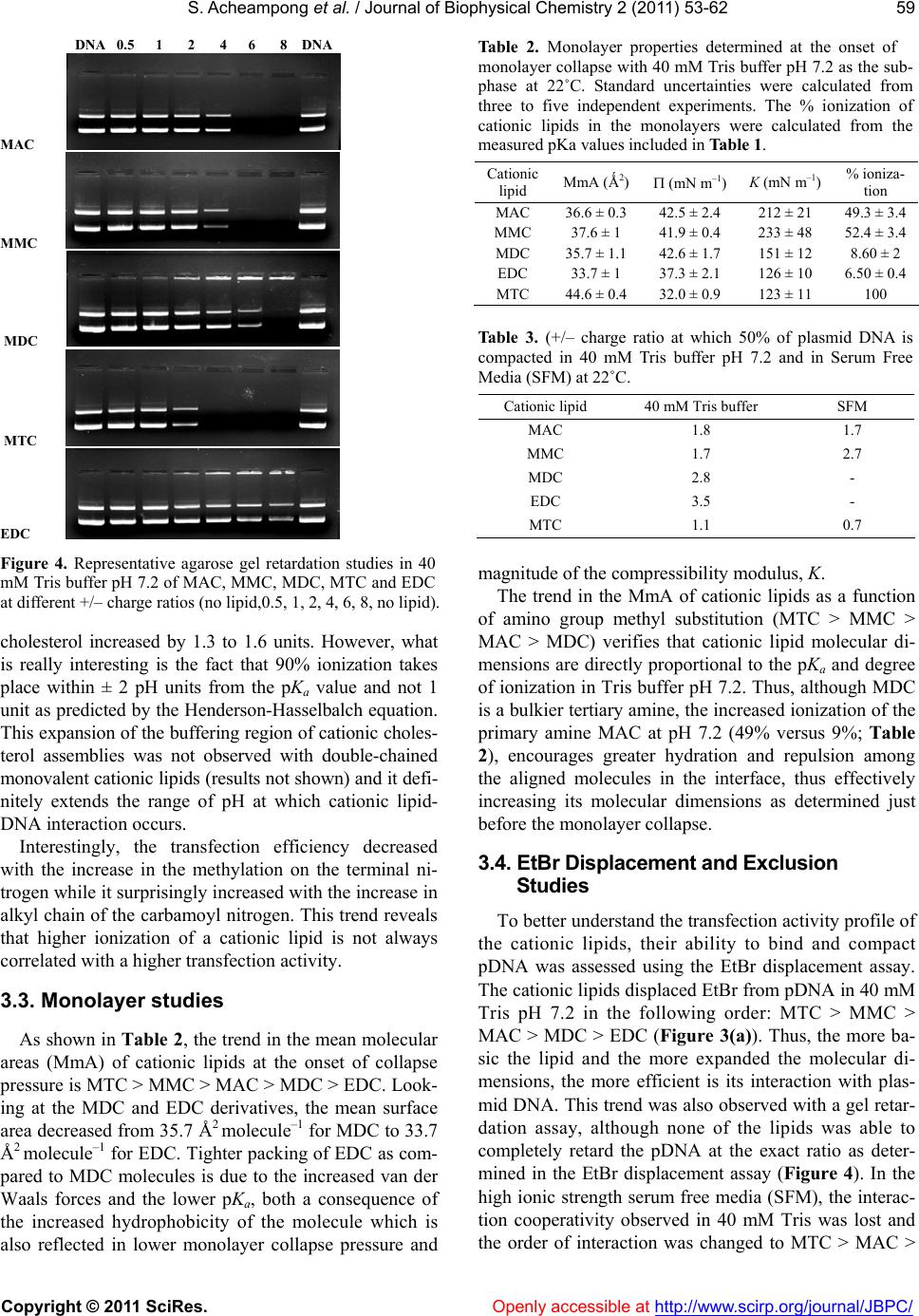 S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 59 DNA 0.5 1 2 4 6 8 DNA MAC MMC MDC MTC EDC Figure 4. Representative agarose gel retardation studies in 40 mM Tris buffer pH 7.2 of MAC, MMC, MDC, MTC and EDC at different +/– charge ratios (no lipid,0.5, 1, 2, 4, 6, 8, no lipid). cholesterol increased by 1.3 to 1.6 units. However, what is really interesting is the fact that 90% ionization takes place within ± 2 pH units from the pKa value and not 1 unit as predicted by the Henderson-Hasselbalch equation. This expansion of the buffering region of cationic choles- terol assemblies was not observed with double-chained monovalent cationic lipids (results not shown) and it defi- nitely extends the range of pH at which cationic lipid- DNA interaction occurs. Interestingly, the transfection efficiency decreased with the increase in the methylation on the terminal ni- trogen while it surprisingly increased with the increase in alkyl chain of the carbamoyl nitrogen. This trend reveals that higher ionization of a cationic lipid is not always correlated with a higher transfection activity. 3.3. Monolayer studies As shown in Table 2, the trend in the mean molecular areas (MmA) of cationic lipids at the onset of collapse pressure is MTC > MMC > MAC > MDC > EDC. Look- ing at the MDC and EDC derivatives, the mean surface area decreased from 35.7 Å2 molecule–1 for MDC to 33.7 Å2 molecule–1 for EDC. Tighter packing of EDC as com- pared to MDC molecules is due to the increased van der Waals forces and the lower pKa, both a consequence of the increased hydrophobicity of the molecule which is also reflected in lower monolayer collapse pressure and Table 2. Monolayer properties determined at the onset of monolayer collapse with 40 mM Tris buffer pH 7.2 as the sub- phase at 22˚C. Standard uncertainties were calculated from three to five independent experiments. The % ionization of cationic lipids in the monolayers were calculated from the measured pKa values included in Table 1. Cationic lipid MmA (Ǻ2) (mN m–1) K (mN m–1) % ioniza- tion MAC 36.6 ± 0.342.5 ± 2.4 212 ± 21 49.3 ± 3.4 MMC 37.6 ± 1 41.9 ± 0.4 233 ± 48 52.4 ± 3.4 MDC 35.7 ± 1.142.6 ± 1.7 151 ± 12 8.60 ± 2 EDC 33.7 ± 1 37.3 ± 2.1 126 ± 10 6.50 ± 0.4 MTC 44.6 ± 0.432.0 ± 0.9 123 ± 11 100 Table 3. (+/– charge ratio at which 50% of plasmid DNA is compacted in 40 mM Tris buffer pH 7.2 and in Serum Free Media (SFM) at 22˚C. Cationic lipid 40 mM Tris buffer SFM MAC 1.8 1.7 MMC 1.7 2.7 MDC 2.8 - EDC 3.5 - MTC 1.1 0.7 magnitude of the compressibility modulus, K. The trend in the MmA of cationic lipids as a function of amino group methyl substitution (MTC > MMC > MAC > MDC) verifies that cationic lipid molecular di- mensions are directly proportional to the pKa and degree of ionization in Tris buffer pH 7.2. Thus, although MDC is a bulkier tertiary amine, the increased ionization of the primary amine MAC at pH 7.2 (49% versus 9%; Table 2), encourages greater hydration and repulsion among the aligned molecules in the interface, thus effectively increasing its molecular dimensions as determined just before the monolayer collapse. 3.4. EtBr Displace ment and Exclusi on Stud ies To better understand the transfection activity profile of the cationic lipids, their ability to bind and compact pDNA was assessed using the EtBr displacement assay. The cationic lipids displaced EtBr from pDNA in 40 mM Tris pH 7.2 in the following order: MTC > MMC > MAC > MDC > EDC (Figure 3(a)). Thus, the more ba- sic the lipid and the more expanded the molecular di- mensions, the more efficient is its interaction with plas- mid DNA. This trend was also observed with a gel retar- dation assay, although none of the lipids was able to completely retard the pDNA at the exact ratio as deter- mined in the EtBr displacement assay (Figure 4). In the high ionic strength serum free media (SFM), the interac- tion cooperativity observed in 40 mM Tris was lost and the order of interaction was changed to MTC > MAC >  S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 60 MMC. MDC and EDC failed to significantly condense the plasmid DNA (Figure 3(b)). Most importantly, the charge ratio at which 50% of EtBr is displaced from pDNA decreased from 1.1 in 40 mM Tris to 0.7 for MTC, remained the same around 1.7 to 1.8 for the MAC and increased from 1.7 to 2.7 for MMC (Table 3). Evidently, the increased ionic strength of the media did not ad- versely affect the electrostatic interaction of the more hydrophilic quaternary ammonium salt MTC and pri- mary amine derivative MAC, but it seriously reduced the reactivity of the more lipophilic secondary and tertiary amine derivatives MMC and MDC and EDC, respec- tively, due to reduced hydration. 3.5. Particle Size and Electrophoretic Mobility Studies Cationic lipid dispersions in the absence of pDNA were in general below 200 nm in 40 mM Tris buffer pH 7.2. The smallest particle diameter was exhibited by the quaternary ammonium derivative MTC presumably due to increased ionization and polar head expansion. Owing to reduced hydration, the particle size of cationic lipid dispersions and corresponding zeta potential increased and decreased, respectively, in the high ionic strength SFM (Tabl e 4). The lipoplexes exhibited a very different, but predictable, zeta potential and particle size distribu- tion (Table 5). Lipoplex particles were slightly bigger than the size of cationic lipid dispersions in the absence of pDNA in 40 mM Tris and SFM, until the point of charge neutrality where a big jump in the lipoplex size was observed. Specifically, as previously determined by the EtBr displacement assay, complete charge neutraliza- tion occurs at +/– charge ratio of 2 and 4 for MTC and MAC and MMC, respectively. A drastic increase in lipo- Table 4. Hydrodynamic characterization of lipid dispersions (in the absence of DNA) carried out in 40 mM Tris buffer pH 7.4 and serum free media (SFM) at 22˚C. Standard uncertainties were calculated from the average values measured from three separate ex- periments. Composition Hydrodynamic Diameter (nm) PDI Zeta Potential (mV) 40 mM Tris SFM 40 mM Tris SFM 40 Mm Tris SFM MAC 160 ± 6 258 ± 78 0.26 ± 0.02 0.15 ± 0.01 +53 ± 3 +19.5 ± 1 MMC 113 ± 7 160 ± 9 0.24 ± 0.01 0.26 ± 0.02 +55 ± 1 +24 ± 2 MDC 169 ± 14 183 ± 11 0.39 ± 0.07 0.36 ± 0.04 +45 ± 1.5 +21 ± 3 EDC 203 ± 26 302 ± 83 0.45 ± 0.03 0.40 ± 0.01 +45 ± 2.6 +22 ± 2 MTC 77 ± 5 98 ± 3 0.26 ± 0.04 0.24 ± 0.02 +31 ± 7 +23 ± 1 Table 5. Particle size and zeta potential of lipoplexes at various charge ratios in Tris 40 mM buffer pH 7.2 and in Serum Free Me- dium (SFM) at 22˚C. Standard uncertainties were calculated from the average values measured from three independent experiments. Cationic lipid +/– charge ratio Hydrodynamic Diameter (nm) Polydispersity index Zeta Potential (mV) 40 mM Tris SFM 40 mM Tris SFM 40 mM Tris SFM MAC 1/1 204 ± 4 199 ± 7 0.26 ± 0.01 0.20 ± 0.01 –52 ± 2.3 –27 ± 3 MAC 2/1 328 ± 40 247 ± 14 0.30 ± 0.03 0.22 ± 0.01 –51 ± 1.5 –31 ± 3 MAC 4/1 1173 ± 714 731 ± 266 0.71 ± 0.4 0.34 ± 0.1 –6.35 ± 31.3 –33 ± 4 MMC 1/1 200 ± 59 250 ± 25 0.25 ± 0.04 0.26 ± 0.01 –50 ± 2.6 –29 ± 2 MMC 2/1 174 ± 32 273 ± 14 0.18 ± 0.07 0.25 ± 0.02 –49 ± 1.5 –31 ± 3 MMC 4/1 1137 ± 68 658 ± 166 0.53 ± 0.04 0.32 ± 0.16 +36 ± 4 –35 ± 1.3 MDC 1/1 229 ± 23 260 ± 25 0.28 ± 0.01 0.26 ± 0.02 –55 ± 2 –30 ± 2.3 MDC 2/1 236 ± 26 304 ± 31 0.31 ± 0.05 0.32 ± 0.08 –55 ± 2.6 –31 ± 2 MDC 4/1 411 ± 26 383 ± 35 0.27 ± 0.03 0.28 ± 0.07 –52 ± 0.4 –31 ± 2 EDC 1/1 454 ± 31 418 ± 112 0.19 ± 0.03 0.26 ± 0.09 –61 ± 2.6 –29 ± 2 EDC 2/1 323 ± 78 448 ± 149 0.26 ± 0.03 0.20 ± 0.05 –60 ± 2.6 –31 ± 0.2 EDC 4/1 421 ± 172 484 ± 116 0.20 ± 0.08 0.29 ± 0.01 –59 ± 2 –33 ± 2 MTC 1/1 270 ± 97 737 ± 437 0.37 ± 0.1 0.54 ± 0.16 –38 ± 0.6 –25 ± 2 MTC 2/1 1350 ± 1077 3782 ± 925 0.70 ± 0.2 0.62 ± 0.15 –30 ± 11 –18 ± 6.5 MTC 4/1 172 ± 13 475 ± 236 0.33 ± 0.05 0.41 ± 0.06 +39 ± 1 +26 ± 1  S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 61 plex particle size was observed close to these charge ra- tios (Table 5). Charge neutralization in lipoplexes com- posed of these cationic lipids was verified in 40 mM Tris pH 7.2, where the zeta potential reverses sign and be- comes positive or it is very close to zero as the charge ratio increases from 1 : 1 to 2 : 1 and 4 : 1. In summary, it was found that increased hydration of cholesterol-based cationic lipids correlates with increased ionization, increased polar head group dimensions due to higher repulsive forces among the lipids and greater inter- action with plasmid DNA, as shown by EtBr displace- ment and exclusion assays. Thus, hydration was found to be the primary factor controlling ionization of cationic lipids and their interaction with DNA. Factors affecting the hydration of the cationic lipids, such as increased io- nic strength, were shown to adversely affect primarily the ionization and DNA interaction of the more hydro- phobic tertiary (MDC, EDC) and secondary (MMC) amine derivatives. Furthermore, ionization and pKa of the cholesterol-based cationic lipids increased tremen- dously by 1.3 to 1.5 pH units in the presence of other lipids due to increased spacing, improved hydration and reduced electronic overlap. No correlation was found between the pKa of cationic assemblies and the transfec- tion activity. Also, the similarity in lipoplex particle size and zeta potential precludes predicting a cationic lipid trend in mediating transfection activity. However, com- parison of this work with our published report on secon- dary carbamate isomers AC, MC, DC and TC [14] sub- stantiates the following: Firstly, methylating or ethylat- ing the carbamoyl nitrogen of DC decreases the pKa of cationic assemblies and their interaction with plasmid DNA, but it substantially increases their transfection ac- tivity in the absence of the helper lipid DOPE. Secondly, the transfection activity of the tertiary carbamate analog MMC is significantly lower than that of the secondary carbamate MC, presumably due to toxicity issue. Thirdly, the transfection activity of the primary amine derivatives, AC and MAC, was highest, while the quaternary ammo- nium derivatives, TC and MTC were essentially transfec- tion incompetent despite their highly efficient interaction with plasmid DNA. 4. CONCLUSIONS Our success of measuring the apparent pKa of pure ca- tionic assemblies and demonstrating that membrane ioni- zation in pure cationic assemblies is much lower than that in mixed membranes was followed by the unfortu- nate realization that transfection activity is not correlated with the pKa of basic cationic lipids. The appropriate test to build structure-activity relationships is still to be dis- covered, but as our studies indicated, the key lies in the three analogs MMA, DC and MDC which their physico- chemical properties and their transfection activities differ drastically from each other. Rather a delicate bal- ance between the hydrophilicity and hydrophobicity of these cationic lipids must be controlling their transfection potency. REFERENCES [1] Gao, X.A. and Huang, L. (1991) A novel cationic lipo- some reagent for efficient transfection of mammalian cells. Biochemical and Biophysical Research Communi- cations, 179, 280-285. doi:10.1016/0006-291X(91)91366-K [2] Middleton, P.G., Caplen, N.J., Gao, X., Huang, L., Gaya, H., Geddes, D.M. and Alton, E.W.F.W. (1994) Nasal ap- plication of the cationic liposome DC-Chol: DOPE does not alter ion transport, lung function or bacteria growth. European Respiratory Journal, 7, 442-445. doi:10.1183/09031936.94.07030442 [3] Blagbrough, I.S., Geall, A.J. and Neal, A.P. (2003) Poly- amines and novel polyamine conjugates interact with DNA in ways that can be exploited in non-viral gene therapy. Biochemical Society Transactions, 31, 397-406. doi:10.1042/BST0310397 [4] Griesenbach, U., Kitson, C., Garcia, S.E., Farley, R., Singh, C., Somerton, L., Painter, H., Smith, R.L., Gill, D. R., Hyde, S.C., Chow, Y.-H., Hu, J., Gray, M., Edbrooke, M., Ogilvie, V., MacGregor, G., Scheule, R.K., Cheng, S.H., Caplen, N.J. and Alton, E.W.F.W (2006) Inefficient cationic lipid-mediated siRNA and antisense oligonucleo- tide transfer to airway epithelial cells in vivo. Respiratory Research, 7, 1-15. [5] Vigneron, J.-P., Oudrhiri, N., Fauquet, M., Vergely, L., Bradley, J.-C., Basseville, M., Lehn, P. and Lehn, J.-M. (1996) Guanidinium-cholesterol cationic lipids: Efficient vectors for the transfection of eukaryotic cells. Proceed- ings of the National Academy of Sciences of the United States of America, 93, 9682-9686. doi:10.1073/pnas.93.18.9682 [6] Fang, N., Wang, J., Mao, H.-Q., Leong, K.W. and Chan, V. (2003) BHEM-Chol/DOPE liposome induced pertur- bation of phospholipid bilayer. Colloids and Surfaces B: Biointerfaces, 29, 233-245. doi:10.1016/S0927-7765(02)00207-2 [7] Ghosh, Y.K, Visweswariah, S.S. and Bhattacharya, S. (2000) Nature of linkage between the cationic head group and cholesterol skeleton controls gene transfection effi- ciency. Federation of European Biochemical Societies Letters, 473, 341-344. doi:10.1016/S0014-5793(00)01558-1 [8] Bajaj, A., Mishra, S.K., Kondaiah, P. and Bhattacharya, S. (2008) Effect of the headgroup variation on the gene transfer properties of cholesterol based cationic lipids possessing ether linkage. Biochimica et Biophysica Acta: Biomembranes, 1778, 1222-1236. doi:10.1016/j.bbamem.2007.12.010 [9] Esposito, C., Generosi, J., Mossab, G., Masotti, A. and Castellano, A.C. (2006) The analysis of serum effects on structure, size and toxicity of DDAB-DOPE and DC- Chol-DOPE lipoplexes contributes to explain their differ- ent transfection efficiency. Colloid Surface B Biointer-  S. Acheampong et al. / Journal of Biophysical Chemistry 2 (2011) 53-62 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/JBPC/ 62 faces, 53, 187-192. [10] Koltover, I., Salditt, T. and Safinya, C.R. (1999) Phase diagram, stability, and overcharging of lamellar cationic lipid-DNA self-assembled complexes. Biophysical Jour- nal, 77, 915-924. doi:10.1016/S0006-3495(99)76942-0 [11] Felgner, P.L., Gadek, T.R., Holm, M., Roman, R., Chan, W., Wenz, M., Northorp, J.P., Ringold, G.M. and Daniel- sen, M. (1987) Lipofection: A highly efficient lipid- mediated DNA transfection procedure. Proceedings of the National Academy of Sciences of the United States of America, 84, 7413-7417. doi:10.1073/pnas.84.21.7413 [12] Israelachvili, J.N., Marcelja, S. and Horn, R.G. (1980) Physical principles of membrane organization. Quarterly reviews of Biophysics, 13, 121-200. doi:10.1017/S0033583500001645 [13] Gruner, S.M., Cullis, P.R., Hope, M.J. and Tilcock, P.S. (1985) Lipid polymorphism: The molecular basis of non- bilayer phases. Annual Review of Biophysics and Bio- physical Chemistry, 14, 211. doi:10.1146/annurev.bb.14.060185.001235 [14] Kearns, M., Donkor, A.M. and Savva, M. (2008) Struc- ture—Transfection activity studies of novel cationic cho- lesterol-based amphiphiles. Molecular Pharmaceutics, 5, 128-139. doi:10.1021/mp700131c [15] Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Mo- lecular cloning: A laboratory manual. 2nd Edition, Cold Spring Harbor Laboratory Press, New York. [16] Savva, M. and Acheampong, S. (2009) The interaction energies of cholesterol and 1,2-dioleoyl-phosphoetha- nolamine in spread mixed monolayers at the air-water in- terface. Journal of Physical Chemistry B, 113, 9811-9820. doi:10.1021/jp902748s [17] Spelios, M. and Savva, M. (2008) Novel N,N′-1,3- diaminopropyl-2-carbamoyl bivalent cationic lipids for gene delivery-synthesis, in vitro transfection activity, and physicochemical characterization. Federation of Euro- pean Biochemical Societies Journal, 275, 148-162. [18] Wang, J.L. and Edelman, G.M. (1971) Fluorescent probes for confromational states of proteins. Journal of Biologi- cal Chemistry, 246, 1185-1191. [19] Bailey, A.L. and Cullis, P.R. (1994) Modulation of mem- brane fusion by asymmetric transbilayer distributions of amino lipids. Biochemistry, 33, 12573-12580. doi:10.1021/bi00208a007 [20] Asokan, A. and Cho, M.J. (2003) Cytosolic delivery of macromolecules II. Mechanistic studies with pH-sensitive morpholine lipids. Biochimica et Biophysica Acta: Bio- membranes, 161, 151-160. [21] Heyes, J., Palmer L., Bremner, K. and MacLachlan, I. (2005) Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. Journal of Con- trolled Release, 107, 276-287. doi:10.1016/j.jconrel.2005.06.014 [22] Marchington, A.F., Moore, S.C.R. and Richards, W.G. (1979) The inductive effect in molecules and ions. Jour- nal of the American Chemical Society, 101, 5529-5532. doi:10.1021/ja00513a012 [23] Miessler, G.L. and Tarr, D.A. (2003) Inorganic Chemis- try, Prentice Hall, New Jersey. |

