R. J. DELLE-BOVI ET AL.
Copyright © 2011 SciRes. AJAC
216
altered in order to decrease relative colony size, see Ta-
ble 1 and Figure 3.
The overall colony size increased as incubation time
increased. It was beneficial to observe the carbohydrate
production over extended period of incubation times in
order to determine the optimal incubation period for
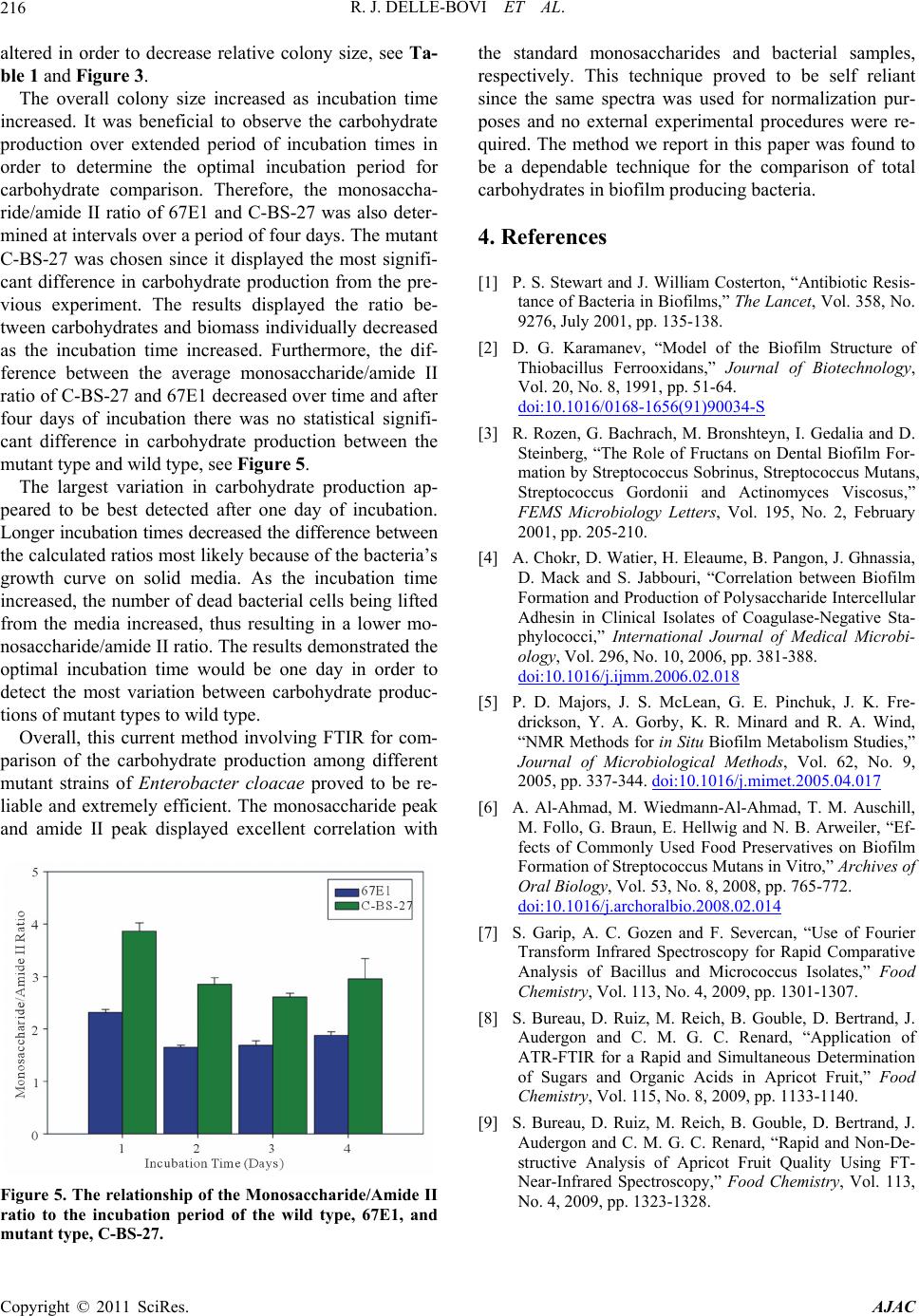
carbohydrate comparison. Therefore, the monosaccha-
ride/amide II ratio of 67E1 and C-BS-27 was also deter-
mined at intervals over a period of four days. The mutant
C-BS-27 was chosen since it displayed the most signifi-
cant difference in carbohydrate production from the pre-
vious experiment. The results displayed the ratio be-
tween carbohydrates and biomass individually decreased
as the incubation time increased. Furthermore, the dif-
ference between the average monosaccharide/amide II
ratio of C-BS-27 and 67E1 decreased over time and after
four days of incubation there was no statistical signifi-
cant difference in carbohydrate production between the
mutant type and wild type, see Figure 5.
The largest variation in carbohydrate production ap-
peared to be best detected after one day of incubation.
Longer incubation times decreased the difference between
the calculated ratios most likely because of the bacteria’s
growth curve on solid media. As the incubation time
increased, the number of dead bacterial cells being lifted
from the media increased, thus resulting in a lower mo-
nosaccharide/amide II ratio. The results demonstrated the
optimal incubation time would be one day in order to
detect the most variation between carbohydrate produc-
tions of mutant types to wild type.
Overall, this current method involving FTIR for com-
parison of the carbohydrate production among different
mutant strains of Enterobacter cloacae proved to be re-
liable and extremely efficient. The monosaccharide peak
and amide II peak displayed excellent correlation with
Figure 5. The relationship of the Monosaccharide/Amide II
ratio to the incubation period of the wild type, 67E1, and
utant type, C-BS-27. m
the standard monosaccharides and bacterial samples,
respectively. This technique proved to be self reliant
since the same spectra was used for normalization pur-
poses and no external experimental procedures were re-
quired. The method we report in this paper was found to
be a dependable technique for the comparison of total
carbohydrates in biofilm producing bacteria.
4. References
[1] P. S. Stewart and J. William Costerton, “Antibiotic Resis-
tance of Bacteria in Biofilms,” The Lancet, Vol. 358, No.
9276, July 2001, pp. 135-138.
[2] D. G. Karamanev, “Model of the Biofilm Structure of
Thiobacillus Ferrooxidans,” Journal of Biotechnology,
Vol. 20, No. 8, 1991, pp. 51-64.
doi:10.1016/0168-1656(91)90034-S
[3] R. Rozen, G. Bachrach, M. Bronshteyn, I. Gedalia and D.
Steinberg, “The Role of Fructans on Dental Biofilm For-
mation by Streptococcus Sobrinus, Streptococcus Mutans,
Streptococcus Gordonii and Actinomyces Viscosus,”
FEMS Microbiology Letters, Vol. 195, No. 2, February
2001, pp. 205-210.
[4] A. Chokr, D. Watier, H. Eleaume, B. Pangon, J. Ghnassia,
D. Mack and S. Jabbouri, “Correlation between Biofilm
Formation and Production of Polysaccharide Intercellular
Adhesin in Clinical Isolates of Coagulase-Negative Sta-
phylococci,” International Journal of Medical Microbi-
ology, Vol. 296, No. 10, 2006, pp. 381-388.
doi:10.1016/j.ijmm.2006.02.018
[5] P. D. Majors, J. S. McLean, G. E. Pinchuk, J. K. Fre-
drickson, Y. A. Gorby, K. R. Minard and R. A. Wind,
“NMR Methods for in Situ Biofilm Metabolism Studies,”
Journal of Microbiological Methods, Vol. 62, No. 9,
2005, pp. 337-344. doi:10.1016/j.mimet.2005.04.017
[6] A. Al-Ahmad, M. Wiedmann-Al-Ahmad, T. M. Auschill,
M. Follo, G. Braun, E. Hellwig and N. B. Arweiler, “Ef-
fects of Commonly Used Food Preservatives on Biofilm
Formation of Streptococcus Mutans in Vitro,” Archives of
Oral Biology, Vol. 53, No. 8, 2008, pp. 765-772.
doi:10.1016/j.archoralbio.2008.02.014
[7] S. Garip, A. C. Gozen and F. Severcan, “Use of Fourier
Transform Infrared Spectroscopy for Rapid Comparative
Analysis of Bacillus and Micrococcus Isolates,” Food
Chemistry, Vol. 113, No. 4, 2009, pp. 1301-1307.
[8] S. Bureau, D. Ruiz, M. Reich, B. Gouble, D. Bertrand, J.
Audergon and C. M. G. C. Renard, “Application of
ATR-FTIR for a Rapid and Simultaneous Determination
of Sugars and Organic Acids in Apricot Fruit,” Food
Chemistry, Vol. 115, No. 8, 2009, pp. 1133-1140.
[9] S. Bureau, D. Ruiz, M. Reich, B. Gouble, D. Bertrand, J.
Audergon and C. M. G. C. Renard, “Rapid and Non-De-
structive Analysis of Apricot Fruit Quality Using FT-
Near-Infrared Spectroscopy,” Food Chemistry, Vol. 113,
No. 4, 2009, pp. 1323-1328.