Paper Menu >>
Journal Menu >>
 Natural Science, 2009, 1, 55-62 NS http://dx.doi.org/10.4236/ns.2009.11010 Copyright © 2009 SciRes. OPEN ACCESS Synthesis of biodiesel from Jatropha curcas L. seed oil using artificial zeolites loaded with CH3COOK as a heterogeneous catalyst Wei Xue1, You-Chun Zhou1, Bao-An Song1, Xia Shi1, Jun Wang1, Shi-Tao Yin1, De-Yu Hu1, Lin-Hong Jin1, Song Yang1,* 1Key Laboratory of Green Pesticide and Bioengineering, Ministry of Education of China, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025, P. R. China; To whom correspondence should be addressed: Tel.: +86-851- 362-0521, Fax: +86-851-362-2211. Email: *jhzx.msm@gmail.com, *yangsdqj@gmail.com Received 18 May 2009; revised 30 May 2009; accepted 10 June 2009. ABSTRACT An environmentally benign process was devel- oped for the transesterification of Jatropha curcas L. seed oil with methanol using artificial zeolites loaded with potassium acetate as a heterogeneous catalyst. After calcination for 5 h at 823 K, the catalyst loaded with 47 wt.% CH3COOK exhibited the highest efficiency and best catalytic activity. The easily prepared cata- lysts were characterized by means of X-ray dif- fraction and IR spectroscopy, as well as Hammett indicator titration. The results revealed a strong dependence of catalytic activity on ba- sicity. The optimum reaction conditions for transesterification of J. curcas oil were also in- vestigated. The methyl ester content in the bio- diesel product exceeded 91% after 4h reaction at reflux temperature in the presence of 2% solid catalyst and no water washing process is needed during workup. Keywords: Biodiesel; Heterogeneous Catalyst; Artificial Zeolites; Jatropha Curcas L. seed Oil 1. INTRODUCTION Biodiesel is a biodegradable and non-toxic renewable alternative to diesel fuel that is composed of mono-alkyl esters of long-chain fatty acids derived from vegetable oils or animal fats. Biodiesel is increasing in importance because of its benign impact on the environment. [1,2] Biodiesel is produced mainly through the transesterifica- tion of vegetable oils using short-chain alcohols, typi- cally methanol or ethanol [3], because these are cheap and readily available from syngas, in which methanol is usually preferred. [4,5] Transesterification of vegetable oils with methanol, also called methanolysis, is typically carried out in the presence of homogeneous base or acid catalysts. Homogeneous base catalysts, including potas- sium hydroxide, sodium hydroxide and potassium and sodium alkoxides, such as NaOCH3 [1], have higher catalytic activity than acid catalysts. Furthermore, since acid catalysts are more corrosive than base catalysts, base catalysis is usually preferred in commercial proc- esses. [6] In the conventional homogeneous reaction, removal of the base after reaction is a major problem, since aqueous quenching results in the formation of sta- ble emulsions and saponification, making the separation of methyl esters difficult and producing waste water. [7] However, the use of solid base catalysts can not only overcome those disadvantages, but also confer some advantages, such as the elimination of a quenching step (and associated contaminated water waste) during the work-up process and the scope to operate in continuous mode. [8,9] Therefore, environmentally friendly hetero- geneous catalysts are promising for biodiesel production because of environmental constraints and potential sim- plification of existing processes. Recently, heterogeneous catalysts used to catalyze the transesterification of vegetable oils to prepare fatty acid methyl esters (FAME) have attracted considerable atten- tion. Wilson et al. [5] prepared a series of Li-promoted CaO catalysts with Li loading in the range 0.26-4.0 wt.% to catalyze the transesterification of glyceryl tributyrate and methanol. They found that the optimum loading correlated with the formation of an elec- tron-deficient surface Li+ species and associated-OH species at defect sites on the support. Lee et al. [10] re- ported a process for the production of biodiesel from vegetable oils using an Na/NaOH/γ-Al2O3 heterogene- ous catalyst. Under optimized reaction conditions, this Na/NaOH/γ-Al2O3 heterogeneous base catalyst showed almost the same activity as a conventional homogeneous NaOH catalyst. However, the base catalyst had to be  56 W. Xue et al. / Natural Science 1 (2009) 55-62 Copyright © 2009 SciRes. OPEN ACCESS prepared in special apparatus equipped with a nitrogen flow line and cold circulating water flow. These disad- vantages restrict potential industrial applications. Xie et al. [11] developed a type of Al2O3-loaded KNO3 solid base catalyst for the transesterification of soybean oil. The catalytic activity was ascribed to the presence of K2O and K-O-Al groups derived from KNO3 or other potassium compounds during high-temperature calcina- tion. Zeolites have attracted much attention in the prepara- tion of solid base catalysts. [12,13,14,15] The basicity and catalytic activity of these zeolites can be modulated through ion exchange of alkali and the occlusion of al- kali metal oxides in zeolite cages by decomposition. However, the preparation procedures for these modified zeolites have some disadvantages. For ion-exchanged zeolites, the exchanged samples need to be washed with distilled, deionized water to remove excess alkali re- maining in the ETS-10 zeolite cages. [13] Furthermore, the treatment process requires a long time and produces much waste water. In the present study, a modified artificial zeolite for catalysis of the transesterification of Jatropha curcas L. seed oil was developed using a simple preparation proc- ess. This catalyst preparation technique has many ad- vantages: (1) compared with ETS-10 and NaX zeolites, artificial zeolites are cheaper; (2) artificial zeolites are used directly as a support without any pretreatment; and (3) after impregnation with potassium acetate, artificial zeolite samples were dried and then simply calcined in a muffle furnace. Unlike NaX occluded catalysts, [13,14] artificial zeolite-supported potassium acetate was ther- mally decomposed in an uncontrolled manner and the catalyst sample was prepared easily. To the best of our knowledge, this is the first time that a catalyst of artifi- cial zeolites loaded with CH3COOK has been adopted for biodiesel production from J. curcas oil, a plant be- longing to the Euphorbiaceae family with seed oil that is non-edible and a good material for industrial biodiesel production. [16] After calcination for 5 h at 823 K, the artificial zeolite catalyst loaded with 47 wt.% CH3COOK exhibited the highest basicity and the best catalytic activity for the reaction. The catalytic activity was evaluated in terms of the methyl ester content of the product after transesterification of J. curcas oil. The op- timum reaction conditions were determined using an orthogonal experimental method. 2. EXPERIMENTAL 2.1. Catalyst Preparation Artificial zeolites (Na2OAl2O3xSiO2yH2O) were ob- tained from Shanghai Qingxi Chemical Science and Technology Ltd. (Shanghai, China). CH3COOK/artificial zeolite supported catalysts were prepared by impregnat- ing artificial zeolites with an aqueous solution of potas- sium acetate. Samples with various CH3COOK loadings were impregnated for 12 h to ensure that the CH3COOK diffused and dispersed thoroughly over the surface and through the pores of the artificial zeolites. The samples were then dried overnight at 383 K and calcined (typi- cally at 823 K) in air for 5 h to yield readily usable cata- lysts. In experiments, catalyst samples with loading amounts of 9,23,33,41,44,47 and 50 wt.% (relative to the total mass of the catalyst before calcination) were des- ignated as 9%, 23%, 33%, 41%, 44%, 47% and 50% CH3COOK/artificial zeolites, respectively. [11] 2.2. Catalyst Characterization The basic strength (H_) of the solid base was assessed using Hammett indicators. [17,18,19] In our experiments, the following Hammett indicators were used: bromthy- mol blue (H_=7.2), phenolphthalein (H_=9.8), 2, 4-di- nitroa-niline (H_=15.0), and nitroaniline (H_=18.4). Approximately 50 mg of the catalyst sample was shaken with 10 mL of anhydrous ethanolic solution of Hammett indicator and left to equilibrate for 2 h. [20] Then the color change of the solution was observed. When the solution exhibits a color change, this indicates that the basic strength of the catalyst is stronger than the indica- tor used. However, when the solution produces no color change, the basic strength of the catalyst is weaker than that of the indicator used. The basicity of the catalysts was determined by titration with a Hammett indicator and a 0.02 mol/L anhydrous ethanolic solution of ben- zene carboxylic acid. [6] It should be noted that Hammett indicator titration can only give qualitative information about the basic properties of catalysts. X-Ray diffraction (XRD) patterns of the samples were recorded on a Rigaku D/MAX-2200 powder X-ray dif- fractometer with Cu K (λ=0.154nm) radiation using an acceleration voltage of 40 kV and a current of 30mA, over a 2 range of 0-65° with a step size of 0.04° at a scanning speed of 3°/min. The data were processed us- ing DiffracPlus software. The phases were identified using the Power Diffraction File (PDF) database (JCPDS, International Center for Diffraction Data). IR spectra of the samples were measured using the KBr pellet technique. Spectra were recorded on a Shi- madzu IR-Prestige-21 spectrometer with resolution of 4 cm–1 over the range 400-4000cm–1. 2.3. Transesterification Reaction Jatropha curcas oil was prepared by squeezing seeds of J. curcas from Luodian county, Guizhou Province, south- west China. The crude oil obtained was then further pu- rified by filtering out solid impurities and refined to re- duce the water content. According to gas chromatogra- phy (GC) analysis, the fatty acids of J. curcas oil con- sisted of: palmitic acid (12.47%), palmitoleic acid (2.10%), stearic acid (6.42%), oleic acid (32.04%), and 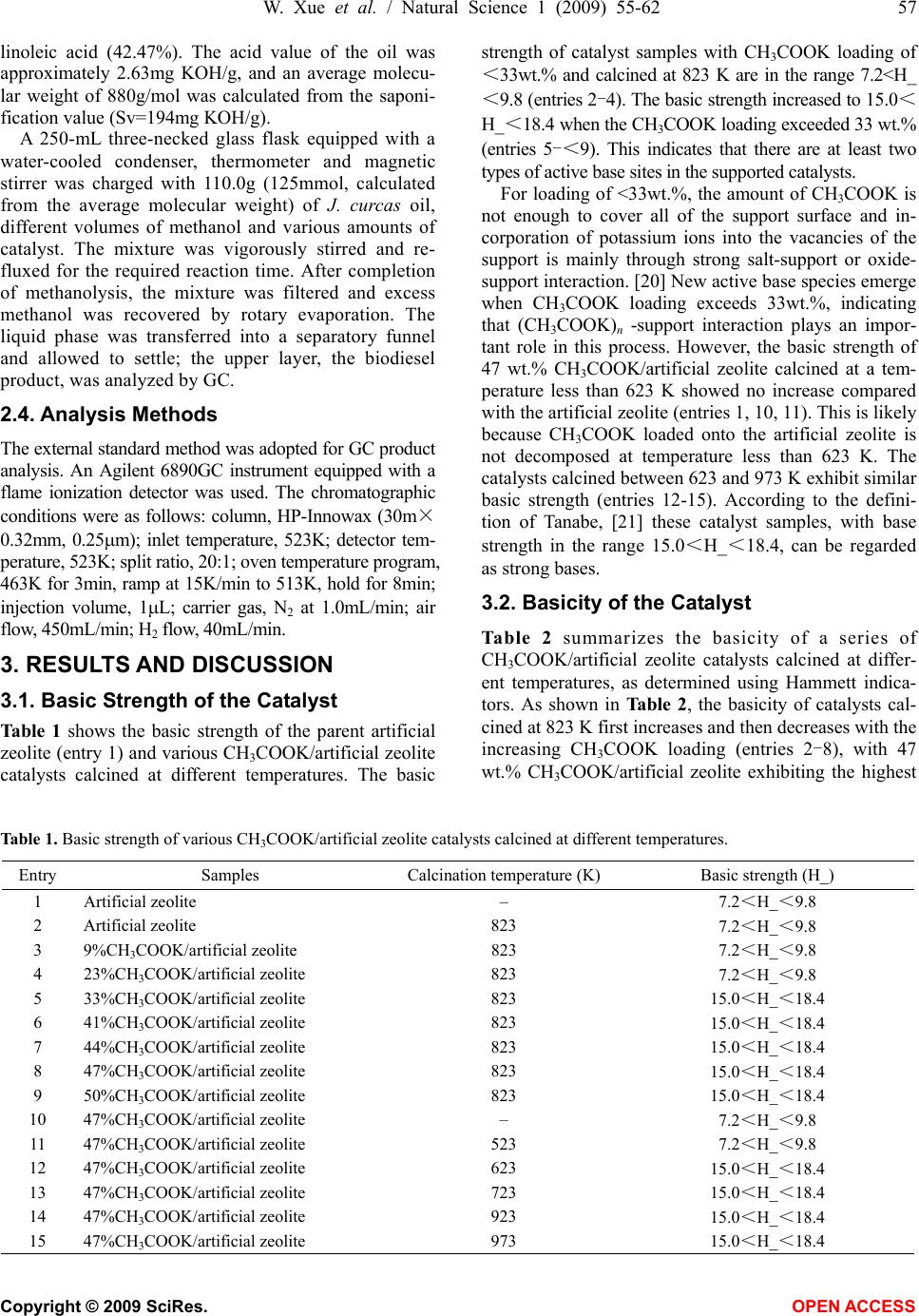 W. Xue et al. / Natural Science 1 (2009) 55-62 57 Copyright © 2009 SciRes. OPEN ACCESS linoleic acid (42.47%). The acid value of the oil was approximately 2.63mg KOH/g, and an average molecu- lar weight of 880g/mol was calculated from the saponi- fication value (Sv=194mg KOH/g). A 250-mL three-necked glass flask equipped with a water-cooled condenser, thermometer and magnetic stirrer was charged with 110.0g (125mmol, calculated from the average molecular weight) of J. curcas oil, different volumes of methanol and various amounts of catalyst. The mixture was vigorously stirred and re- fluxed for the required reaction time. After completion of methanolysis, the mixture was filtered and excess methanol was recovered by rotary evaporation. The liquid phase was transferred into a separatory funnel and allowed to settle; the upper layer, the biodiesel product, was analyzed by GC. 2.4. Analysis Methods The external standard method was adopted for GC product analysis. An Agilent 6890GC instrument equipped with a flame ionization detector was used. The chromatographic conditions were as follows: column, HP-Innowax (30m× 0.32mm, 0.25m); inlet temperature, 523K; detector tem- perature, 523K; split ratio, 20:1; oven temperature program, 463K for 3min, ramp at 15K/min to 513K, hold for 8min; injection volume, 1L; carrier gas, N2 at 1.0mL/min; air flow, 450mL/min; H2 flow, 40mL/min. 3. RESULTS AND DISCUSSION 3.1. Basic Strength of the Catalyst Table 1 shows the basic strength of the parent artificial zeolite (entry 1) and various CH3COOK/artificial zeolite catalysts calcined at different temperatures. The basic strength of catalyst samples with CH3COOK loading of <33wt.% and calcined at 823 K are in the range 7.2<H_ <9.8 (entries 2-4). The basic strength increased to 15.0< H_<18.4 when the CH3COOK loading exceeded 33 wt.% (entries 5-<9). This indicates that there are at least two types of active base sites in the supported catalysts. For loading of <33wt.%, the amount of CH3COOK is not enough to cover all of the support surface and in- corporation of potassium ions into the vacancies of the support is mainly through strong salt-support or oxide- support interaction. [20] New active base species emerge when CH3COOK loading exceeds 33wt.%, indicating that (CH3COOK)n -support interaction plays an impor- tant role in this process. However, the basic strength of 47 wt.% CH3COOK/artificial zeolite calcined at a tem- perature less than 623 K showed no increase compared with the artificial zeolite (entries 1, 10, 11). This is likely because CH3COOK loaded onto the artificial zeolite is not decomposed at temperature less than 623 K. The catalysts calcined between 623 and 973 K exhibit similar basic strength (entries 12-15). According to the defini- tion of Tanabe, [21] these catalyst samples, with base strength in the range 15.0<H_<18.4, can be regarded as strong bases. 3.2. Basicity of the Catalyst Table 2 summarizes the basicity of a series of CH3COOK/artificial zeolite catalysts calcined at differ- ent temperatures, as determined using Hammett indica- tors. As shown in Table 2, the basicity of catalysts cal- cined at 823 K first increases and then decreases with the increasing CH3COOK loading (entries 2-8), with 47 wt.% CH3COOK/artificial zeolite exhibiting the highest Table 1. Basic strength of various CH3COOK/artificial zeolite catalysts calcined at different temperatures. Entry Samples Calcination temperature (K) Basic strength (H_) 1 Artificial zeolite – 7.2<H_<9.8 2 Artificial zeolite 823 7.2<H_<9.8 3 9%CH3COOK/artificial zeolite 823 7.2<H_<9.8 4 23%CH3COOK/artificial zeolite 823 7.2<H_<9.8 5 33%CH3COOK/artificial zeolite 823 15.0<H_<18.4 6 41%CH3COOK/artificial zeolite 823 15.0<H_<18.4 7 44%CH3COOK/artificial zeolite 823 15.0<H_<18.4 8 47%CH3COOK/artificial zeolite 823 15.0<H_<18.4 9 50%CH3COOK/artificial zeolite 823 15.0<H_<18.4 10 47%CH3COOK/artificial zeolite – 7.2<H_<9.8 11 47%CH3COOK/artificial zeolite 523 7.2<H_<9.8 12 47%CH3COOK/artificial zeolite 623 15.0<H_<18.4 13 47%CH3COOK/artificial zeolite 723 15.0<H_<18.4 14 47%CH3COOK/artificial zeolite 923 15.0<H_<18.4 15 47%CH3COOK/artificial zeolite 973 15.0<H_<18.4  58 W. Xue et al. / Natural Science 1 (2009) 55-62 Copyright © 2009 SciRes. OPEN ACCESS Table 2. Basicity of various CH3COOK/artificial zeolite catalysts calcined at different temperatures. Entry Samples Calcination temperature (K) Basicity (mmol KOH/g) 1 Artificial zeolite – 0 2 Artificial zeolite 873 0 3 9%CH3COOK/artificial zeolite 823 0.0246 4 23%CH3COOK/artificial zeolite 823 0.1393 5 33%CH3COOK/artificial zeolite 823 0.3122 6 41%CH3COOK/artificial zeolite 823 0.3527 7 47%CH3COOK/artificial zeolite 823 0.4058 8 50%CH3COOK/artificial zeolite 823 0.3786 9 47%CH3COOK/artificial zeolite – 0.2079 10 47%CH3COOK/artificial zeolite 523 0.2540 11 47%CH3COOK/artificial zeolite 623 0.2832 12 47%CH3COOK/artificial zeolite 723 0.3689 13 47%CH3COOK/artificial zeolite 923 0.2956 14 47%CH3COOK/artificial zeolite 973 0.1654 basicity (0.4058 mmol KOH/g; entry 7). This is similar to the findings of Xie et al. [11] The calcination tem- perature also affects catalyst basicity. It is evident from Table 2 that catalysts calcined at 823K had the highest basicity (entries 7, 9-14). It was noted that 47% CH3 COOK/artificial zeolite without calcination had higher basicity than 23% CH3COOK/artificial zeolite calcined at 823 K (0.2079 vs. 0.1393 mmol KOH/g, entries 9 and 4). 3.2 Basicity of the Catalyst Table 2 summarizes the basicity of a series of CH3COOK/artificial zeolite catalysts calcined at differ- ent temperatures, as determined using Hammett indica- tors. As shown in Table 2, the basicity of catalysts cal- cined at 823 K first increases and then decreases with the increasing CH3COOK loading (entries 2-8), with 47 wt.% CH3COOK/artificial zeolite exhibiting the highest basicity (0.4058 mmol KOH/g; entry 7). This is similar to the findings of Xie et al. [11] The calcination tem- perature also affects catalyst basicity. It is evident from Table 2 that catalysts calcined at 823 K had the highest basicity (entries 7, 9-14). It was noted that 47% CH3COOK/artificial zeolite without calcination had higher basicity than 23% CH3COOK/artificial zeolite calcined at 823 K (0.2079 vs. 0.1393 mmol KOH/g, en- tries 9 and 4). 3.3. Lnfluence of Catalyst Preparation Conditions 3.3.1. Lnfluence of CH3COOK Loading The catalytic activity of the catalysts was evaluated by comparing the FAME content of the transesterification products. The effect of CH3COOK loading on the cata- lytic activity is shown Fig. 1. As the CH3COOK loading increases from 9% to 47%, the methyl ester content in- creases. The highest methyl ester content (91.58%) was obtained for 47 wt.% CH3COOK loading. However, the FAME content decreased slightly for a further increase in CH3COOK loading, which may be due to partial cov- ering of basic sites by K2O species from the excess CH3COOK on the surface of the composite. It is impor- tant to point out that the conversion decreased signifi- cantly when potassium salt loading exceeded the critical limit for the transesterification of soybean. [11] 3.3.2. Influence of Calcination Temperature Figure 2 shows the influence of calcination temperature on the catalytic activity in the temperature range from 523 to 923 K. The optimal calcination temperature ob- served was 823 K. At this temperature, the methyl ester content reached 91.58%. However, when the calcination temperature increased to 923 K, the catalyst activity de- creased. Such results are in line with the basicity proper- ties shown in Table 2, indicating that higher basicity results in higher conversion and higher methyl ester content. However, the FAME content obtained using 47 wt.% CH3COOK/artificial zeolite calcined at 523 K is lower than that obtained using 23 wt.% CH3COOK/arti- ficial zeolite calcined at 823 K, although the basicity of the former is higher than that of the latter (0.2540 vs. 0.1393 mmol KOH/g). This implies that basicity is the most important, but not the only factor affecting the ac- tivity of these supported catalysts. 3.4. FTIR Analysis IR spectroscopy was used to investigate the CH3COOK/ artificial zeolite catalysts. The results are shown in Fig. 3. For the parent artificial zeolite, two absorption bands at 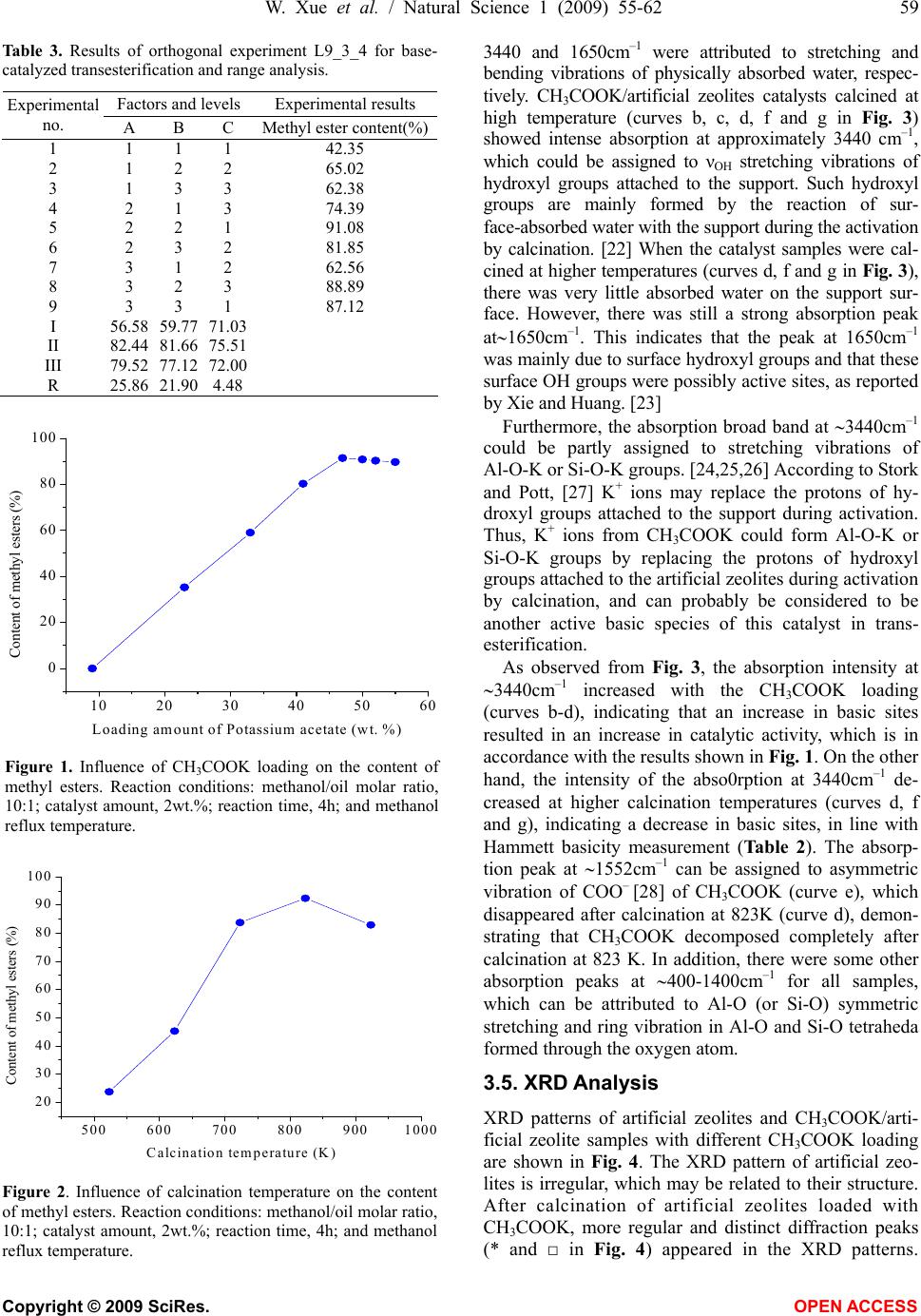 W. Xue et al. / Natural Science 1 (2009) 55-62 59 Copyright © 2009 SciRes. OPEN ACCESS Table 3. Results of orthogonal experiment L9_3_4 for base- catalyzed transesterification and range analysis. Factors and levelsExperimental results Experimental no. A B C Methyl ester content(%) 1 1 1 1 42.35 2 1 2 2 65.02 3 1 3 3 62.38 4 2 1 3 74.39 5 2 2 1 91.08 6 2 3 2 81.85 7 3 1 2 62.56 8 3 2 3 88.89 9 3 3 1 87.12 I 56.58 59.77 71.03 II 82.44 81.66 75.51 III 79.52 77.12 72.00 R 25.86 21.90 4.48 1020 30 40 50 60 0 20 40 60 80 100 Content of methyl esters (%) Loading amount of Potassium acetate (wt. %) Figure 1. Influence of CH3COOK loading on the content of methyl esters. Reaction conditions: methanol/oil molar ratio, 10:1; catalyst amount, 2wt.%; reaction time, 4h; and methanol reflux temperature. 500 600 700 800 9001000 20 30 40 50 60 70 80 90 100 Content of methyl esters (%) Calcination temperature (K) Figure 2. Influence of calcination temperature on the content of methyl esters. Reaction conditions: methanol/oil molar ratio, 10:1; catalyst amount, 2wt.%; reaction time, 4h; and methanol reflux temperature. 3440 and 1650cm–1 were attributed to stretching and bending vibrations of physically absorbed water, respec- tively. CH3COOK/artificial zeolites catalysts calcined at high temperature (curves b, c, d, f and g in Fig. 3) showed intense absorption at approximately 3440 cm–1, which could be assigned to νOH stretching vibrations of hydroxyl groups attached to the support. Such hydroxyl groups are mainly formed by the reaction of sur- face-absorbed water with the support during the activation by calcination. [22] When the catalyst samples were cal- cined at higher temperatures (curves d, f and g in Fig. 3), there was very little absorbed water on the support sur- face. However, there was still a strong absorption peak at1650cm–1. This indicates that the peak at 1650cm–1 was mainly due to surface hydroxyl groups and that these surface OH groups were possibly active sites, as reported by Xie and Huang. [23] Furthermore, the absorption broad band at 3440cm–1 could be partly assigned to stretching vibrations of Al-O-K or Si-O-K groups. [24,25,26] According to Stork and Pott, [27] K+ ions may replace the protons of hy- droxyl groups attached to the support during activation. Thus, K+ ions from CH3COOK could form Al-O-K or Si-O-K groups by replacing the protons of hydroxyl groups attached to the artificial zeolites during activation by calcination, and can probably be considered to be another active basic species of this catalyst in trans- esterification. As observed from Fig. 3, the absorption intensity at 3440cm–1 increased with the CH3COOK loading (curves b-d), indicating that an increase in basic sites resulted in an increase in catalytic activity, which is in accordance with the results shown in Fig. 1. On the other hand, the intensity of the abso0rption at 3440cm–1 de- creased at higher calcination temperatures (curves d, f and g), indicating a decrease in basic sites, in line with Hammett basicity measurement (Table 2). The absorp- tion peak at 1552cm–1 can be assigned to asymmetric vibration of COO– [28] of CH3COOK (curve e), which disappeared after calcination at 823K (curve d), demon- strating that CH3COOK decomposed completely after calcination at 823 K. In addition, there were some other absorption peaks at 400-1400cm–1 for all samples, which can be attributed to Al-O (or Si-O) symmetric stretching and ring vibration in Al-O and Si-O tetraheda formed through the oxygen atom. 3.5. XRD Analysis XRD patterns of artificial zeolites and CH3COOK/arti- ficial zeolite samples with different CH3COOK loading are shown in Fig. 4. The XRD pattern of artificial zeo- lites is irregular, which may be related to their structure. After calcination of artificial zeolites loaded with CH3COOK, more regular and distinct diffraction peaks (* and □ in Fig. 4) appeared in the XRD patterns. 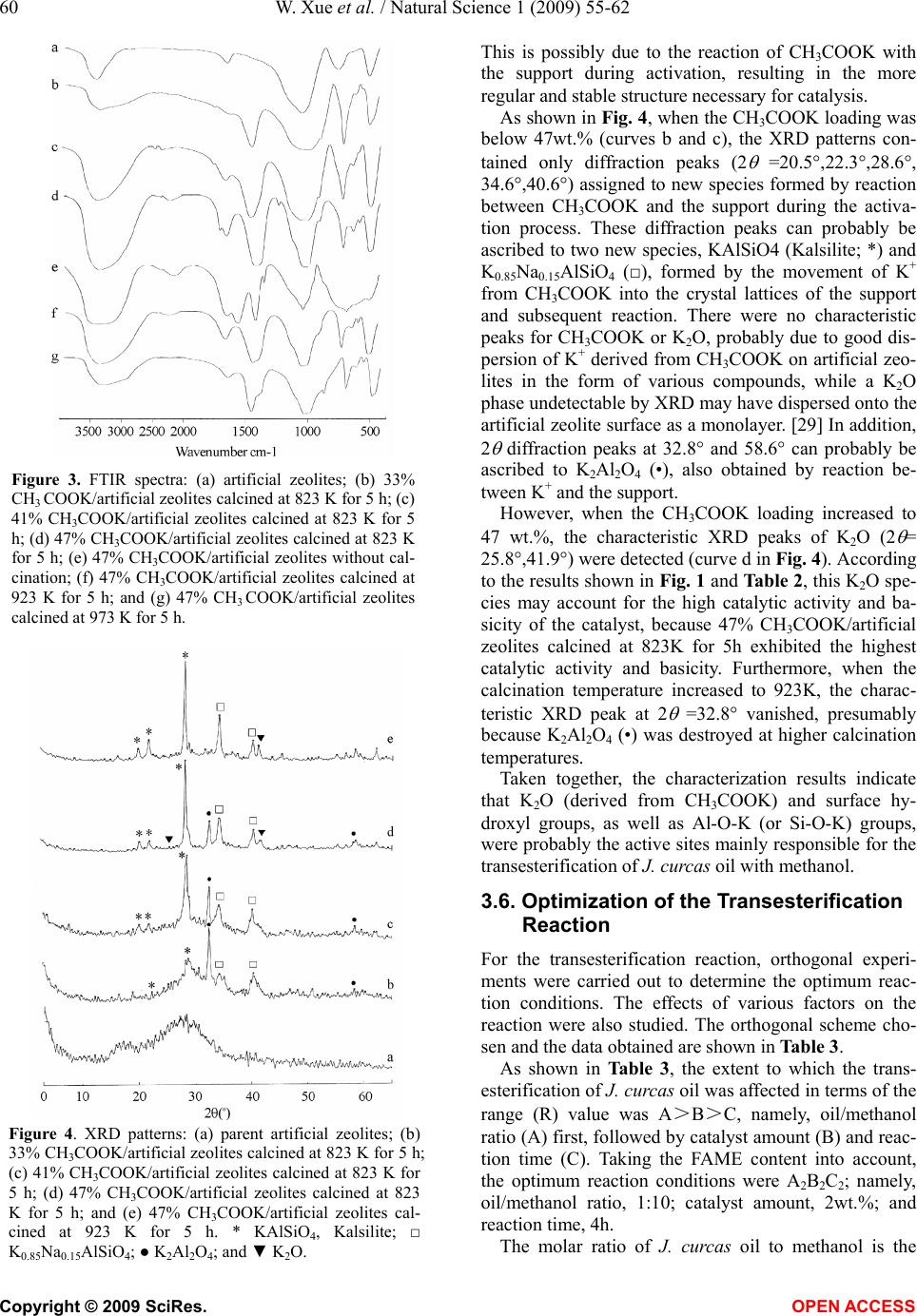 60 W. Xue et al. / Natural Science 1 (2009) 55-62 Copyright © 2009 SciRes. OPEN ACCESS Figure 3. FTIR spectra: (a) artificial zeolites; (b) 33% CH3 COOK/artificial zeolites calcined at 823 K for 5 h; (c) 41% CH3COOK/artificial zeolites calcined at 823 K for 5 h; (d) 47% CH3COOK/artificial zeolites calcined at 823 K for 5 h; (e) 47% CH3COOK/artificial zeolites without cal- cination; (f) 47% CH3COOK/artificial zeolites calcined at 923 K for 5 h; and (g) 47% CH3 COOK/artificial zeolites calcined at 973 K for 5 h. Figure 4. XRD patterns: (a) parent artificial zeolites; (b) 33% CH3COOK/artificial zeolites calcined at 823 K for 5 h; (c) 41% CH3COOK/artificial zeolites calcined at 823 K for 5 h; (d) 47% CH3COOK/artificial zeolites calcined at 823 K for 5 h; and (e) 47% CH3COOK/artificial zeolites cal- cined at 923 K for 5 h. * KAlSiO4, Kalsilite; □ K0.85Na0.15AlSiO4; ● K2Al2O4; and ▼ K2O. This is possibly due to the reaction of CH3COOK with the support during activation, resulting in the more regular and stable structure necessary for catalysis. As shown in Fig. 4, when the CH3COOK loading was below 47wt.% (curves b and c), the XRD patterns con- tained only diffraction peaks (2 =20.5°,22.3°,28.6°, 34.6°,40.6°) assigned to new species formed by reaction between CH3COOK and the support during the activa- tion process. These diffraction peaks can probably be ascribed to two new species, KAlSiO4 (Kalsilite; *) and K0.85Na0.15AlSiO4 (□), formed by the movement of K+ from CH3COOK into the crystal lattices of the support and subsequent reaction. There were no characteristic peaks for CH3COOK or K2O, probably due to good dis- persion of K+ derived from CH3COOK on artificial zeo- lites in the form of various compounds, while a K2O phase undetectable by XRD may have dispersed onto the artificial zeolite surface as a monolayer. [29] In addition, 2 diffraction peaks at 32.8° and 58.6° can probably be ascribed to K2Al2O4 (•), also obtained by reaction be- tween K+ and the support. However, when the CH3COOK loading increased to 47 wt.%, the characteristic XRD peaks of K2O (2 = 25.8°,41.9°) were detected (curve d in Fig. 4). According to the results shown in Fig. 1 and Table 2, this K2O spe- cies may account for the high catalytic activity and ba- sicity of the catalyst, because 47% CH3COOK/artificial zeolites calcined at 823K for 5h exhibited the highest catalytic activity and basicity. Furthermore, when the calcination temperature increased to 923K, the charac- teristic XRD peak at 2 =32.8° vanished, presumably because K2Al2O4 (•) was destroyed at higher calcination temperatures. Taken together, the characterization results indicate that K2O (derived from CH3COOK) and surface hy- droxyl groups, as well as Al-O-K (or Si-O-K) groups, were probably the active sites mainly responsible for the transesterification of J. curcas oil with methanol. 3.6. Optimization of the Transesterification Reaction For the transesterification reaction, orthogonal experi- ments were carried out to determine the optimum reac- tion conditions. The effects of various factors on the reaction were also studied. The orthogonal scheme cho- sen and the data obtained are shown in Table 3. As shown in Table 3, the extent to which the trans- esterification of J. curcas oil was affected in terms of the range (R) value was A>B>C, namely, oil/methanol ratio (A) first, followed by catalyst amount (B) and reac- tion time (C). Taking the FAME content into account, the optimum reaction conditions were A2B2C2; namely, oil/methanol ratio, 1:10; catalyst amount, 2wt.%; and reaction time, 4h. The molar ratio of J. curcas oil to methanol is the  W. Xue et al. / Natural Science 1 (2009) 55-62 61 Copyright © 2009 SciRes. OPEN ACCESS most important factor affecting the transesterification to methyl esters (Table 3). Theoretically, three moles of methanol are required for one mole of triglyceride to yield one mole of glycerol and three moles of FAME. However, a slight excess of methanol is required to drive the equilibrium to the product side, because the trans- esterification reaction is reversible. [1] With an increase in the oil/methanol molar ratio, the conversion increased considerably. However, further addition of methanol not only had no significant effect on the conversion, but also seriously affected glycerine separation due to the in- crease in solubility. [30] Thus, the optimum molar ratio of J. curcas oil to methanol to produce methyl esters was 1:10. The amount of catalyst is another important factor that affects the reaction. With no catalyst, transesterification does not occur. When the amount of catalyst was insuf- ficient, the FAME content was very low (entries 1, 4, 7). Saponification took place when the amount of catalyst was increased to 3%, leading to product emulsion and making separation difficult. The FAME content and product yield were also influenced (entries 3, 6, 9). As revealed in orthogonal experiments, 2 wt.% catalyst was appropriate for this transesterification reaction. The final factor affecting transesterification is the re- action time. When the reaction time is too short, the con- version of vegetable oil to methyl esters is incomplete, and conversion increases with the reaction time. How- ever, when the reaction reaches equilibrium, prolonged reaction time does not increase the FAME content of the product, but increases the cost for biodiesel production. In homogeneous base-catalyzed transesterification, the reaction time is usually less than 1 h. For heterogeneous transesterification the reaction time needs to be longer, because the system components, the base catalyst, methanol and J. curcas oil in the present study, require a longer contact time. According to orthogonal experi- ments, an optimum reaction time of 4 h was chosen. An experiment was carried out under above optimum reaction conditions, a product yield of 94.27% and methyl ester content of 91.58% were obtained after transesterification. 4. CONCLUSIONS Easily prepared solid-base catalysts using artificial zeo- lites as a support were developed for the transesterifica- tion of J. curcas oil with methanol to produce biodiesel. The CH3COOK/artificial zeolites solid catalysts exhib- ited high catalytic activity in the transesterification process. The methyl ester content exceeded 91% when the catalyst with 47 wt.% CH3COOK was calcined at 823 K for 5 h. Catalyst characterization revealed that K2O, surface hydroxyl groups and Al-O-K (or Si-O-K) groups are the main basic sites. Furthermore, the activity of the catalysts depends strongly on their basicity. Or- thogonal experiments revealed the following optimum reaction conditions: oil/methanol ratio, 1:10; catalyst amount, 2wt.%; and reaction time, 4h. No water washing step is needed thus no waster water was produced during this process. The information mentioned above estab- lishes certain basis for industrial biodiesel production from J. curcas seed oil using the heterogeneous catalysts. Further investigations on the reaction mechanism and active sites are under way in our laboratory. ACKNOWLEDGEMENT This work is financially supported by Program for New Century Ex- cellent Talents in Chinese University (NCET-05-0818), Key Science and Technology Project of Guizhou Province (No. 20076004), and the National Key Technology R&D Program (2006BAD07A12). REFERENCES [1] Ma, F. and Hanna, M. A. (1999) Biodiesel production: A review. Bioresour Technol, 70, 1-15. [2] Ramadhas, A. S., Jayaraj, S. and Muraleedharan C. (2005) Biodiesel production from high FFA rubber seed oil. Fuel, 84, 335-340. [3] Schuchardt, U., Sercheli, R. and Vargas, R. M. (1998) Transesterification of vegetable oils: A review. J Braz Chem Soc, 9, 199-210. [4] Graboski, M. S. and McCormick, R. L. (1998) Combus- tion of fat and vegetable oil derived fuels in diesel en- gines. Prog Energy Combust Sci, 24, 125-164. [5] Watkins, R. S., Lee, A. F. and Wilson, K. (2004) Li-CaO catalysed tri-glyceride transesterification for biodiesel applications. Green Chem, 6, 335-340. [6] Xie, W. L. and Li, H. T. (2006) Alumina-supported po- tassium iodide as a heterogeneous catalyst for biodiesel production from soybean oil. J Mol Catal A: Chem, 255, 1-9. [7] Gryglewicz, S. (1999) Rapeseed oil methyl esters prepa- ration using heterogeneous catalysts. Bioresour Technol, 70, 249-253. [8] Ono, Y. and Baba, T. (1997) Selective reactions over solid base catalysts. Catal Today, 38, 321-337. [9] Clark, J. H. and Macquarrie, D. J. (1996) Environmen- tally friendly catalytic methods. Chem Soc Rev, 25, 303-310. [10] Kim H J, Kang B S, Kim M J, Park Y M, Kim D K, Lee J S, Lee K Y. Transesterification of vegetable oil to bio- diesel using heterogeneous base catalyst. Catal Today 2004; 93-95: 315-320. [11] Xie, W. L., Peng, H. and Chen, L. G. (2006) Trans- esterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl Catal A: Gen, 300, 67-74. [12] Bordawekar, S. V. and Davis, R. J. (2000) Probing the basic character of alkali-modified zeolites by CO2 ad- sorption microcalorimetry, butene isomerization, and toluene alkylation with ethylene. J Catal, 189, 79-90. [13] Suppes, G. J., Dasari, M. A., Doskocil, E. J., Mankidy, P. J. and Goff, M. J. (2004) Transesterification of soybean  62 W. Xue et al. / Natural Science 1 (2009) 55-62 Copyright © 2009 SciRes. OPEN ACCESS oil with zeolite and metal catalysts. Appl Catal A: Gen, 257, 213-223. [14] Doskocil, E. J. and Mankidy, P. J. (2003) Effects on solid basicity for sodium metal and metal oxide occluded NaX zeolites. Appl Catal A: Gen, 252, 119–132. [15] Leclercq, E., Finiels, A. and Moreau C. (2001) Trans- esterification of rapeseed oil in the presence of basic zeo- lites and related solid catalysts. J Am Oil Chem Soc, 78, 1161-1165. [16] Gubitz, G. M., Mittelbach, M. and Trabi, M. (1999) Ex- ploitation of the tropical oil seed plant Jatropha curcas L. Bioresour Technol, 67, 73-82. [17] Forni, L. (1974) Comparison of the methods for the de- termination of surface acidity of solid catalysts. Catal Rev, 8, 65-115. [18] Gorzawski, H. and Hoelderich, W. F. (1999) Preparation of superbases and their use as catalysts for double-bond isomerization. J Mol Catal A: Chem, 144, 181-187. [19] Xie, W. L., Huang, X. M. and Li, H. T. (2007) Soybean oil methyl esters preparation using NaX zeolites loaded with KOH as a heterogeneous catalyst. Bioresour Tech- nol, 98, 936-939. [20] Chen, Y. and Zhang, L. F. (1992) Surface interaction model of γ-alumina-supported metal oxides. Catal Lett, 12, 51-62. [21] Tanabe, K. (1985) Catalysis by acids and bases. in: B. Imelik, C. Nacceche, G. Condurier, Y. BenTaarti, J. C. Vedrine (Eds.), Elsevier, Amsterdam, 1-1. [22] Nakamoto, K. (1970) Infrared spectra of inorganic and coordination compounds. John Wiley, New York, 98-98. [23] Xie, W. L. and Huang, X. M. (2006) Synthesis of bio- diesel from soybean oil using heterogeneous KF/ZnO catalyst. Catal Lett, 107, 53-59. [24] Krupay, B. W. and Amenomiya, Y. (1981) Al- kali-promoted alumina catalysts: I. Chemisorption and oxygen exchange of carbon monoxide and carbon diox- ide on potassium-promoted alumina catalysts. J Catal, 67, 362-370. [25] Iordan, A., Kappenstein, C., Colnay, E. and Zaki, M. I. (1998) Surface contribution to the interfacial chemistry of potassium modified oxide catalysts. J Chem Soc Faraday Trans, 94, 1149-1156. [26] Amenomiya, Y. and Pleizier, G. (1982) Alkali-promoted alumina catalysts: II. Water-gas shift reaction. J Catal, 76, 345-353. [27] Stork, W. H. J. and Pott, G. T. (1974) Studies of com- pound formation on alkali/γ-aluminum oxide catalyst systems using chromium, iron, and manganese lumines- cence. J Phys Chem B, 78, 2496-2506. [28] Bilger, S., Syskakis, E., Naoumidis, A. and Nickel, H. (1992) Sol-gel synthesis of strontium-doped lanthanum manganite. J Am Ceram Soc, 75, 964-970. [29] Jiang, D. E., Zhao, B. Y., Xie, Y. C., Pan, G. C., Ran, G. P. and Min, E. Z. (2001) Structure and basicity of γ-Al2O3-supported MgO and its application to mercap- tan oxidation. Appl Catal A: Gen, 219, 69-78. [30] Meher, L. C., Sagar, D. V. and Naik, S. N. (2006) Tech- nical aspects of biodiesel production by transesterifica- tion-a review. Renew Sust Energy Rev, 10, 248-268. |

