Paper Menu >>
Journal Menu >>
 Natural Science, 2009, 1, 47-54 NS http://dx.doi.org/10.4236/ns.2009.11009 Copyright © 2009 SciRes. OPEN ACCESS Adaptation fermentation of Pichia stipitis and combination detoxification on steam exploded lignocellulosic prehydrolyzate Jun-Jun Zhu1, Qiang Yong1, Yong Xu1, Shang-Xing Chen2, Shi-Yuan Yu1* 1Ministry of Education, Key Laboratory of Forest Genetics & Biotechnology, Nanjing Forestry University, Nanjing 210037, China; 2College of Forestry, Jiangxi Agricultural University, Nanchang 330045, China. *Corresponding author: Tel: +86 25 85427255; Fax: +86 25 85418873. E-mail: *shiyuanyu.nfu@gmail.com Received 25 May 2009; revised 5 June 2009; accepted 8 June 2009. ABSTRACT Yeast Pichia stipitis CBS 5776 was developed through adaptation fermentation step by step in steam exploded corn stover prehydrolyzate because high concentration of weak acids and other inhibitors present in the prehydrolyzate could degrade the fermentability. However, the adaptability of Pichia stipitis CBS 5776 in the prehydrolyzate was so limited that steam strip- ping and overliming were applied to remove these inhibitors from it. Corn stover was steam exploded; the filtrate of steam exploded corn stover was hydrolyzed with dilute sulfuric acid, and then the acid hydrolyzate was detoxified and fermented by Pichia stipitis CBS 5776. Steam stripping could remove volatile com- pounds from the acid hydrolyzate and the fil- trate. At a steam stripping time of 120min, 81% acetic acid and 59% formic acid were removed from the acid hydrolyzate, 77% acetic acid and 45% formic acid were removed from the filtrate, while furfural was stripped off completely from the acid hydrolyzate and the filtrate. Overliming could reduce the contents of furfural and phe- nolics present in the acid hydrolyzate, however, sugars, especially pentoses, were also removed partially. It was necessary to detoxify the acid hydrolyzate in order to ferment the sugars to ethanol. Acid hydrolyzate detoxified with a combination of steam stripping for 120 min and overliming at pH11 and 60℃ for 90 min, its fer- mentability was significantly improved. Xylose was consumed nearly completely in 24h with an ethanol yield of 15.92g/l, 80.34% of theoretical. Keywords: Steam Explosion; Steam Stripping; Overliming; Inhibitor; Acid Hydrolyzate 1. INTRODUCTION For several decades, ethanol has been promoted as a promising alternative fuel for transportation. The use of fossil fuels has contributed to the buildup of carbon di- oxide in the atmosphere; however ethanol is a clean- burning fuel that makes no net contribution to global warming because the carbon dioxide produced by the combustion of ethanol is consumed by plant growth which continues the carbon cycle balance in the nature. Ethanol can be produced from any material containing simple or complex sugars, such as sugar cane and vari- ous starchy materials (corn, wheat, and potatoes). How- ever, the most promising raw material is represented by lignocellulose because of its renewable nature, abun- dance and low cost [1]. Lignocelluloses are mainly comprised of cellulose, hemicellulose, and lignin. The substrate must be pre- treated so that it is more amenable to conversion. A number of pretreatments such as dilute acid hydrolysis [2], alkali treatment [3], sodium sulphite treatment [4], steam explosion [5], ammonia fiber explosion [6], lime treatment [7], wet oxidation [8], liquid hot water pre- treatment [9], organic solvent treatment [10], and biolo- gial pretreatment [11], and so on, have been used fre- quently to improve the saccharification of the carbohy- drates. Of these methods, steam explosion has been rec- ognized as one of the most cost- effective and potential pretreatment methods [12,13]. During the pretreatment process with high temperature, a part of cellulose, hemi- cellulose and lignin is degraded to produce compounds which inhibit enzymatic hydrolysis and ethanol fermen- tation. The main degraded products from steam exploded corn stover have been identified by HPLC and GC-MS analysis [14]. The inhibiting compounds are divided into three main groups based on origin: weak acids, furan derivatives, and phenolic compounds. Weak acids (formic, acetic, and levulinic acid), furan derivatives (furfural, 5-hy-  48 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 Copyright © 2009 SciRes. OPEN ACCESS droxymethylfurfural) from sugar degradation, phenol compounds from lignin degradation are considered to be potential fermentation inhibitors from pretreated ligno- cellulose [15]. In order to improve the fermentability of yeast, it can be done from the following two aspects, one is to make the yeast to adapt to the prehydrolyzate step by step, and another is to adopt methods to remove the inhibitors from the prehydrolyzate. Nigam investigated a mutant Pichia stipitis NRRL Y-7124 to adapt in acid hydrolyzate. When it was tested for its ability to ferment acid hydrolyzate, it showed shorter fermentation time, better tolerance to acid and could ferment at lower pH. The ethanol yield and productivity were increased 1.3 and 2.1 fold, respectively [16]. However, more research- ers investigated detoxification methods to detoxify the hydrolyzate to improve fermentation efficiency. Detoxi- fication methods include neutralization [17], overliming [18], steam stripping [19], ion-exchange [20], treatment with activated carbon [20], wood charcoal [21], and lac- case and peroxidase [22]. In many instances, the most economical and widely used method of detoxification involves treatment of hydrolyzates with solid calcium hydroxide [23]. This process of “overliming” is reported as an effective method of reducing toxicity of various hydrolyzates [24]. However, different yeasts endure the ability of inhibitors differently. Previous investigation in this laboratory indicated that only “overliming” was not an effective method to detoxify hydrolyzate fermented by Yeast Pichia stipitis CBS 5776 (data not shown), probably because overliming could not remove acetic and formic acid which inhibited the fermentability. In this work, Pichia stipitis CBS 5776 was adapted in the filtrate with addition of xylose 30g/l step by step, and at the same time, steam stripping was firstly used to re- move the volatile compounds such as formic and acetic acid in the acid hydrolyzate or filtrate, then overliming was performed to remove more other inhibitors. Xylose was added up to 45g/l to three kinds of hydrolyzates, undetoxified hydrolyzate, the hydrolzyate treated with steam stripping, and the hydrolyzate treated with a com- bination of steam stripping and overliming. The fer- mentabilities of the three kinds of hydrolyzates with yeast Pichia stipitis CBS 5776 were investigated. 2. MATERIALS AND METHODS 2.1. Preparation of Acid Hydrolyzate Corn stover was obtained from Zhaodong city, Heilong- jiang Province, China. The main composition of the raw material was (w/w% of the dry weight): glucan, 40.10; xylan, 22.30; and lignin, 18.80. The corn stover was crushed to a granularity of 3 to 5cm, and then steam treated at 1.8MPa for 5min before explosion. After steam explosion, 100g (dry weight) exploded corn stover was washed and filtered three times with 1L distilled water. The filtrate was then hydrolyzed with dilute sulfuric acid 30g/l at 121℃ for 45min. 2.2. Detoxification of the Acid Hydrolyzate and the Filtrate The acid hydrolyzate or the filtrate was heated to boiling and kept at 100℃. Steam was then put into the bottom of the vessel with a distributor to perform stripping. Steam stripping was operated for 15, 30, 45, 60, 90, and 120 min, respectively. Overliming was carried out by initially adjusting the pH of the acid hydrolyzate to pH9, 10 and 11, respec- tively, using solid calcium hydroxide. The samples were then heated to 40 or 60℃ in a water bath for 90min fol- lowed by centrifuging at 5000 rpm for 10min. The upper liquid was collected and stored at 4℃. A combination of steam stripping and overliming was also performed for detoxification of the acid hydrolyzate. The hydrolyzate was firstly detoxified by steam strip- ping as described above; the sample’s volume was ad- justed to its original volume by the addition of water. The sample was then detoxified by overliming proce- dure. 2.3. Yeast Strain and Media The yeast Pichia stipitis CBS 5776 was conserved in Nanjing Forestry University. It was maintained at 4℃ in a medium containing (g/l): xylose, 20; yeast extract, 5; peptone, 3; and agar, 20. Inoculation medium contained 30g/l xylose, 5g/l pep- tone and 3g/l yeast extract at natural pH. Multiplication medium was similar to the inoculation medium. The xylose fermentation medium was described previously by Yu [25]. Inoculum was prepared in 250ml shaking flask with 100ml medium, and incubated on a rotary shaker at 170 rpm and 30℃ for several batches (24 hours per batch). When the optical density (OD) of yeast cells reached 10, the cells were harvested by centrifugation, and the pellet was inoculated into the fermentation media. 2.4. Adaptable Fermentation Adaptation medium was prepared by adding 30g/l xy- lose and the other compositions were described by Yu [25] in 10%, 20%, 30%, 40% and 50% (v/v) filtrate of steam exploded corn stover. The cells from inoculum preparation were firstly in- oculated into 100 ml of the adaptation fermentation me- dium of 10% filtrate in 250ml shaking flask at 30℃ on a rotary shaker at 150 rpm for 22h, then repeated fermen- tation of 10 % filtrate for two times, finally the cells was inoculated into 20%, 30%, 40% and 50% filtrate and fermented for three times, respectively. 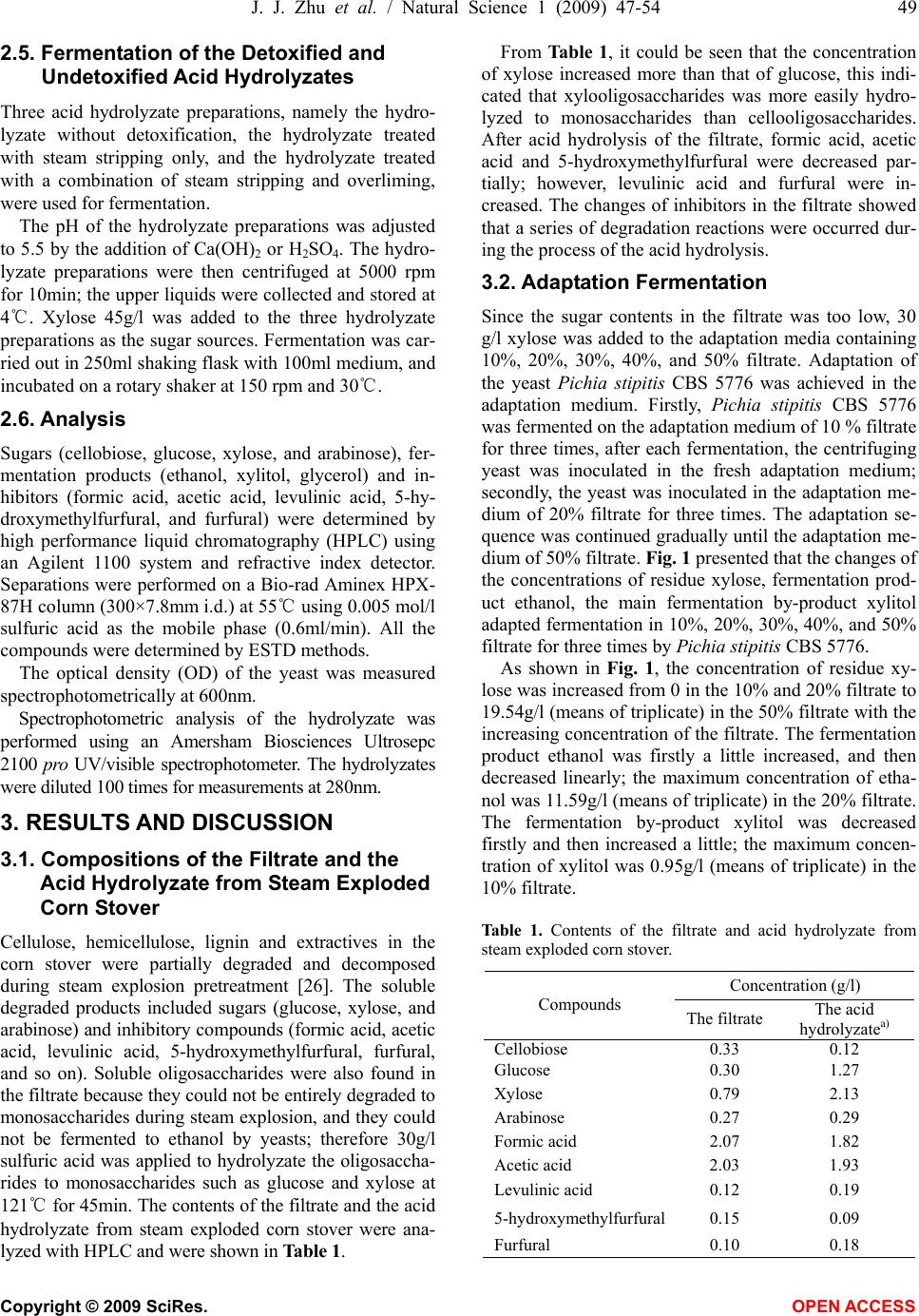 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 49 Copyright © 2009 SciRes. OPEN ACCESS 2.5. Fermentation of the Detoxified and Undetoxified Acid Hydrolyzates Three acid hydrolyzate preparations, namely the hydro- lyzate without detoxification, the hydrolyzate treated with steam stripping only, and the hydrolyzate treated with a combination of steam stripping and overliming, were used for fermentation. The pH of the hydrolyzate preparations was adjusted to 5.5 by the addition of Ca(OH)2 or H2SO4. The hydro- lyzate preparations were then centrifuged at 5000 rpm for 10min; the upper liquids were collected and stored at 4℃. Xylose 45g/l was added to the three hydrolyzate preparations as the sugar sources. Fermentation was car- ried out in 250ml shaking flask with 100ml medium, and incubated on a rotary shaker at 150 rpm and 30℃. 2.6. Analysis Sugars (cellobiose, glucose, xylose, and arabinose), fer- mentation products (ethanol, xylitol, glycerol) and in- hibitors (formic acid, acetic acid, levulinic acid, 5-hy- droxymethylfurfural, and furfural) were determined by high performance liquid chromatography (HPLC) using an Agilent 1100 system and refractive index detector. Separations were performed on a Bio-rad Aminex HPX- 87H column (300×7.8mm i.d.) at 55℃ using 0.005 mol/l sulfuric acid as the mobile phase (0.6ml/min). All the compounds were determined by ESTD methods. The optical density (OD) of the yeast was measured spectrophotometrically at 600nm. Spectrophotometric analysis of the hydrolyzate was performed using an Amersham Biosciences Ultrosepc 2100 pro UV/visible spectrophotometer. The hydrolyzates were diluted 100 times for measurements at 280nm. 3. RESULTS AND DISCUSSION 3.1. Compositions of the Filtrate and the Acid Hydrolyzate from Steam Exploded Corn Stover Cellulose, hemicellulose, lignin and extractives in the corn stover were partially degraded and decomposed during steam explosion pretreatment [26]. The soluble degraded products included sugars (glucose, xylose, and arabinose) and inhibitory compounds (formic acid, acetic acid, levulinic acid, 5-hydroxymethylfurfural, furfural, and so on). Soluble oligosaccharides were also found in the filtrate because they could not be entirely degraded to monosaccharides during steam explosion, and they could not be fermented to ethanol by yeasts; therefore 30g/l sulfuric acid was applied to hydrolyzate the oligosaccha- rides to monosaccharides such as glucose and xylose at 121℃ for 45min. The contents of the filtrate and the acid hydrolyzate from steam exploded corn stover were ana- lyzed with HPLC and were shown in Table 1. From Table 1, it could be seen that the concentration of xylose increased more than that of glucose, this indi- cated that xylooligosaccharides was more easily hydro- lyzed to monosaccharides than cellooligosaccharides. After acid hydrolysis of the filtrate, formic acid, acetic acid and 5-hydroxymethylfurfural were decreased par- tially; however, levulinic acid and furfural were in- creased. The changes of inhibitors in the filtrate showed that a series of degradation reactions were occurred dur- ing the process of the acid hydrolysis. 3.2. Adaptation Fermentation Since the sugar contents in the filtrate was too low, 30 g/l xylose was added to the adaptation media containing 10%, 20%, 30%, 40%, and 50% filtrate. Adaptation of the yeast Pichia stipitis CBS 5776 was achieved in the adaptation medium. Firstly, Pichia stipitis CBS 5776 was fermented on the adaptation medium of 10 % filtrate for three times, after each fermentation, the centrifuging yeast was inoculated in the fresh adaptation medium; secondly, the yeast was inoculated in the adaptation me- dium of 20% filtrate for three times. The adaptation se- quence was continued gradually until the adaptation me- dium of 50% filtrate. Fig. 1 presented that the changes of the concentrations of residue xylose, fermentation prod- uct ethanol, the main fermentation by-product xylitol adapted fermentation in 10%, 20%, 30%, 40%, and 50% filtrate for three times by Pichia stipitis CBS 5776. As shown in Fig. 1, the concentration of residue xy- lose was increased from 0 in the 10% and 20% filtrate to 19.54g/l (means of triplicate) in the 50% filtrate with the increasing concentration of the filtrate. The fermentation product ethanol was firstly a little increased, and then decreased linearly; the maximum concentration of etha- nol was 11.59g/l (means of triplicate) in the 20% filtrate. The fermentation by-product xylitol was decreased firstly and then increased a little; the maximum concen- tration of xylitol was 0.95g/l (means of triplicate) in the 10% filtrate. Table 1. Contents of the filtrate and acid hydrolyzate from steam exploded corn stover. Concentration (g/l) Compounds The filtrate The acid hydrolyzatea) Cellobiose 0.33 0.12 Glucose 0.30 1.27 Xylose 0.79 2.13 Arabinose 0.27 0.29 Formic acid 2.07 1.82 Acetic acid 2.03 1.93 Levulinic acid 0.12 0.19 5-hydroxymethylfurfural0.15 0.09 Furfural 0.10 0.18 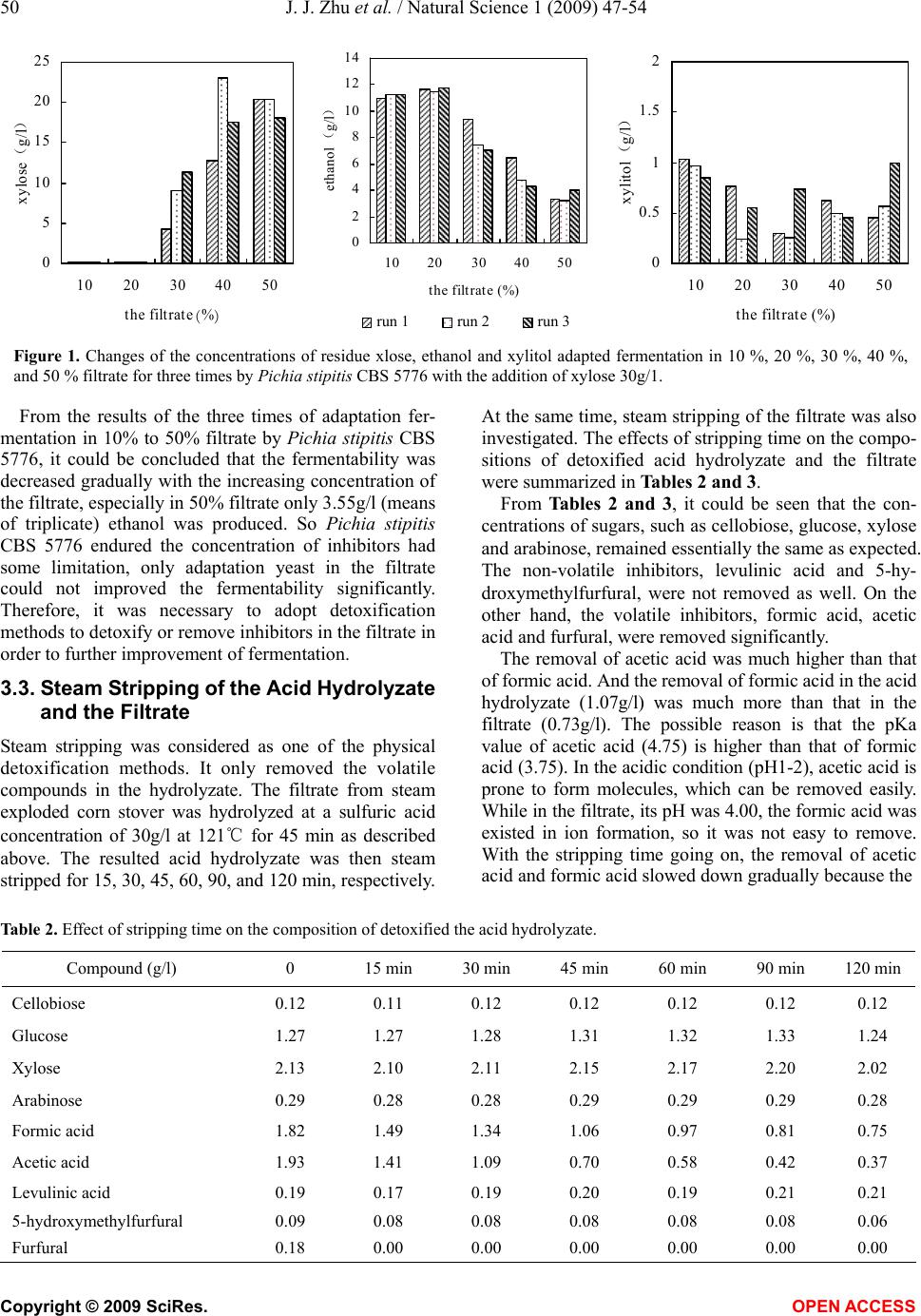 50 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 Copyright © 2009 SciRes. OPEN ACCESS 0 5 10 15 20 25 10 20 3040 50 the filtrate(%) xylose(g/l ) 0 2 4 6 8 10 12 14 10 2030 40 50 the filtrate (%) ethanol (g/l ) run 1run 2run 3 0 0.5 1 1.5 2 10 20 30 40 50 the filtrate (%) xylitol(g/l ) Figure 1. Changes of the concentrations of residue xlose, ethanol and xylitol adapted fermentation in 10 %, 20 %, 30 %, 40 %, and 50 % filtrate for three times by Pichia stipitis CBS 5776 with the addition of xylose 30g/1. From the results of the three times of adaptation fer- mentation in 10% to 50% filtrate by Pichia stipitis CBS 5776, it could be concluded that the fermentability was decreased gradually with the increasing concentration of the filtrate, especially in 50% filtrate only 3.55g/l (means of triplicate) ethanol was produced. So Pichia stipitis CBS 5776 endured the concentration of inhibitors had some limitation, only adaptation yeast in the filtrate could not improved the fermentability significantly. Therefore, it was necessary to adopt detoxification methods to detoxify or remove inhibitors in the filtrate in order to further improvement of fermentation. 3.3. Steam Stripping of the Acid Hydrolyzate and the Filtrate Steam stripping was considered as one of the physical detoxification methods. It only removed the volatile compounds in the hydrolyzate. The filtrate from steam exploded corn stover was hydrolyzed at a sulfuric acid concentration of 30g/l at 121℃ for 45 min as described above. The resulted acid hydrolyzate was then steam stripped for 15, 30, 45, 60, 90, and 120 min, respectively. At the same time, steam stripping of the filtrate was also investigated. The effects of stripping time on the compo- sitions of detoxified acid hydrolyzate and the filtrate were summarized in Tables 2 and 3. From Tables 2 and 3, it could be seen that the con- centrations of sugars, such as cellobiose, glucose, xylose and arabinose, remained essentially the same as expected. The non-volatile inhibitors, levulinic acid and 5-hy- droxymethylfurfural, were not removed as well. On the other hand, the volatile inhibitors, formic acid, acetic acid and furfural, were removed significantly. The removal of acetic acid was much higher than that of formic acid. And the removal of formic acid in the acid hydrolyzate (1.07g/l) was much more than that in the filtrate (0.73g/l). The possible reason is that the pKa value of acetic acid (4.75) is higher than that of formic acid (3.75). In the acidic condition (pH1-2), acetic acid is prone to form molecules, which can be removed easily. While in the filtrate, its pH was 4.00, the formic acid was existed in ion formation, so it was not easy to remove. With the stripping time going on, the removal of acetic acid and formic acid slowed down gradually because the Table 2. Effect of stripping time on the composition of detoxified the acid hydrolyzate. Compound (g/l) 0 15 min 30 min 45 min 60 min 90 min 120 min Cellobiose 0.12 0.11 0.12 0.12 0.12 0.12 0.12 Glucose 1.27 1.27 1.28 1.31 1.32 1.33 1.24 Xylose 2.13 2.10 2.11 2.15 2.17 2.20 2.02 Arabinose 0.29 0.28 0.28 0.29 0.29 0.29 0.28 Formic acid 1.82 1.49 1.34 1.06 0.97 0.81 0.75 Acetic acid 1.93 1.41 1.09 0.70 0.58 0.42 0.37 Levulinic acid 0.19 0.17 0.19 0.20 0.19 0.21 0.21 5-hydroxymethylfurfural 0.09 0.08 0.08 0.08 0.08 0.08 0.06 Furfural 0.18 0.00 0.00 0.00 0.00 0.00 0.00 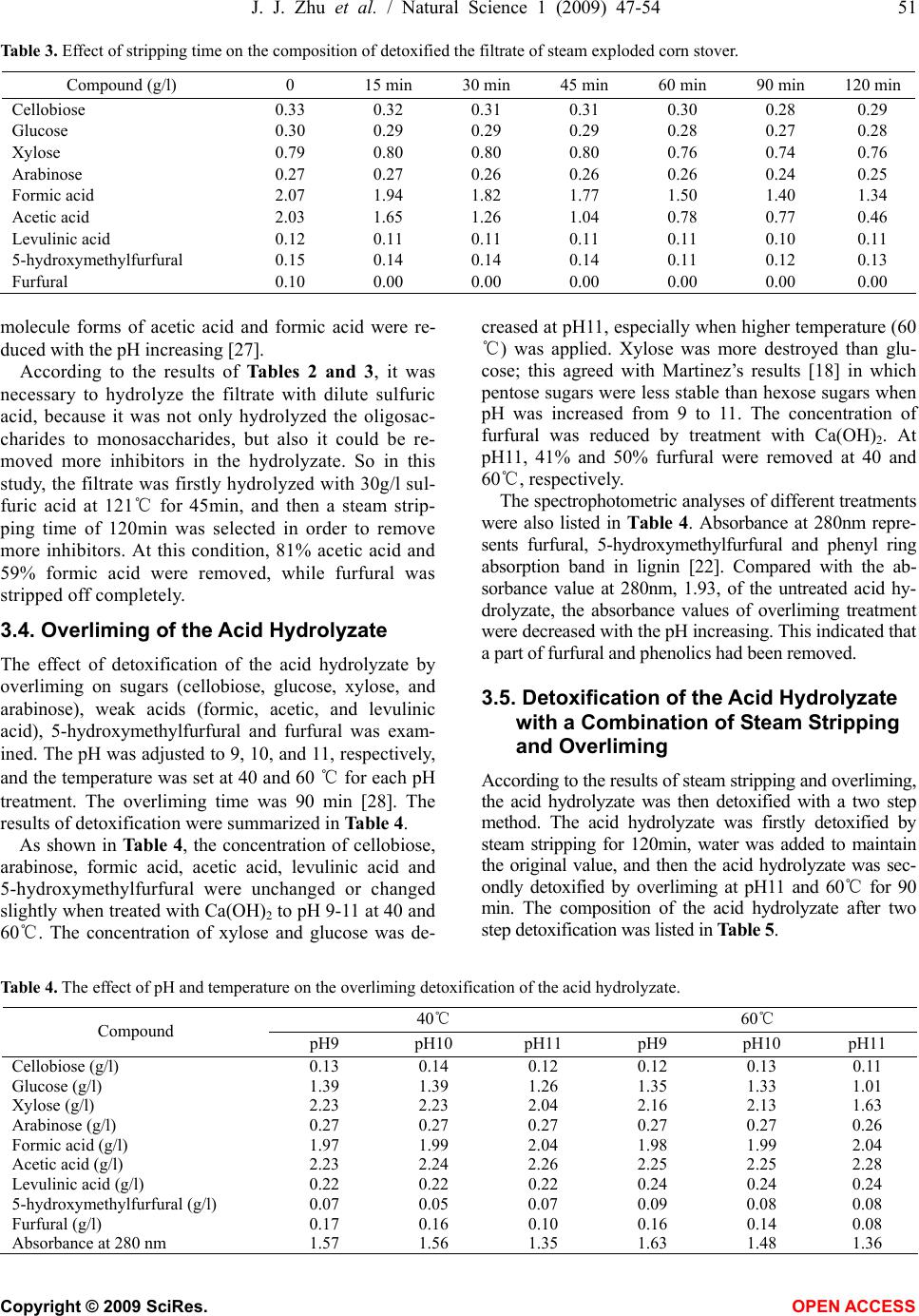 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 51 Copyright © 2009 SciRes. OPEN ACCESS Table 3. Effect of stripping time on the composition of detoxified the filtrate of steam exploded corn stover. Compound (g/l) 0 15 min 30 min 45 min 60 min 90 min 120 min Cellobiose 0.33 0.32 0.31 0.31 0.30 0.28 0.29 Glucose 0.30 0.29 0.29 0.29 0.28 0.27 0.28 Xylose 0.79 0.80 0.80 0.80 0.76 0.74 0.76 Arabinose 0.27 0.27 0.26 0.26 0.26 0.24 0.25 Formic acid 2.07 1.94 1.82 1.77 1.50 1.40 1.34 Acetic acid 2.03 1.65 1.26 1.04 0.78 0.77 0.46 Levulinic acid 0.12 0.11 0.11 0.11 0.11 0.10 0.11 5-hydroxymethylfurfural 0.15 0.14 0.14 0.14 0.11 0.12 0.13 Furfural 0.10 0.00 0.00 0.00 0.00 0.00 0.00 molecule forms of acetic acid and formic acid were re- duced with the pH increasing [27]. According to the results of Tables 2 and 3, it was necessary to hydrolyze the filtrate with dilute sulfuric acid, because it was not only hydrolyzed the oligosac- charides to monosaccharides, but also it could be re- moved more inhibitors in the hydrolyzate. So in this study, the filtrate was firstly hydrolyzed with 30g/l sul- furic acid at 121℃ for 45min, and then a steam strip- ping time of 120min was selected in order to remove more inhibitors. At this condition, 81% acetic acid and 59% formic acid were removed, while furfural was stripped off completely. 3.4. Overliming of the Acid Hydrolyzate The effect of detoxification of the acid hydrolyzate by overliming on sugars (cellobiose, glucose, xylose, and arabinose), weak acids (formic, acetic, and levulinic acid), 5-hydroxymethylfurfural and furfural was exam- ined. The pH was adjusted to 9, 10, and 11, respectively, and the temperature was set at 40 and 60 ℃ for each pH treatment. The overliming time was 90 min [28]. The results of detoxification were summarized in Table 4. As shown in Table 4, the concentration of cellobiose, arabinose, formic acid, acetic acid, levulinic acid and 5-hydroxymethylfurfural were unchanged or changed slightly when treated with Ca(OH)2 to pH 9-11 at 40 and 60℃. The concentration of xylose and glucose was de- creased at pH11, especially when higher temperature (60 ℃) was applied. Xylose was more destroyed than glu- cose; this agreed with Martinez’s results [18] in which pentose sugars were less stable than hexose sugars when pH was increased from 9 to 11. The concentration of furfural was reduced by treatment with Ca(OH)2. At pH11, 41% and 50% furfural were removed at 40 and 60℃, respectively. The spectrophotometric analyses of different treatments were also listed in Table 4. Absorbance at 280nm repre- sents furfural, 5-hydroxymethylfurfural and phenyl ring absorption band in lignin [22]. Compared with the ab- sorbance value at 280nm, 1.93, of the untreated acid hy- drolyzate, the absorbance values of overliming treatment were decreased with the pH increasing. This indicated that a part of furfural and phenolics had been removed. 3.5. Detoxification of the Acid Hydrolyzate with a Combination of Steam Stripping and Overliming According to the results of steam stripping and overliming, the acid hydrolyzate was then detoxified with a two step method. The acid hydrolyzate was firstly detoxified by steam stripping for 120min, water was added to maintain the original value, and then the acid hydrolyzate was sec- ondly detoxified by overliming at pH11 and 60℃ for 90 min. The composition of the acid hydrolyzate after two step detoxification was listed in Table 5. Table 4. The effect of pH and temperature on the overliming detoxification of the acid hydrolyzate. 40℃ 60℃ Compound pH9 pH10 pH11 pH9 pH10 pH11 Cellobiose (g/l) 0.13 0.14 0.12 0.12 0.13 0.11 Glucose (g/l) 1.39 1.39 1.26 1.35 1.33 1.01 Xylose (g/l) 2.23 2.23 2.04 2.16 2.13 1.63 Arabinose (g/l) 0.27 0.27 0.27 0.27 0.27 0.26 Formic acid (g/l) 1.97 1.99 2.04 1.98 1.99 2.04 Acetic acid (g/l) 2.23 2.24 2.26 2.25 2.25 2.28 Levulinic acid (g/l) 0.22 0.22 0.22 0.24 0.24 0.24 5-hydroxymethylfurfural (g/l) 0.07 0.05 0.07 0.09 0.08 0.08 Furfural (g/l) 0.17 0.16 0.10 0.16 0.14 0.08 Absorbance at 280 nm 1.57 1.56 1.35 1.63 1.48 1.36 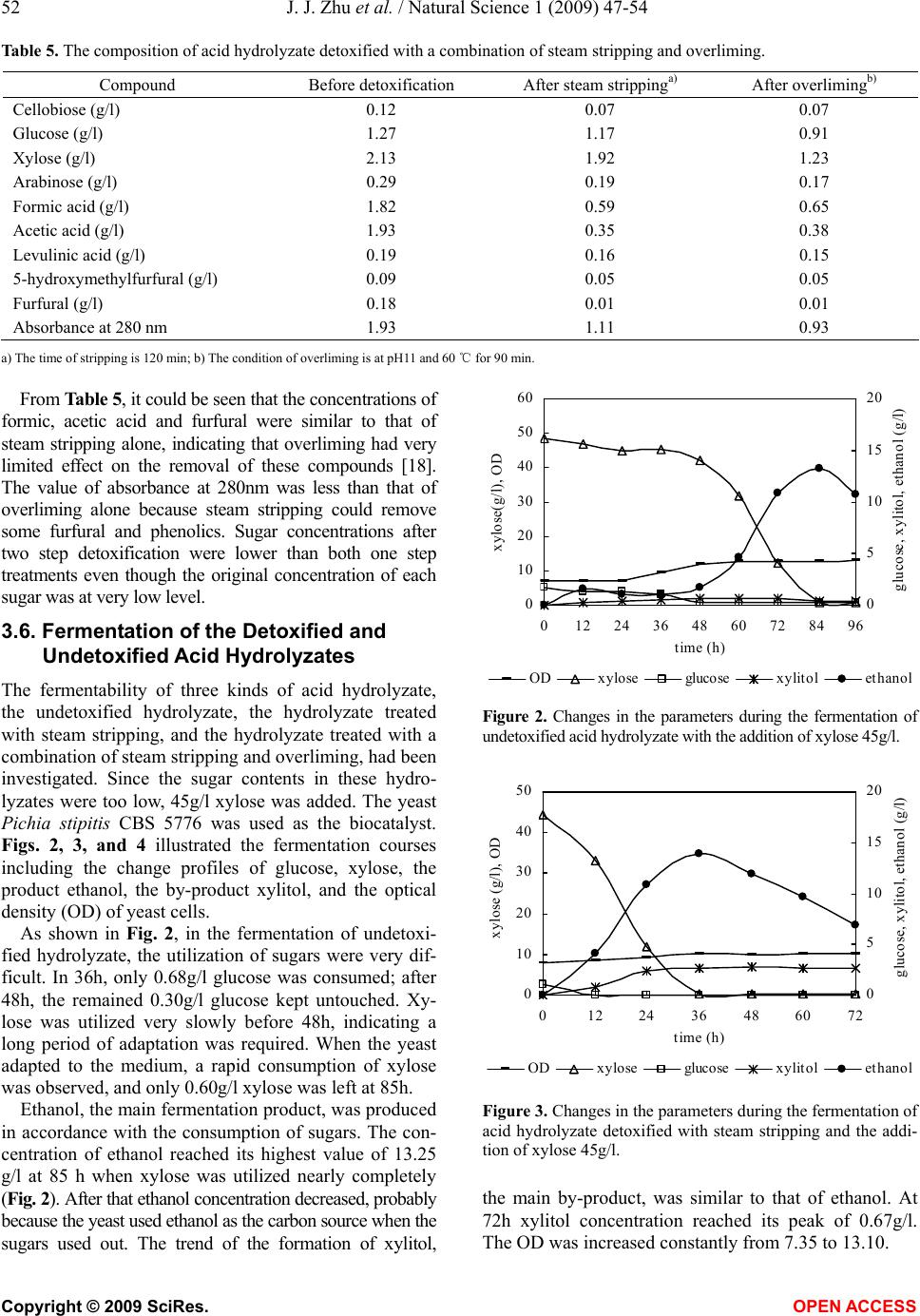 52 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 Copyright © 2009 SciRes. OPEN ACCESS Table 5. The composition of acid hydrolyzate detoxified with a combination of steam stripping and overliming. Compound Before detoxification After steam strippinga) After overlimingb) Cellobiose (g/l) 0.12 0.07 0.07 Glucose (g/l) 1.27 1.17 0.91 Xylose (g/l) 2.13 1.92 1.23 Arabinose (g/l) 0.29 0.19 0.17 Formic acid (g/l) 1.82 0.59 0.65 Acetic acid (g/l) 1.93 0.35 0.38 Levulinic acid (g/l) 0.19 0.16 0.15 5-hydroxymethylfurfural (g/l) 0.09 0.05 0.05 Furfural (g/l) 0.18 0.01 0.01 Absorbance at 280 nm 1.93 1.11 0.93 a) The time of stripping is 120 min; b) The condition of overliming is at pH11 and 60 ℃ for 90 min. From Table 5, it could be seen that the concentrations of formic, acetic acid and furfural were similar to that of steam stripping alone, indicating that overliming had very limited effect on the removal of these compounds [18]. The value of absorbance at 280nm was less than that of overliming alone because steam stripping could remove some furfural and phenolics. Sugar concentrations after two step detoxification were lower than both one step treatments even though the original concentration of each sugar was at very low level. 3.6. Fermentation of the Detoxified and Undetoxified Acid Hydrolyzates The fermentability of three kinds of acid hydrolyzate, the undetoxified hydrolyzate, the hydrolyzate treated with steam stripping, and the hydrolyzate treated with a combination of steam stripping and overliming, had been investigated. Since the sugar contents in these hydro- lyzates were too low, 45g/l xylose was added. The yeast Pichia stipitis CBS 5776 was used as the biocatalyst. Figs. 2, 3, and 4 illustrated the fermentation courses including the change profiles of glucose, xylose, the product ethanol, the by-product xylitol, and the optical density (OD) of yeast cells. As shown in Fig. 2, in the fermentation of undetoxi- fied hydrolyzate, the utilization of sugars were very dif- ficult. In 36h, only 0.68g/l glucose was consumed; after 48h, the remained 0.30g/l glucose kept untouched. Xy- lose was utilized very slowly before 48h, indicating a long period of adaptation was required. When the yeast adapted to the medium, a rapid consumption of xylose was observed, and only 0.60g/l xylose was left at 85h. Ethanol, the main fermentation product, was produced in accordance with the consumption of sugars. The con- centration of ethanol reached its highest value of 13.25 g/l at 85 h when xylose was utilized nearly completely (Fig. 2). After that ethanol concentration decreased, probably because the yeast used ethanol as the carbon source when the sugars used out. The trend of the formation of xylitol, 0 10 20 30 40 50 60 0 1224364860728496 time (h) xylose(g/l), OD 0 5 10 15 20 glucose, xylitol, ethanol (g/l) OD xylose glucose xylitol ethanol Figure 2. Changes in the parameters during the fermentation of undetoxified acid hydrolyzate with the addition of xylose 45g/l. 0 10 20 30 40 50 0 122436486072 time (h) xylose (g/l), OD 0 5 10 15 20 glucose, xylitol, ethanol (g/l) OD xylose glucose xylitol ethanol Figure 3. Changes in the parameters during the fermentation of acid hydrolyzate detoxified with steam stripping and the addi- tion of xylose 45g/l. the main by-product, was similar to that of ethanol. At 72h xylitol concentration reached its peak of 0.67g/l. The OD was increased constantly from 7.35 to 13.10.  J. J. Zhu et al. / Natural Science 1 (2009) 47-54 53 Copyright © 2009 SciRes. OPEN ACCESS 0 10 20 30 40 50 0 122436486072 time (h) xylose (g/l), OD 0 5 10 15 20 glucose, xylitol, ethanol (g/l) OD xylose glucose xylitol ethanol Figure 4. Changes in the parameters during the fermentation of acid hydrolyzate detoxified with a combination of steam strip- ping and overliming, and the addition of xylose 45g/l. The fermentation of the hydrolyzate treated with steam stripping was more easily than that of undetoxified hy- drolyzate as shown in Fig. 3. Glucose was exhausted in 12h. Xylose was consumed rapidly as well; in 36h only 0.38g/l xylose was left. Ethanol reached its highest value of 13.91g/l at 36h, compared to 85h in Fig. 2. Xylitol production increased rapidly in 24h, while the OD was increased constantly from 8.50 to 10.43. Compared with Fig. 2, the fermentablity of the hydrolyzate treated with steam stripping was improved significantly. In the fermentation of the hydrolyzate treated with a combination of steam stripping and overliming, the sugar utilization and ethanol production were further improved compared with Fig. 3. As shown in Fig. 4, glucose was exhausted in 12h, while xylose was con- sumed substantially in 24h with a remaining concentra- tion of 2.28g/l. The peak of ethanol concentration, 15.92 g/l, which was 80.34% of theoretical, appeared at 24h, a reasonable fermentation time for practical applications. From the data above (Figs. 2, 3, and 4), it could be concluded that it is necessary to detoxify the acid hy- drolyzate in order to ferment the sugars to ethanol. Steam stripping is an efficient method to detoxify the hydrolyzate. If combined with overliming, steam strip- ping could significantly improve the fermentability of the hydrolyzate in terms of sugar utilization and ethanol production. 4. CONCLUSIONS The ability of yeast Pichia stipitis CBS 5776 adapted gradually on the fermentation medium containing the filtrate of steam exploded was so limited that detoxifica- tion methods must be adopted to remove these inhibitors existed in the filtrate. A combination of steam stripping and overliming was an effective method to remove the inhibitors in the steam exploded corn stover prehydro- lyzate, and could improve the fermentability of Pichia stipitis CBS 5776. Steam stripping could remove volatile compounds and at a stripping time of 120min, 81% ace- tic acid and 59% formic acid were removed while fur- fural was stripped off completely from the acid hydro- lyzate. Overliming could reduce the contents of furfural and phenolics presented in the acid hydrolyzate, how- ever, sugars, especially pentoses, were also removed partially. When the acid hydrolyzate detoxified with a combination of steam stripping for 120min and overlim- ing at pH11 and 60℃ for 90min, its fermentability was significantly improved. Xylose was consumed nearly completely in 24h with an ethanol yield of 15.92g/l, 80.34% of theoretical. ACKNOWLEDGMENTS The authors acknowledge the financial supports from National Natural Science Foundation of China (Grant No.30871992), National High Technology Research and Development Program of China (Grant No. 2008AA 05Z401), and Natural Science Foundation of Jiangsu Higher Education of China (Grant No. 06KJA22015). REFERENCES [1] Saha, B. C., Iten, L. B., Cotta, M. A. and Wu, Y. V. (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Proc. Biochem. 40, 3693-3700. [2] Lloyd, T. A. and Wyman, C. E. (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis. Bioresour. Technol., 96, 1967-1977. [3] Carrillo, F., Lis, M. J., Colom, X., Valldeperas, M. and Valldeperas, J. (2005) Effect of alkali pretreatment on cellulose hydrolysis of wheat straw: Kinetic study. Proc. Biochem., 40, 3360-3364. [4] Kapoor, M., Nair, L. M. and Kuhad, R. C. (2008) Cost-effective xylanase production from free and immo- bilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem. Eng. J., 38, 88-97. [5] Viola, E., Cardinale, M., Santarcangelo, R., Villone, A. and Zimbardi, F. (2008) Ethanol from eel grass via steam explosion and enzymatic hydrolysis. Biomass Bioenerg., 32, 613-618. [6] Teymouri, F., Laureano-Perez, L., Alizadeh, H. and Dale, B. E. (2005) Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour. Technol., 96, 2014- 2018. [7] Saha, B. C. and Cotta, M. A. (2008) Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenerg., 32, 971-977. [8] Klinke, H. B., Thomsen, A. B. and Ahring, B. K. (2001) Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl. Microbiol. Bio- technol., 57, 631-638. [9] Mosier, N., Hendrickson, R., Ho, N., Sedlak, M. and Ladisch, M. R. (2005) Optimization of pH controlled liq-  54 J. J. Zhu et al. / Natural Science 1 (2009) 47-54 Copyright © 2009 SciRes. OPEN ACCESS uid hot water pretreatment of corn stover. Bioresour. Technol., 96, 1986-1993. [10] Xu, F., Sun, J. X., Liu, C. F. and Sun, R. C. (2006) Com- parative study of alkali-and acidic organic sol- vent-soluble hemicellulosic polysaccharides from sugar- cane bagasse. Carbohyd. Res., 341, 253-261. [11] Singh, P., Suman, A., Tiwari, P., Arya, N., Gaur, A. and Shrivastava, A. K. (2008) Biological pretreatment of sugarcane trask for its conversion to fermentable sugars. World J. Microbiol. Biotechnol., 24, 667-673. [12] McMillan, J. D. (1994) Pretreatment of lignocellulosic biomass. In: Himmel, M. E., Baker, J. O., Overend, R. P. (Eds), Enzymatic conversion of biomass for fuels pro- duction. American Chemical Society, Washington, DC, 292-324. [13] Ohgren, K., Galbe, M. and Zacchi, G. (2005) Optimiza- tion of steam pretreatment of SO2-impregnated corn stover for fuel ethanol production. Appl. Biochem. Bio- technol., 124, 1055-1067. [14] Zhu, J., Yong, Q., Chen, S., Xu, Y., Zhang, X. and Yu, S. (2008) Identification of degraded products during corn stover steam-explosion pretreatment. Chemistry and In- dustry of Forest Products, 29(2), 22-26. [15] Roberto, I. C., Lacis, L. S., Barbosa, M. F. S. and de Mancilha, I. M. (1991) Utilization of sugar cane bagasse hemicellulosic hydrolysate by Pichia stipitis for the pro- duction of ethanol. Proc. Biochem., 26, 15-21. [16] Nigam, J. N. (2001) Development of xylose-fermenting yeast Pichia stipitis for ethanol production through ad- aptation on hardwood hemicellulose acid prehydrolysate. J. Appl. Microbiol., 90, 208-215. [17] Yu, Z. and Zhang, H. (2003) Pretreatments of cellulose pyrolysate for ethanol production by Saccharomyces cerevisiae, Pichia sp. YZ-1 and Zymomonas mobilis. Biomass Bioenerg., 24, 257-262. [18] Martinez, A., Rodriguez, M. E., York, S. W., Preston, J. F. and Ingram, L. O. (2000) Effects of Ca(OH)2 treatments (“overliming”) on the composition and toxicity of ba- gasse hemicellulose hydrolysates. Biotechnol. Bioeng., 69(5), 526-536. [19] Yu, S., Wayman, M. and Parekh, S. K. (1987) Fermenta- tion to ethanol of pentose-containing spent sulphite liq- uor. Biotechnol. Bioeng., 29, 1144-1150. [20] Chandel, A. K., Kapoor, R. K., Singh, A. and Kuhad, R. C. (2007) Detoxification of sugarcane bagasse hydrolys- ate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol., 98, 1947-1950. [21] Miyafuji, H., Danner, H., Neureiter, M., Thomasser, C., Bvochora, J., Szolar, O. and Braun, R. (2003) Detoxifi- cation of wood hydrolysates with wood charcoal for in- creasing the fermentability of hydrolysates. Enzyme Mi- crob. Technol., 32, 396-400. [22] Jönsson, L. J., Palmqvist, E., Nilvebrant, N. O. and Hahn-Hägerdal, B. (1998) Detoxification of wood hy- drolysates with laccase and peroxidase from white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol., 49, 691-697. [23] Olsson, L. and Hahn-Hägerdal, B. (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb. Technol., 18, 312-331. [24] Larsson, S., Reimann, A., Nilvebrant, N. O. and Jönsson, L. J. (1999) Comparison of different methods for the de- toxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol., 77-79, 91-103. [25] Yu, S., Jeppsson, H. and Hahn-Hägerdal, B. (1995) Xy- lulose fermentation by Saccharomyces cerevisiae and xylose-fermenting yeast strains. Appl. Microbiol. Bio- technol., 44, 314-320. [26] Saddler, J. N., Ramos, L. P. and Breuil, C. (1993) Steam pretreatment of lignocellulosic residues. In: Bioconversion of forest and agricultural plant residues (Saddler, J. N., Ed.). CAB International Wallingford. UK, 73-91. [27] Palmqvist, E. and Hahn-Hägerdal, B. (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mecha- nisms of inhibition. Bioresour. Technol., 74, 25-33. [28] Purwadi, R., Niklasson, C. and Taherzadeh, M. J. (2004) Kinetic study of detoxification of dilute-acid hydro- lyzates by Ca(OH)2. J. Biotechnol., 114, 187-198. |

