Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 439-443 doi:10.4236/msa.2011.25058 Published Online May 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 439 The Effect of Annealing Treatments on Spherulitic Morphology and Physical Ageing on Glass Transition of Poly Lactic Acid (PLLA) El-Hadi Ahmed Mohamed1,2 1Department of physics, Faculty of Applied Science, Umm al-Qura University, Mecca, Kingdom of Saudi Arabia; 2Department of Basic Science, Higher Institute for Engineering and Technology, El Arisch, Nord Sinai, Egypt. Email: Bioplastics.elhadi1962@yahoo.com Received October 29th, 2010; revised December 24th, 2010; accepted May 9th, 2011. ABSTRACT Spherulitic morpho logy of pure poly lactic acid (PLLA) PLLA have investigated after thermal annea ling. The morphol- ogy of spherulite of pure poly lactic acid (PLLA) PLLA have investigated after thermal annealing. The effect of both annealing temperature and crystallization temperature on the formation of cracks was described by polarized optical microscope (POM). Non banded spherulite (fibrils ) with cracks was detected in PLLA film after annealing at 160˚C (180 min.) and isothermal crystallizatio n temperatures at 140˚C and 150˚C. With increasing temperature after anneal- ing treatment the size of spherulite is increased and more cracks are formed. The maximum growth rate of spherulites was found at 130˚C. The physical ageing was carried out by annealing the PLLA sample at room temperature for sev- eral annealing time (ta) from 0 h to 720 h. The enthalpy relaxation has been studied by differential scanning calorimetry (DSC) through analysis of the endothermic peak of glass transition temperature, which increased and shifted towards higher temperatu re as the annealing time increased. Keywords: Poly Lactic Acid (PLLA), Morphology, Non-Ban ded Spherulites, Annealing, Polarized Optical Microscopy, Physical Ageing 1. Introduction Most of the plastic materials from petrochemicals such as polyethylene (PE), polypropylene (PP), poly vinyl chlo- ride (PVC) and polystyrene (PS) are used in various areas, such as household appliances, auto parts, building materials and packaging of food, because of their excel- lent processing, physical properties and durability. How- ever there is a problem with the disposal of these prod- ucts. It is making many environmental problems (pollu- tion), such as the disposal of municipal solid waste by burning. It leads to the rise of toxic gases associated with rising temperature and increase in the concentration of carbon dioxide in the atmosphere. The last twenty years attracted the attention of many scientists to this problem and they have been looking at the production of bioplastics materials called green po- lymers or environment polymers. The most important of these new materials are poly(3-hydroxybutyrate) (PHB) [1-3] and poly lactic acid (PLLA). PLLA [4] is polyester aliphatic, insoluble in water, biodegradable thermo plas- tic and transparent. PLLA has a crystallinity of about 40%, glass transition temperature of 50˚C - 70˚C and melting temperature of 173˚C - 178˚C. Although the lactic acid has been known for more than a century. Its scientific and commercial interest has increased only in recent years around the world because of environmental pollution. Now, it is the next generations alternative to synthetic polymers from petrochemicals. Lactic acid can produce from renewable resources (molasses from sugar cane and syrup date) using bacterial fermentation (micro-organisms, bacteria), remnants of food and dairy plants. PLLA can be used as sutures and bone implants (screws, pins, plates, fixation rods and clips, etc), pharmaceutical, cosmetic, textiles industries and food packaging applications, shopping bags, especially for deep drawing articles, thermoformed products such as drinking cups, take-away food trays, containers and plant boxes and diapers [4]. Advantages: PLLA is biodegradable and insoluble in  The Effect of Annealing Treatments on Spherulitic Morphology and Physical Ageing on Glass Transition of Poly Lactic Acid (PLLA) Copyright © 2011 SciRes. MSA 440 water, polyester, optics actives, crystalline and higher melting temperature [4-11]. Disadvantages: PLLA is brittle, higher cost of produc- tion, poor mechanical properties and slow crystallization rate [8-11], compared to synthetic polymers like polys- tyrene (PS), polypropylene (PP) and polyethylene tere- phthalate (PET). It is known that semi-crystalline polymers can form spherulites when crystallized from solution or melts. The morphology of PLLA is mainly spherulitic. The spheru- lites made of thin fibrils are oriented along the radius of a circle. With increasing the crystallization time, the sphe- rulites grow and impinge with each other longitudinally. The study of thermal stability and morphology is im- portant [12-17] in polymer processing because control the temperature of the end product during the processing like injection molding or casting film by chill roll is necessary. The physical properties of semi-crystalline polymers de- pend upon the mechanisms of crystallization and mor- phology during processing. Mechanisms of crystallization is the key to the basic information for understanding the relationship between manufacturing processes and fea- tures observed in the final product of the polymer. It is important to study the physical aging [18] to de- sign and develop the polymers with long term durability, i.e. understand the safe use of polymers. The aging process is common in non-crystalline polymer and re- ferred to as structural relaxation and is happening be- cause these materials do not exist in the state of thermo- dynamic equi- librium. Physical aging is a phenomenon that is caused by material evolution towards thermody- namic equilibrium. Physical ageing is called change in amorphous phase by annealed at different temperature (Ta) at near or lower than its glass temperature (Tg). This leads to changing in physical properties such as density, enthalpy, dielectric permittivity, electrical conductivity and refractive index, also make changes in the mechani- cal properties like Young’s modulus and yield stress. This occurs during longer storage time by use of poly- mers. The aim of present work is to investigate the effect of annealing treatments on the morphology of PLLA using polarized optical microscopy (POM). Furthermore, the physical ageing phenomenon and the glass transition temperature were studied using differential scanning ca- lorimetry (DSC). The aim of this study is to investigate the effect of an- nealing treatments of PLLA on morphology using pola- rized optical microscopy (POM) and the physical ageing phenomenon on the glass transition temperature using differential scanning calorimetry (DSC). 2. Experimental Poly (L-Lactic acid) PLLA, grade L9000 was obtained from Biomer®, Germany. PLLA had initially a molar mass of 220 000 g/mol. PLLA as pellet was prepared by dissolving in hot chloroform (0.04 g/ml), and then the solution was casted on microscopy glass slide as a thin film (100 - 300 nm). The casting films held for 24 hour to evaporate the sol- vent and dried at 60˚C for 1 day to remove the solvent completely. The casted film with glass slide was covered with another glass slide. The casted film was placed be- tween two microscopy glass slide as a sandwich and in- serted to a hot stage and melted at 200˚C for 3 min to erase their thermal history and cooled slowly to the iso- thermal crystallization temperature (Tc). The sample was held at both (Ta) and (Tc) for the required time to develop spherulite. The morphology of PLLA was studied by optical mi- croscopy (Nikon Eclipse E600) equipped with hot-stage (Instec STC200). The sample was heated on the hot-stage from room temperature to 200˚C, held 3 min. for com- plete melting of the polymer, and cooled slowly from 200˚C to annealing temperature (Ta = 160˚C) and iso- thermal crystallization Tc of 140˚C and 150˚C where the growing of spherulites started. A new sample was used each time to keep away from any effects due to degrada- tion processing by higher melting processing temperature (Tmp = 200˚C). Glass transition temperature (Tg), heat capacity (Cp) and enthalpy of relaxation (ΔH) are determined by diffe- rential scanning calorimetry (DSC). The sample was heated from room temperature to 200˚C with the a rate of 10˚C·min−1 and kept at ageing times 24 h, 48 h, 96 h and 720 h (first run). Whereas in the second run, the samples were heated from 25˚C to 80˚C with a rate of 10˚C·min−1 (second run). The enthalpy of relaxation was seen as an endotherm near Tg. 3. Results and Discussion 3.1. Morphology and Spherulite Growth Rate Spherulitic textures are morphological features. It ob- served in semi crystalline polymers. A generation of spherulite started from lamellar crystal, which is often formed at the beginning of the crystal flakes (nucleation). Moreover, spherulite can be formed in two or three di- mensions (spherical), crystal circular that displays in maltese cross between polaroids (polarizer and analyzer). Figure 1 is shown the polarized optical micrographs of neat PLLA spherulites with cracks. These cracks depend on the crystallization temperature (Tc) and the annealing temperature (Ta). Cracks appear after melting, annealing 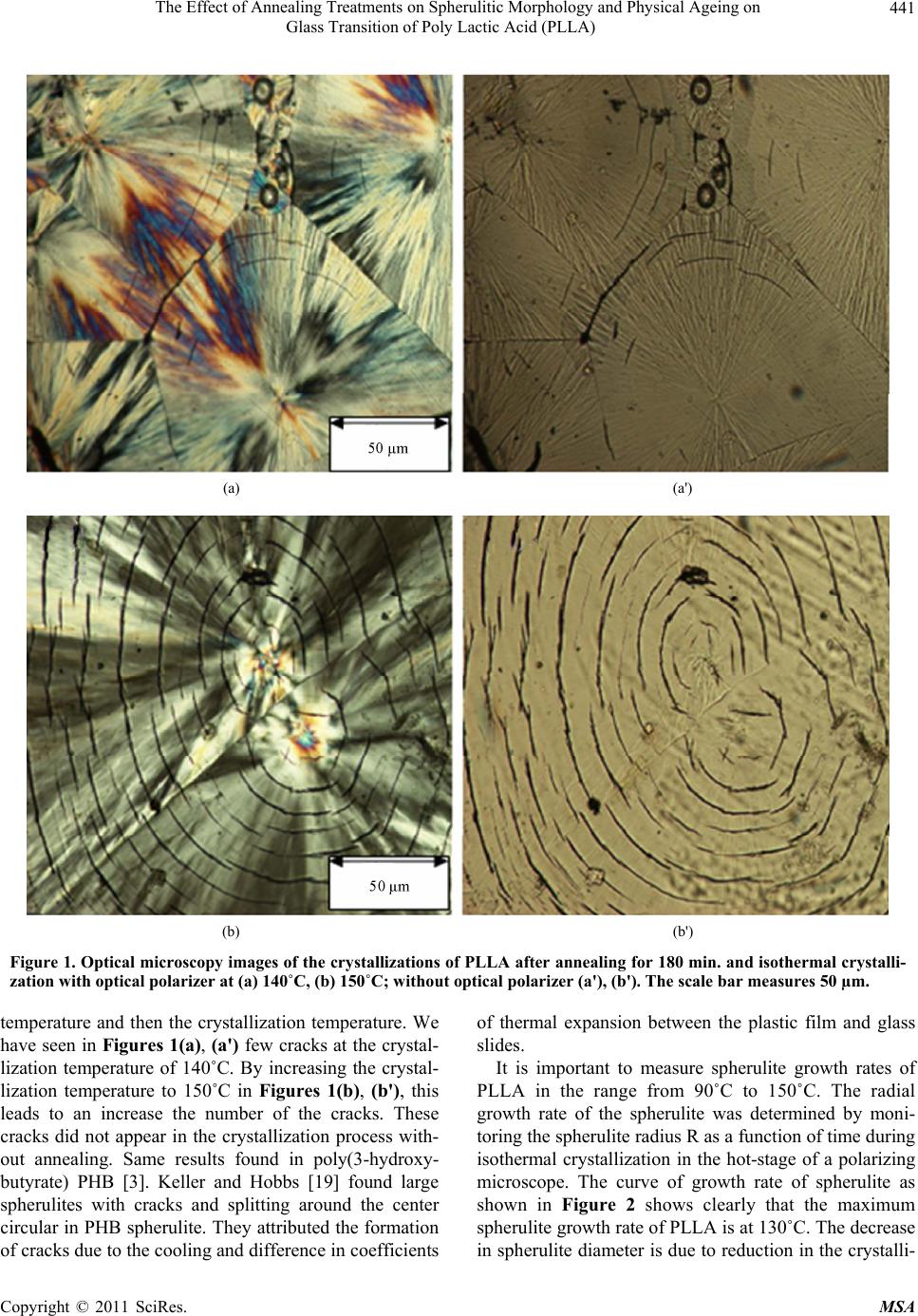 The Effect of Annealing Treatments on Spherulitic Morphology and Physical Ageing on Glass Transition of Poly Lactic Acid (PLLA) Copyright © 2011 SciRes. MSA 441 (a) (a') (b) (b') Figure 1. Optical microscopy images of the crystallizations of PLLA after annealing for 180 min. and isothermal crystalli- zation with optical polarizer at (a) 140˚C, (b) 150˚C; without optical polarizer (a'), (b'). The scale bar measures 50 µm. temperature and then the crystallization temperature. We have seen in Figures 1(a), (a') few cracks at the crystal- lization temperature of 140˚C. By increasing the crystal- lization temperature to 150˚C in Figures 1(b), (b'), this leads to an increase the number of the cracks. These cracks did not appear in the crystallization process with- out annealing. Same results found in poly(3-hydroxy- butyrate) PHB [3]. Keller and Hobbs [19] found large spherulites with cracks and splitting around the center circular in PHB spherulite. They attributed the formation of cracks due to the cooling and difference in coefficients of thermal expansion between the plastic film and glass slides. It is important to measure spherulite growth rates of PLLA in the range from 90˚C to 150˚C. The radial growth rate of the spherulite was determined by moni- toring the spherulite radius R as a function of time during isothermal crystallization in the hot-stage of a polarizing microscope. The curve of growth rate of spherulite as shown in Figure 2 shows clearly that the maximum spherulite growth rate of PLLA is at 130˚C. The decrease in spherulite diameter is due to reduction in the crystalli-  The Effect of Annealing Treatments on Spherulitic Morphology and Physical Ageing on Glass Transition of Poly Lactic Acid (PLLA) Copyright © 2011 SciRes. MSA 442 Figure 2. Spherulite growth rates of PLLA, isothermal crystallization after cooling from the melt as a function of temperature. zation temperature. The sharp peak at crystallization temperature of 120˚C is similar to the results reported in Refences [14,16]. It was also found that the crystalliza- tion kinetics of the investigated PLLA is very slow. This is due to the fact that the number of homogenous nuc- leated is small at temperatures more than 130˚C, which causing longer time period at the processing. We found that the crystallization kinetics of the PLLA is very slow because the number of homogenous nucleation are small at more than 130˚C, causing longer time period at the processing. This means that the spherulites are large and make the material more brittle. 3.2. DSC Analysis Physical aging in polymers can occurs at deferent time at room temperatures below the glass transition temperature (Tg). DSC thermograms were recorded on heating as a function of aging time (ta). Figure 3 shows the relation between heat flow and temperature. It was shown that, the glass transition temperature (Tg) of pure PLLA ap- peared at 60˚C, cold crystallization (Tcc) at 108˚C, melt- ing point (Tm) at 174˚C. Figure 4 shows DSC curves for the PLLA samples with different ageing time and the glass transition temperature was determined. The result the ageing of PLLA has also strong influence on the glass transition temperature. The enthalpy relaxation was found to be increase with ageing time and the glass tran- sition shifted to a higher temperature. At beginning, the value of Tg found to be 61˚C after 24 h and 96 h, it in- creased to 62.5˚C after 15 and 30 days. 4. Conclusion The effect of annealing after melting on morphology of Figure 3. DSC second heating of PLLA after annealing at room temperature for 360 h. Figure 4. Enthalpy relaxation of the PLLA samples with heating rate 10˚C·min−1 after ageing time: (a) 24 h; (b) 96 h; (c) 360 h; and (d) 720 h. pure PLLA by POM has been discussed. Large non banded spherulites with cracks are formed around the center after annealed at higher temperature by longer annealing time and isothermal crystallization. Changes of relaxation enthalpy were detected by DSC after an- nealing near glass transition temperature (Tg). The posi- tion of glass transition shifted to higher values and magnitude of the enthalpy increased by increasing the ageing time. 5. Acknowledgements The author thanks the research and consulting center and institute of scientific research for part supporting this project 43005001 (ministry of higher education of K.S.A).  The Effect of Annealing Treatments on Spherulitic Morphology and Physical Ageing on Glass Transition of Poly Lactic Acid (PLLA) Copyright © 2011 SciRes. MSA 443 REFERENCES [1] A. El-Hadi and R. Schnabel, “Effect of Melt Processing on Crystallization Behavior and Rheology of Poly(3-hydro-xybutyrate) (PHB) and Its Blends,” Macromolecular Materials and Engineering, Vol. 287, No. 5, 2002, pp. 363-372. doi:10.1002/1439-2054(20020501)287:5<363::AID-MA ME363>3.0.CO;2-D [2] A. El-Hadi and R. Schnabel, “Correlation between De- gree of Crystallinity, Morphology, Glass Temperature, Mechanical Properties and Biodegradation of Poly (3-hydroxyal-kanoate) PHAs and Their Blends,” Polymer Test, Vol. 21, No. 6, 2002, pp. 665-674. doi:10.1016/S0142-9418(01)00142-8 [3] A. El-Hadi, “Development of a Biodegradable Material Based on Poly(3-hydroxybutyrate) PHB,” Ph.D. Disserta- tion, Physics Department, University of Halle-Witten- berg, Halle and Wittenberg, 2002. [4] Y. Ikada and H. Tsuji, “Biodegradable Polyesters for Medical and Ecological Applications,” Macromolecules Rapid Communication, Vol. 21, No. 3, 2000, pp. 117-132. [5] S. Petrulyte, “Advanced Textile Materials and Biopoly- mers in Wound Management,” Danish Medical Bulletin, Vol. 55, No. 1, 2008, pp. 72-77. [6] W. H. Hoidy, M. B. Ahmad, E. A. J. Al-Mulla and N. A. B. Ibrahim, “Preparation and Characterization of Polylac- tic Acid and Polycaprolactone Clay Nanocomposites,” Journal of Applied Sciences, Vol. 10, No. 2, 2010, pp. 97- 106. doi:10.3923/jas.2010.97.106 [7] Z. Kulinski and E. Piorkowska, “Plasticization of Poly(L-lactide) with Poly(propylene glycol),” Bioma- cromolecules, Vol. 7, No. 20, September 2006, pp. 2128- 2135. doi:10.1021/bm060089m [8] R. Masirek, E. Piórkowska, A. Gałęski and M. Mucha, “Influence of Thermal history on the Nonisothermal Cry- stallization of Poly(L-lactide),” Journal Applied Polymer Science, Vol. 105, No. 1, 2007, pp. 282-290. doi:10.1002/app.26047 [9] M. Kozłowski, R. Masirek, E. Piórkowska and M. Ga- zicki- Lipman, “Biodegradable Blends of Poly(L-lactide) and Starch,” Journal Applied Polymer Science, Vol. 105, No. 1, 2007, pp. 269-277. doi:10.1002/app.26088 [10] M. Pluta and A. Gałęski, “Plastic Deformation of Amorphous Poly(L/DL-lactide), Structure Evolution and Physical Prorties,” Biomacromolecules, Vol. 8, No. 6 2007, pp. 1836-843. doi:10.1021/bm061229v [11] M. Pluta, “Melt Compounding of Polylactide/Organoclay: Structure and Properties of Nanocomposites,” Journal Polymer Science B: Polymer Physics, Vol. 44, No. 23, 2006, 3392-3405. doi:10.1002/polb.20957 [12] H. D. Keith and F. J. Padden, “The Optical Behavior of Spherulites in Crystalline Polymers, Part I, “Calculation of Theoretical Extinction Patterns in Spherulites with Twisting Crystalline Orientation,” Journal Polymer Science, Vol. 39, No. 135, 1959, pp. 101-122. [13] A. J. Lovinger and D. C. Bassett, “Development in Crys- talline Polymers-1, Chapter 5,” Applied Science Publish- ers, 1982, pp. 195-273. [14] M. L. Di Lorenzo, “Crystallization Behavior of Poly(L-lac- tic acid),” European Polymer Journal, Vol. 41, No. 3, 2005, pp. 569-575. doi:10.1016/j.eurpolymj.2004.10.020 [15] R. Vasanthakumari and A. J. Pennings, “Crystallisation Kinetics of Poly(L-lactic acid),” Polymer, Vol. 24, No. 2, 1983, pp. 175-178. doi:10.1016/0032-3861(83)90129-5 [16] Y. Yuryev, P. Wood-Adams and M. C. Heuzey, “Crystal- lization of Polylactide Films: An Atomic Force Micro- scopy Study of the Effects of Temperature and Blending,” Polymer, Vol. 49, No. 9, 2008, pp. 2306-2320. doi:10.1016/j.polymer.2008.02.023 [17] L. C. E. Struik, “Mechanical Behavior and Physical Age- ing of Semi-Crystalline Polymers,” Polymer, Vol. 30, No. 5, 1989, pp. 815-830. doi:10.1016/0032-3861(89)90177-8 [18] J. K. Hobbs, T. J. McMaster, M. J. Miles and P. J. Bar- ham, “Cracking in Spherulites of Poly(hydroxybutyrate),” Journal Polymer Science Part B: Polymer Physics, Vol. 37, No. 15, 1996, pp. 3241-3246. |

