Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 435-438 doi:10.4236/msa.2011.25057 Published Online May 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 435 Electrochemical Behavior of Nanocrystalline Fe88Si12 Alloy in 3.5 wt% NaCl Solution Licai Fu1,2,*, Jun Yang1, Qinling Bi1, Weimin Liu1 1State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, China; 2Department of Chemical and Materials Engineering, University of Alberta, Edmonton, Canada. Email: licai1@ualberta.ca Received February 9th, 2011; revised April 13th, 2011; accepted April 15th, 2011. ABSTRACT Influence of microstructure on electrochemical behavior of nanocrystalline Fe88Si12 alloy has been investigated in 3.5 wt% NaCl solution. The results show that Fe88Si12 alloy with optimal corrosion resistance is composite of ordered Fe3Si and disordered Fe(Si) phases and grain size of 40 nm. Because the ordered Fe3Si structure is beneficial to form SiO2 film, which possesses good corrosion resistance compared with the Fe2O3 film from disordered Fe(Si). Moreover, al- though the decreased grain size is conducive to form preservative, as the grain size decreases to 10 nm, the grain boundary increases to above 30 vol%, which is the active sites for corrosion attack. Keywords: Fe88Si12 Alloy, Nanocrystalline, Microstructure, Electrochemical Behavior 1. Introduction Corrosion resistance is of great importance in assessing many future applications of the nanocrystalline materials. The corrosion behavior of the nanocrystalline materials has been investigated over the last two decades for a va- riety of materials (pure metals, alloys, and composites) [1-5]. Mishra [6] indicated that the high micro-strain in the electrodeposition Ni with grain size of 8 nm can be related to the lower corrosion rate in the 1 mol/l H2SO4 solution. Owing to the higher grain boundary density, the nanocrystalline Co coating exhibited good corrosion re- sistance comparing with coarse grained Co coating in the NaOH or NaCl solutions [7]. Xu et al. [8] reported that the corrosion resistance of a nanocrystalline Ti5Si3C0.8 film and a nanocrystalline Ti5Si3 film with an average grain size of 15 nm was superior to that of Ti-6Al-4V alloy. However, Vinogradov investigated [9] that the co- rrosion behavior of the nanocrystalline Cu changed slightly compared with the coarse grained Cu. These re- searches indicate that the corrosion resistance of the nano- crystalline materials depends on their unique microstruc- ture. Fe88Si12 (atom ratio) alloy has been extensively inves- tigated due to their excellent soft magnetic properties [10, 11]. However, the corrosion resistance of the Fe88Si12 alloy, especially about the nanocrystalline Fe88Si12 alloy, has not received much attention. In this paper, the elec- trochemical behavior of the nanocrystalline Fe88Si12 alloy has been studied by the electrochemical tests, to research influence of the grain size and phase structure on the electrochemical behavior. 2. Experimental The different microstructures of Fe88Si12 alloys has been fabricated by a self propagating high temperature synthe- sis technique (SHS) [12], and annealed treat at 900˚C and 1000˚C for 1 h with air atmosphere, respectively. The grain size and phase structured of the different Fe88Si12 alloys are shown Table 1. Potentiodynamic polarization curves of the different Fe88Si12 alloys were performed with 3.5 wt% NaCl solu- tion at 25˚C. A three-electrode cell system was employed. All the results are referred to standard hydrogen elec- trode (SHE). The ribbons measuring 40 mm × 8 mm were cut from the samples. They were mechanically po- lished with 600 emery paper and rinsed with ethanol and distilled water prior to the electrochemical test. Linear polarization curves were obtained at a scan rate of 0.01 mV/s. The sample was allowed to reach a stationary open circuit potential (90 min). Then, a potential value 200 mV lower than the corrosion potential was applied for 5 minutes and the potentiodynamic scan was initiated. The specimens were examined by X-ray diffract-meter (XRD) 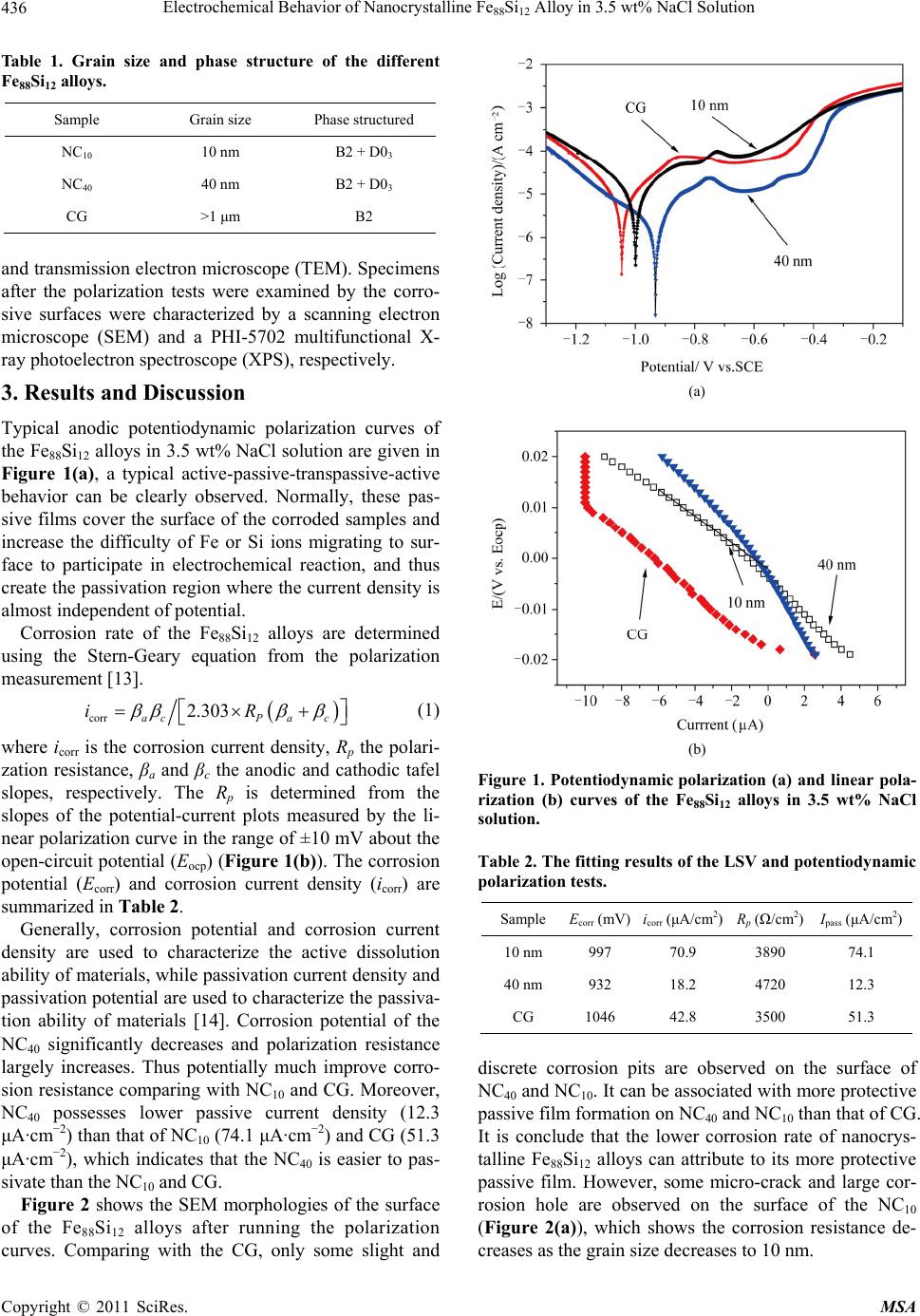 Electrochemical Behavior of Nanocrystalline Fe88Si12 Alloy in 3.5 wt% NaCl Solution Copyright © 2011 SciRes. MSA 436 Table 1. Grain size and phase structure of the different Fe88Si12 alloys. Sample Grain size Phase structured NC10 10 nm B2 + D03 NC40 40 nm B2 + D03 CG >1 μm B2 and transmission electron microscope (TEM). Specimens after the polarization tests were examined by the corro- sive surfaces were characterized by a scanning electron microscope (SEM) and a PHI-5702 multifunctional X- ray photoelectron spectroscope (XPS), respectively. 3. Results and Discussion Typical anodic potentiodynamic polarization curves of the Fe88Si12 alloys in 3.5 wt% NaCl solution are given in Figure 1(a), a typical active-passive-transpassive-active behavior can be clearly observed. Normally, these pas- sive films cover the surface of the corroded samples and increase the difficulty of Fe or Si ions migrating to sur- face to participate in electrochemical reaction, and thus create the passivation region where the current density is almost independent of potential. Corrosion rate of the Fe88Si12 alloys are determined using the Stern-Geary equation from the polarization measurement [13]. corr 2.303 acP ac iR (1) where icorr is the corrosion current density, Rp the polari- zation resistance, βa and βc the anodic and cathodic tafel slopes, respectively. The Rp is determined from the slopes of the potential-current plots measured by the li- near polarization curve in the range of ±10 mV about the open-circuit potential (Eocp) (Figure 1(b)). The corrosion potential (Ecorr) and corrosion current density (icorr) are summarized in Table 2. Generally, corrosion potential and corrosion current density are used to characterize the active dissolution ability of materials, while passivation current density and passivation potential are used to characterize the passiva- tion ability of materials [14]. Corrosion potential of the NC40 significantly decreases and polarization resistance largely increases. Thus potentially much improve corro- sion resistance comparing with NC10 and CG. Moreover, NC40 possesses lower passive current density (12.3 μA·cm−2) than that of NC10 (74.1 μA·cm−2) and CG (51.3 μA·cm−2), which indicates that the NC40 is easier to pas- sivate than the NC10 and CG. Figure 2 shows the SEM morphologies of the surface of the Fe88Si12 alloys after running the polarization curves. Comparing with the CG, only some slight and (a) (b) Figure 1. Potentiodynamic polarization (a) and linear pola- rization (b) curves of the Fe88Si12 alloys in 3.5 wt% NaCl solution. Table 2. The fitting results of the LSV and potentiodynamic polarization tests. Sample Ecorr (mV)icorr (μA/cm2) Rp (Ω/cm2) Ipass (μA/cm2) 10 nm 997 70.9 3890 74.1 40 nm 932 18.2 4720 12.3 CG 1046 42.8 3500 51.3 discrete corrosion pits are observed on the surface of NC40 and NC10. It can be associated with more protective passive film formation on NC40 and NC10 than that of CG. It is conclude that the lower corrosion rate of nanocrys- talline Fe88Si12 alloys can attribute to its more protective passive film. However, some micro-crack and large cor- rosion hole are observed on the surface of the NC10 (Figure 2(a)), which shows the corrosion resistance de- creases as the grain size decreases to 10 nm. 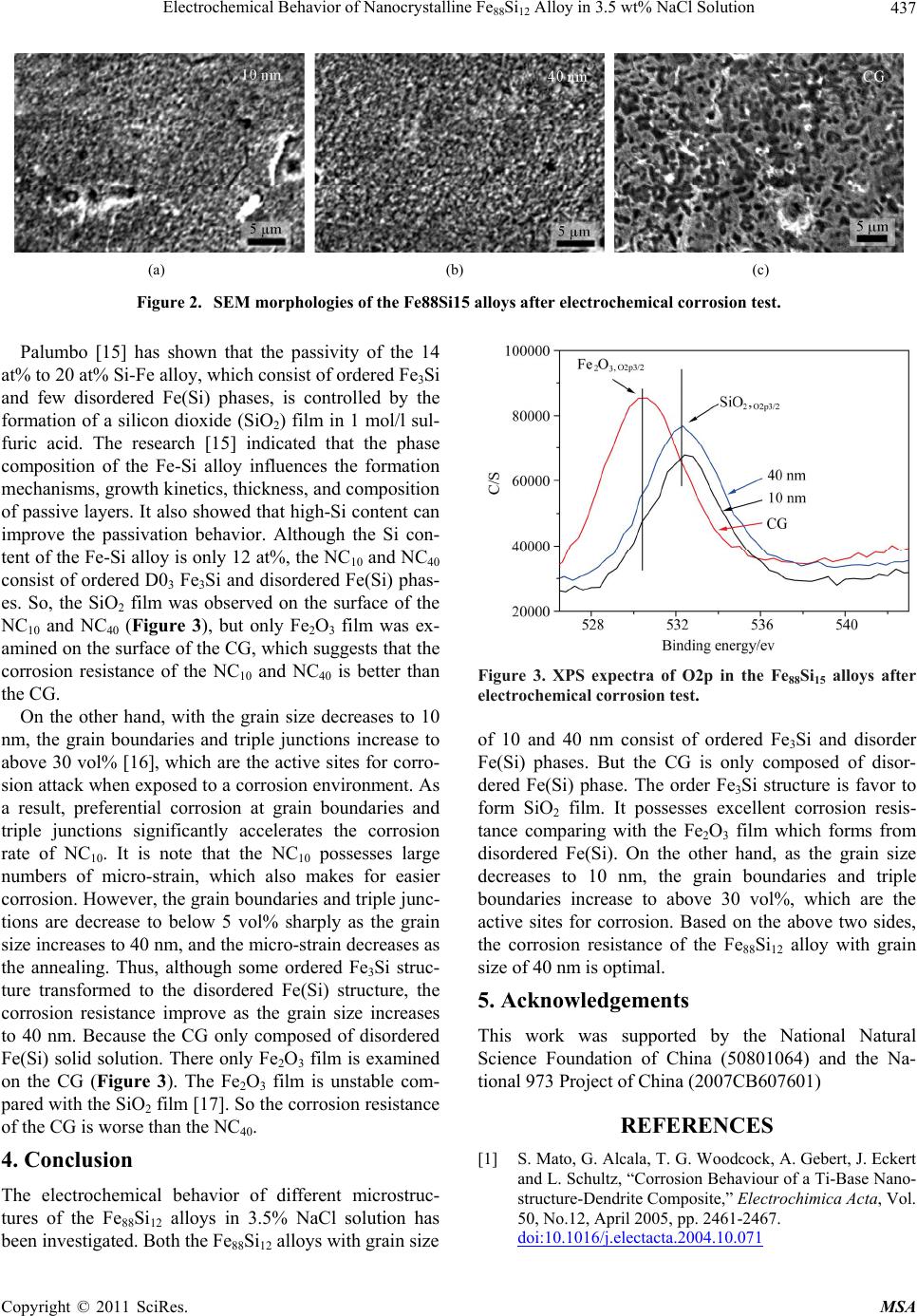 Electrochemical Behavior of Nanocrystalline Fe88Si12 Alloy in 3.5 wt% NaCl Solution Copyright © 2011 SciRes. MSA 437 (a) (b) (c) Figure 2. SEM morphologies of the Fe88Si15 alloys after electrochemical corrosion test. Palumbo [15] has shown that the passivity of the 14 at% to 20 at% Si-Fe alloy, which consist of ordered Fe3Si and few disordered Fe(Si) phases, is controlled by the formation of a silicon dioxide (SiO2) film in 1 mol/l sul- furic acid. The research [15] indicated that the phase composition of the Fe-Si alloy influences the formation mechanisms, growth kinetics, thickness, and composition of passive layers. It also showed that high-Si content can improve the passivation behavior. Although the Si con- tent of the Fe-Si alloy is only 12 at%, the NC10 and NC40 consist of ordered D03 Fe3Si and disordered Fe(Si) phas- es. So, the SiO2 film was observed on the surface of the NC10 and NC40 (Figure 3), but only Fe2O3 film was ex- amined on the surface of the CG, which suggests that the corrosion resistance of the NC10 and NC40 is better than the CG. On the other hand, with the grain size decreases to 10 nm, the grain boundaries and triple junctions increase to above 30 vol% [16], which are the active sites for corro- sion attack when exposed to a corrosion environment. As a result, preferential corrosion at grain boundaries and triple junctions significantly accelerates the corrosion rate of NC10. It is note that the NC10 possesses large numbers of micro-strain, which also makes for easier corrosion. However, the grain boundaries and triple junc- tions are decrease to below 5 vol% sharply as the grain size increases to 40 nm, and the micro-strain decreases as the annealing. Thus, although some ordered Fe3Si struc- ture transformed to the disordered Fe(Si) structure, the corrosion resistance improve as the grain size increases to 40 nm. Because the CG only composed of disordered Fe(Si) solid solution. There only Fe2O3 film is examined on the CG (Figure 3). The Fe2O3 film is unstable com- pared with the SiO2 film [17]. So the corrosion resistance of the CG is worse than the NC40. 4. Conclusion The electrochemical behavior of different microstruc- tures of the Fe88Si12 alloys in 3.5% NaCl solution has been investigated. Both the Fe88Si12 alloys with grain size Figure 3. XPS expectra of O2p in the Fe88Si15 alloys after electrochemical corrosion test. of 10 and 40 nm consist of ordered Fe3Si and disorder Fe(Si) phases. But the CG is only composed of disor- dered Fe(Si) phase. The order Fe3Si structure is favor to form SiO2 film. It possesses excellent corrosion resis- tance comparing with the Fe2O3 film which forms from disordered Fe(Si). On the other hand, as the grain size decreases to 10 nm, the grain boundaries and triple boundaries increase to above 30 vol%, which are the active sites for corrosion. Based on the above two sides, the corrosion resistance of the Fe88Si12 alloy with grain size of 40 nm is optimal. 5. Acknowledgements This work was supported by the National Natural Science Foundation of China (50801064) and the Na- tional 973 Project of China (2007CB607601) REFERENCES [1] S. Mato, G. Alcala, T. G. Woodcock, A. Gebert, J. Eckert and L. Schultz, “Corrosion Behaviour of a Ti-Base Nano- structure-Dendrite Composite,” Electrochimica Acta, Vol. 50, No.12, April 2005, pp. 2461-2467. doi:10.1016/j.electacta.2004.10.071  Electrochemical Behavior of Nanocrystalline Fe88Si12 Alloy in 3.5 wt% NaCl Solution Copyright © 2011 SciRes. MSA 438 [2] F. Renner, A. Stierle, H. Dosch, D. M. Kolb, T. L. Lee and J. Zegenhagen, “Initial Corrosion Observed on the Atomic Scale,” Nature, Vol. 439, No. 9, February 2006, pp. 707-710. doi:10.1038/nature04465 [3] J. Balaraju, V. E. Selvi and K. Rajam, “Electrochemical Behavior of Nanocrystalline Ni-P Alloys Containing Tin and Tungsten,” Protection of Metals and Physical Che- mistry of Surfaces, Vol. 46, No. 6, 2010, pp. 686-691. doi:10.1134/S2070205110060109 [4] S. Mathur, R. Vyas, P. Kulriya, K. Asokan, K. Sachdev and S. K. Sharma, “Effects of Irradiation on the Electro- chemical Behavior of the Alloy Ti60Ni40,” Journal of Al- loys and Compounds, Vol. 503, No. 1, 30 July 2010, pp. 192-193. doi:10.1016/j.jallcom.2010.04.231 [5] V. Cremaschi, I. Avram, T. Perez and H. Sirkin, “Elec- trochemical Studies of Amorphous, Nanocrystalline, and Crystalline FeSiB Based Alloys,” Scripta Material, Vol. 46, No. 1, January 2002, pp. 95-100. doi:10.1016/S1359-6462(01)01204-0 [6] R. Mishra and R. Balasubramaniam, “Effect of Nano- crystalline Grain Size on the Electrochemical and Corro- sion Behavior of Nickel,” Corrosion Science, Vol. 46, No. 12, December 2004, pp. 3019-3029. doi:10.1016/j.corsci.2004.04.007 [7] L. Wang, Y. Lin, Z. Zeng, W. Liu, Q. Xue, L. Hu and J. Zhang, “Electrochemical Corrosion Behavior of Nanocry- stalline Co Coatings Explained by Higher Grain Boun- dary Density,” Electrochimica Acta, Vol. 52, No. 13, Mar- ch 2007, pp. 4342-4350. doi:10.1016/j.electacta.2006.12.009 [8] J. Xu, L. Liu, X. Lu and S. Jiang, “Effect of Carbon Doping on Electrochemical Behaviour of Nanocrystalline Ti5Si3 Film in NaCl Solution,” Electrochemistry Commu- nications, Vol. 13, No. 1, January 2011, pp. 102-105. doi:10.1016/j.elecom.2010.11.028 [9] A. Vinogradov, T. Mimaki and S. Hashimoto, “On the Corrosion Behaviour of Ultra-Fine Grain Copper,” Scrip- ta Material, Vol. 41, No. 3, July 1999, pp. 319-326. doi:10.1016/S1359-6462(99)00170-0 [10] R. Li, Q. Shen, L. Zhang and T. Zhang, “Magnetic Prop- erties of High Silicon Iron Sheet Fabricated by Direct Powder Rolling,” Journal of Magnetism and Magnetic Materials, Vol. 281, No. 2-3, October 2004, pp. 135-139. doi:10.1016/j.jmmm.2004.04.098 [11] M. Komatsubara, K. Sadahiro, O. Kondo, T. Takamiya and A. Honda, “Newly Developed Electrical Steel for High-Frequency Use,” Journal of Magnetism and Mag- netic Materials, Vol. 242-245, No. 1, April 2002, pp. 2l2- 215. [12] L. C. Fu, J. Yang, Q. L. Bi, J. Q. Ma and W. M. Liu, “Combustion Synthesis and Characterization of Bulk Nanocrystalline Fe88Si12 Alloy,” IEEE Transactions on Nanotechnology, Vol. 9, No. 2, March 2010, pp. 218-222. doi:10.1109/TNANO.2009.2028023 [13] M. Stern and A. L. Geary, “Electrochemical Polariza- tion,” Journal of the Electrochemical Society, Vol. 104, No. 1, January 1957, pp. 56-63. doi:10.1149/1.2428438 [14] G. Z. Meng, Y. Li and F. H. Wang, “The Corrosion Be- havior of Fe–10Cr Nanocrystalline Coating,” Electrochi- mica Acta, Vol. 51, No. 20, May 2006, pp. 4277-4284. doi:10.1016/j.electacta.2005.12.015 [15] U. Wolff, F. Schneider, K. Mummert and L. Schultz, “Stability and Electrochemical Properties of Passive Lay- ers on Fe-Si Alloys,” Corrosion, Vol. 56, No. 12, De- cember 2000, pp. 1195-1201. doi:10.5006/1.3280507 [16] G. Palumbo and K. T. Aust, “Structure-Dependence of Intergranular Corrosion in High Purity Nickel,” Acta Me- tallurgica et Materialia, Vol. 24, No. 11, November 1990, pp. 2343-2352. doi:10.1016/0956-7151(90)90101-L [17] A. Atkinson, “A Theoretical Analysis of the Oxidation of Fe-Si Alloys,” Corrosion Science, Vol. 22, No. 2, Febru- ary 1982, pp. 87-102. doi:10.1016/0010-938X(82)90071-3 |

