Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 250-257 doi:10.4236/jbnb.2011.23032 Published Online July 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Qin Wang1, Jie Wu2, Wenbo Wang1, A iqin Wang1,2* 1Center of Eco-material and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, China; 2Key Laboratory for Attapulgite Science and Applied Technology of Jiangsu Province, Huaiyin Institute of Technology, Huaian, China. Email: *aqwang@licp.cas.cn Received February 28th, 2011; revised March 20th 2011; accepted April 20th, 2011. ABSTRACT A series of chitosan/attapulgite (CTS/APT) hybrid microspheres were prepared by a facile spray-drying technique. The developed hybrid microspheres were characterized by Fourier transform infrared spectra (FTIR), X-ray powder dif- fraction (XRD), scanning electron microscopy (SEM) and the zeta potential. The encapsulation efficiency and in vitro controlled release properties of the microspheres for drug were evaluated using diclofenac sodium (DS) as a model drug. Results indicated that the introduction of APT into crosslinked CTS microspheres can achieve narrow size distri- bution and make them more uniform. The isoelectric point of the microspheres increased from 8.14 to 9.18 with in- creasing the content of APT to 10 wt%. DS loaded in hybrid microspheres is hardly released in simulated gastric fluid, but quickly released in simulated intestinal fluid. The electrostatic interaction between hybrid microspheres and DS can improve the encapsulation efficiency and controlled release behavior of CTS/APT microspheres, and the release mechanism fits Fickian diffusion. Keywords: Microsphere, Chitosan, Attapulgite, Spray Drying, Controlled Release 1. Introduction During recent decades, microspheres have been widely used in pharmaceutical applications due to their numer- ous advantages such as higher bioavailability, prolonging drug release, high dispersibility and fast permeability as well as providing an optimal microenvironment for ef- fective drug delivery [1]. Microspheres are mainly pro- duced by different technological procedures like emul- sion-crosslinking [2], ionic gelation [3], sieve method [4] and spray-drying technique [5,6], etc. Among them, the spray-drying technique is a one-step constructive process, which provides greater control over particle size, particle morphology and powder density and avoids tedious processes. Hence, spray-drying becomes the most facile and preferred technique for the fabrication of micro- spheres, and it has been used widely in medical industry due to the low cost and available equipment [7]. Chitosan (CTS) is one of the most extensively studied polysaccharides and has shown great potential as a drug carrier [8-10]. CTS microspheres obtained by spray- drying technique draw considerable attention for both oral and systemic applications in the drug delivery sys- tem. These drug-loading microspheres not only have a de- sirable size range in favor of respiration and absorption, but also can provide a high loading efficiency [6,11]. However, the burst effect of drug loaded in pure CTS mi- crospheres is undesirable in neutral or basic conditions, which limited the application of CTS-based drug carriers [12-14]. Recently, polymer with layered silicate or poly- mer/layered silicate composites have received significant attentions from both the academic and industrial area. The introduction of clay minerals into organic matrix not only solved the burst problem of the loaded drugs, but also enhanced the stability of the delivery system in simulated gastric fluid [15-17]. Attapulgite (APT) is a  Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 251 type of naturally occurred nano-scale fibrous silicate clay, consisting of two double chains of the pyroxene-type (SiO3)2− like amphibole (Si4O11)6− running parallel to the fibre axis, and used in drug delivery system [18]. To date, the application of polymer/clay microspheres prepared by a spray-drying technique drug as a delivery system has not been reported. So, it is expected that the network structure and drug-delivery properties of the microspheres could be improved by the introduction of nanoscale APT into crosslinked CTS microspheres. In this work, APT was introduced into crosslinked CTS microspheres to derive hybrid microspheres in order to alleviate the burst problem of pure CTS microspheres for DS in the gastrointestinal environment. The resultant microsphere was characterized by Fourier transform in- frared spectra (FTIR), X-ray powder diffraction (XRD) and scanning electron microscopy (SEM). The encapsu- lation efficiency and in vitro release behaviors were sys- tematically evaluated using diclofenac sodium (DS) as a model drug. 2. Materials and Methods 2.1. Materials Chitosan (CTS, degree of deacetylation is 0.90, average molecular weight is 90 × 104) was supplied by Zhejiang Yuhuan Ocean Biology Co. (Zhejiang, China). Attapul- gite (APT) was supplied by Mingguang Colloidal Co. (Anhui, China). Diclofenac sodium (DS) was purchased from Jiuzhou pharmaceutical factory (He’nan, China). All the other reagents used were of analytical grade and all solutions were prepared with distilled water. 2.2. Preparation of Crosslinked CTS/APT Hybrid Microspheres The CTS/APT hybrid microspheres were prepared by a spray drying technique as follows. First, CTS (1.0 g) was dissolved in 100 mL 1 w/w% acetic acid solution, and a calculated amount of APT was dispersed into the solu- tion with a continuous stirring at 200 rpm for 4 h to get a homogenous dispersion. Second, a moderate amount of crosslinking agent glutaraldehyde was added into the above mixture and was further stirred at 1000 rpm for 30 min at room temperature. The resultant suspension was then spray-dried (Mini Spray Dryer, Type SY1600, China) through a 0.7 mm nozzle at a feed rate of 12 mL/min, and the inlet temperature was controlled at 150˚C. Third, the yellow hybrid microsphere power can be obtained, and then dried to constant weight in an oven at 70˚C. A series of crosslinked CTS/APT hybrid microspheres with different composition were prepared as shown in Table 1. 2.3. Preparation of DS-Loaded Hybrid Microspheres and Determination of Encapsulation Efficiency 0.2 g DS was dissolved in 100 mL distilled water and then 1.0 g of hybrid microsphere was dispersed in the above solution. After shaking for 12h in a thermostatic shaker bath (THZ-98A) set at 150 rpm and 30˚C, DS- loaded hybrid microspheres were isolated by centrifuga- tion and then dried to constant weight in an oven at 70˚C. To evaluate the amount of the DS entrapped inside the hybrid microspheres, an indirect method was used [19]. Aliquots from the filtered solutions remaining after the removal of the hybrid microspheres were assayed using a UV-vis spectrophotometer (SPECORD 200, ANALYTIK JENA AG) at 280 nm. The encapsulation efficiency of DS entrapped was calculated according to the following equation: Encapsulation efficiency (%) = (w–cv) × 100/w (1) where w is the mass of DS used for drug loading, g; c is the concentration of DS in the filtrate (g mL−1) and v is the volume of the filtrate (mL). 2.4. In Vitro Drug Release In vitro release experiments were carried out using an intelligent dissolution apparatus by immersing 0.25 g of the dried DS-loaded hybrid microspheres in 500 mL dis- solution media. The dissolution media (pH 2.1 or pH 6.8) were prepared by combining HCl, KH2PO4 and NaOH solution according to the Chinese Pharmacopoeia 2005. The mixture was stirred at 50 rpm and kept at 37 ± 1˚C. At predetermined time intervals, 5 mL of the solution was taken and the same amount of the fresh dissolution media was added back to maintain a constant volume. The collected 5 mL of solution was filtrated through a membrane with a pore diameter of 0.45 μm. The filtrate (3 mL) was diluted to 25 mL with the fresh dissolution media. The concentration of DS was determined by a UV-vis spectrophotometer at the wavelength 280 nm, and then the cumulative release percentage of DS was obtained. 2.5. Characterization FTIR spectra of CTS/APT hybrid microspheres were recorded on a Thermo Nicolet NEXUS TM spectropho- tometer using a KBr pellet. SEM observations were taken using scanning electron microscopy (JSM-5600LV, JEOL, Ltd.) after the samples were dispersed in ethanol and coated by a gold film. Power XRD analyses were per- formed using a diffractometer with Cu anode (PAN alyti- cal X’pert PRO), running at 40 kV and 30 mA, scanning from 3˚ to 40˚ at 3˚/min. The zeta potential was measured  Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 252 by Zetasizer Nano ZS (Malvern Instruments Ltd., Wor- cestershire, UK) after the samples (0.1 g) were dispersed in 10 ml distilled water. Main particle size was deter- mined using OMEC LS-pop (6) laser particle analyzer (Omec technology Ltd., Zhuhai, China) after the samples (0.1 g) were dispersed in 2 ml ethanol. 2.6. Statistical Analysis All the tests including measurements of drug content and in vitro drug cumulative release properties were carried out three times and the averages were reported. Statistical data analysis was performed using the Student’s t-test with p < 0.05 as the minimal level of significance. 3. Results and Discussion 3.1. Encapsulation Efficiency of Crosslinked CTS/APT Hybrid Microspheres for DS and Their Sizes Table 1 lists composition of prepared formulations, en- capsulation efficiency and mean particle size of the hy- brid microspheres. It can be seen that the encapsulation efficiency of the hybrid microspheres for DS is always higher than 98%. This may be mainly attributed to elec- trostatic interaction between the microsphere carriers and the DS drug. In order to prove the electrostatic interac- tion, the zeta potentials of the hybrid microspheres and DS drug were determined at various pHs and are showed in Figure 1. It is shown that the isoelectric points of crosslinked CTS, CTS/APT-1 and CTS/APT-2 micro- spheres are pHs 8.14, 9.18 and 8.28, respectively; while the isoelectric point of DS is pH 4.03. This indicates that the hybrid microspheres show positive charge, and DS possesses negative charge in distilled water (pH 6.85). Therefore, a strong electrostatic interaction between the hybrid microspheres and DS leads to the higher encapsu- lation efficiency of the hybrid microspheres for DS. Drug carrier systems with particle size below 200 µm can prolong the gastrointestinal transit time and control the release of the encapsulated drug. But in fact, the op- timal particle size for prolonging residence time of mi- crospheres in the colon is between 4 and 15 µm [20]. From Table 1, it is observed that the mean particle sizes of the hybrid microspheres DS loaded range from 3.70 ± 0.001 to 5.27 ± 0.007 µm (D50). This result suggests that CTS/APT hybrid microspheres can prolong and control the DS release in the colon. Moreover, microspheres in that size range are also able to attach more efficiently to the mucus layer and accumulate in the inflamed region without the need for macrophage uptake [21]. The size distribution of the hybrid microspheres was investigated and is presented in Figure 2. As can be seen, the size distribution of crosslinked CTS microspheres changes from 0.2 to 19.5 µm, but from 0.2 to 16.11 µm for CTS/APT-1 hybrid microsphere and from 0.2 to 13.31 µm for CTS/APT-2 hybrid microsphere. This in- dicates that the introduction of APT into crosslinked CTS microsphere can achieve narrow size distribution and make them more uniform. In addition, the particle size distribution is also expressed in terms of SPAN factor determined as: SPAN = (D90 – D10)/D50 (2) where D10, D50 and D90 are the diameter sizes and the given percentage value is the percentage of particles smaller than that size. A high SPAN value indicates a wide size distribution and a high polydispersity [22]. According to Gottlieb and Schwartzbach’s report [23], a relative SPAN less than 2.00 for spray-dried with D50 below 10 μm, is normally considered as a narrow distri- bution in spray drying. It was seen from Table 1 that all SPAN factor values are lower than 2.00, indicating that CTS/APT hybrid microspheres have narrow size distri- bution. 3.2. FTIR Spectra To prove the interaction among crosslinked CTS, APT and DS, the FTIR spectra of DS, APT, crosslinked CTS, CTS/APT-1 and DS-loaded CTS/APT-1 hybrid micro- spheres are shown in Figure 3. As can be seen from Table 1. Composition of prepared formulations, encapsulation efficiency and mean particle sizes of the hybrid microspheres. Mean particle size (µm) Samples CTS (g) APT (g) Glutaraldehyde (mL) Encapsulation Efficiency (%)D10 D 50 D 90 SPAN Crosslinked CTS 4.0 0.0 3.0 98.97 ± 0.01 2.33 ± 0.057 5.27 ± 0.007 8.82 ± 0.156 1.23 ± 0.021 CTS/APT-1 4.0 0.4 3.0 99.04 ± 0.01 2.11 ± 0.035 4.56 ± 0.003 7.47 ± 0.092 1.18 ± 0.030 CTS/APT-2 4.0 0.8 3.0 99.05 ± 0.02 2.35 ± 0.007 4.77 ± 0.028 7.49 ± 0.085 1.09 ± 0.013 CTS/APT-3 4.0 0.4 2.0 98.35 ± 0.04 2.50 ± 0.001 4.92 ± 0.001 7.57 ± 0.014 1.03 ± 0.003 CTS/APT-4 4.0 0.4 4.0 98.87 ± 0.01 1.92 ± 0.007 3.70 ± 0.001 5.64 ± 0.007 1.00 ± 0.004 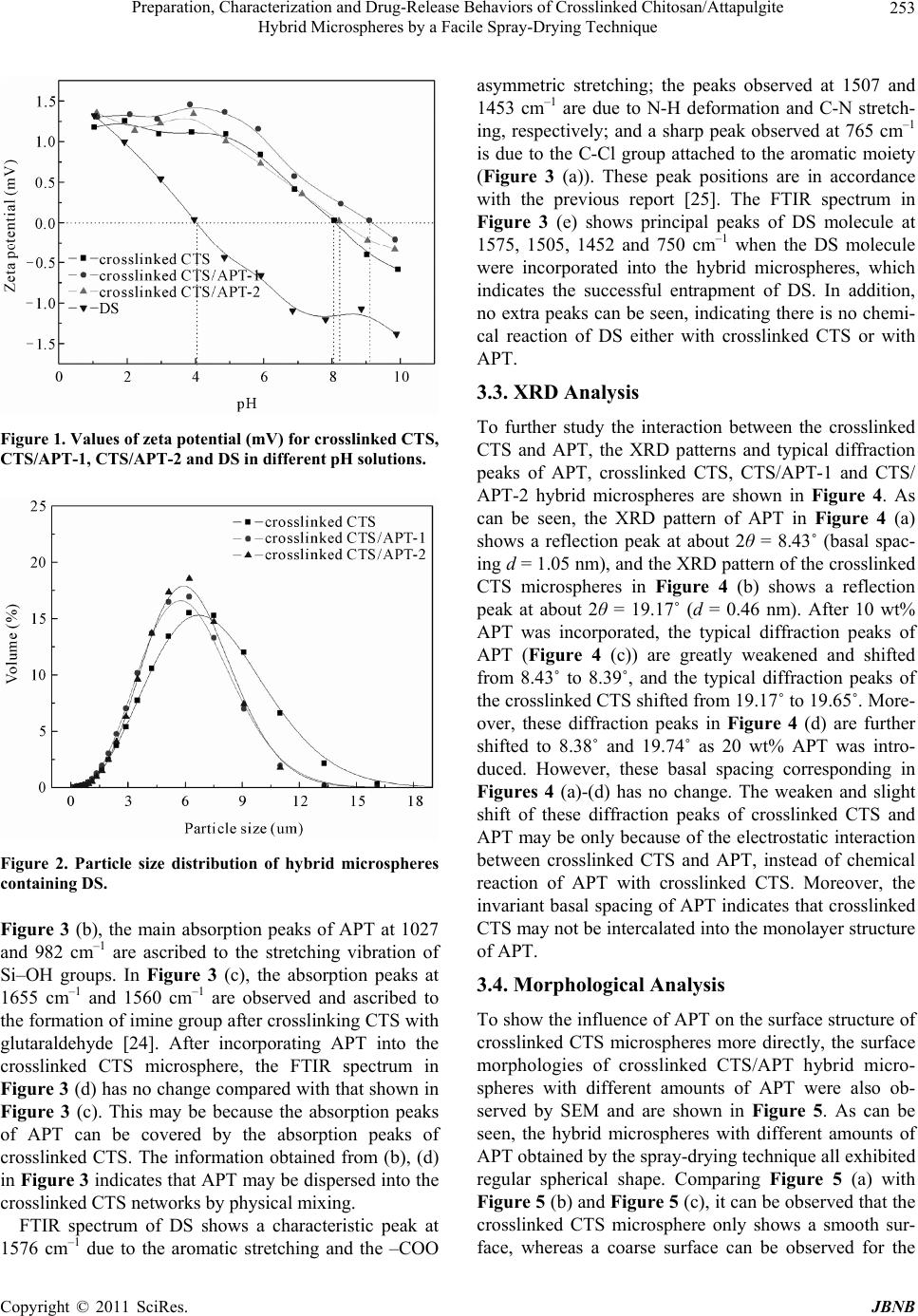 Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 253 Figure 1. Values of zeta potential (mV) for crosslinked CTS, CTS/APT-1, CTS/APT-2 and DS in different pH solutions. Figure 2. Particle size distribution of hybrid microspheres containing DS. Figure 3 (b), the main absorption peaks of APT at 1027 and 982 cm–1 are ascribed to the stretching vibration of Si–OH groups. In Figure 3 (c), the absorption peaks at 1655 cm–1 and 1560 cm–1 are observed and ascribed to the formation of imine group after crosslinking CTS with glutaraldehyde [24]. After incorporating APT into the crosslinked CTS microsphere, the FTIR spectrum in Figure 3 (d) has no change compared with that shown in Figure 3 (c). This may be because the absorption peaks of APT can be covered by the absorption peaks of crosslinked CTS. The information obtained from (b), (d) in Figure 3 indicates that APT may be dispersed into the crosslinked CTS networks by physical mixing. FTIR spectrum of DS shows a characteristic peak at 1576 cm–1 due to the aromatic stretching and the –COO asymmetric stretching; the peaks observed at 1507 and 1453 cm–1 are due to N-H deformation and C-N stretch- ing, respectively; and a sharp peak observed at 765 cm–1 is due to the C-Cl group attached to the aromatic moiety (Figure 3 (a)). These peak positions are in accordance with the previous report [25]. The FTIR spectrum in Figure 3 (e) shows principal peaks of DS molecule at 1575, 1505, 1452 and 750 cm–1 when the DS molecule were incorporated into the hybrid microspheres, which indicates the successful entrapment of DS. In addition, no extra peaks can be seen, indicating there is no chemi- cal reaction of DS either with crosslinked CTS or with APT. 3.3. XRD Analysis To further study the interaction between the crosslinked CTS and APT, the XRD patterns and typical diffraction peaks of APT, crosslinked CTS, CTS/APT-1 and CTS/ APT-2 hybrid microspheres are shown in Figure 4. As can be seen, the XRD pattern of APT in Figure 4 (a) shows a reflection peak at about 2θ = 8.43˚ (basal spac- ing d = 1.05 nm), and the XRD pattern of the crosslinked CTS microspheres in Figure 4 (b) shows a reflection peak at about 2θ = 19.17˚ (d = 0.46 nm). After 10 wt% APT was incorporated, the typical diffraction peaks of APT (Figure 4 (c)) are greatly weakened and shifted from 8.43˚ to 8.39˚, and the typical diffraction peaks of the crosslinked CTS shifted from 19.17˚ to 19.65˚. More- over, these diffraction peaks in Figure 4 (d) are further shifted to 8.38˚ and 19.74˚ as 20 wt% APT was intro- duced. However, these basal spacing corresponding in Figures 4 (a)-(d) has no change. The weaken and slight shift of these diffraction peaks of crosslinked CTS and APT may be only because of the electrostatic interaction between crosslinked CTS and APT, instead of chemical reaction of APT with crosslinked CTS. Moreover, the invariant basal spacing of APT indicates that crosslinked CTS may not be intercalated into the monolayer structure of APT. 3.4. Morphological Analysis To show the influence of APT on the surface structure of crosslinked CTS microspheres more directly, the surface morphologies of crosslinked CTS/APT hybrid micro- spheres with different amounts of APT were also ob- served by SEM and are shown in Figure 5. As can be seen, the hybrid microspheres with different amounts of APT obtained by the spray-drying technique all exhibited regular spherical shape. Comparing Figure 5 (a) with Figure 5 (b) and Figure 5 (c), it can be observed that the crosslinked CTS microsphere only shows a smooth sur- face, whereas a coarse surface can be observed for the 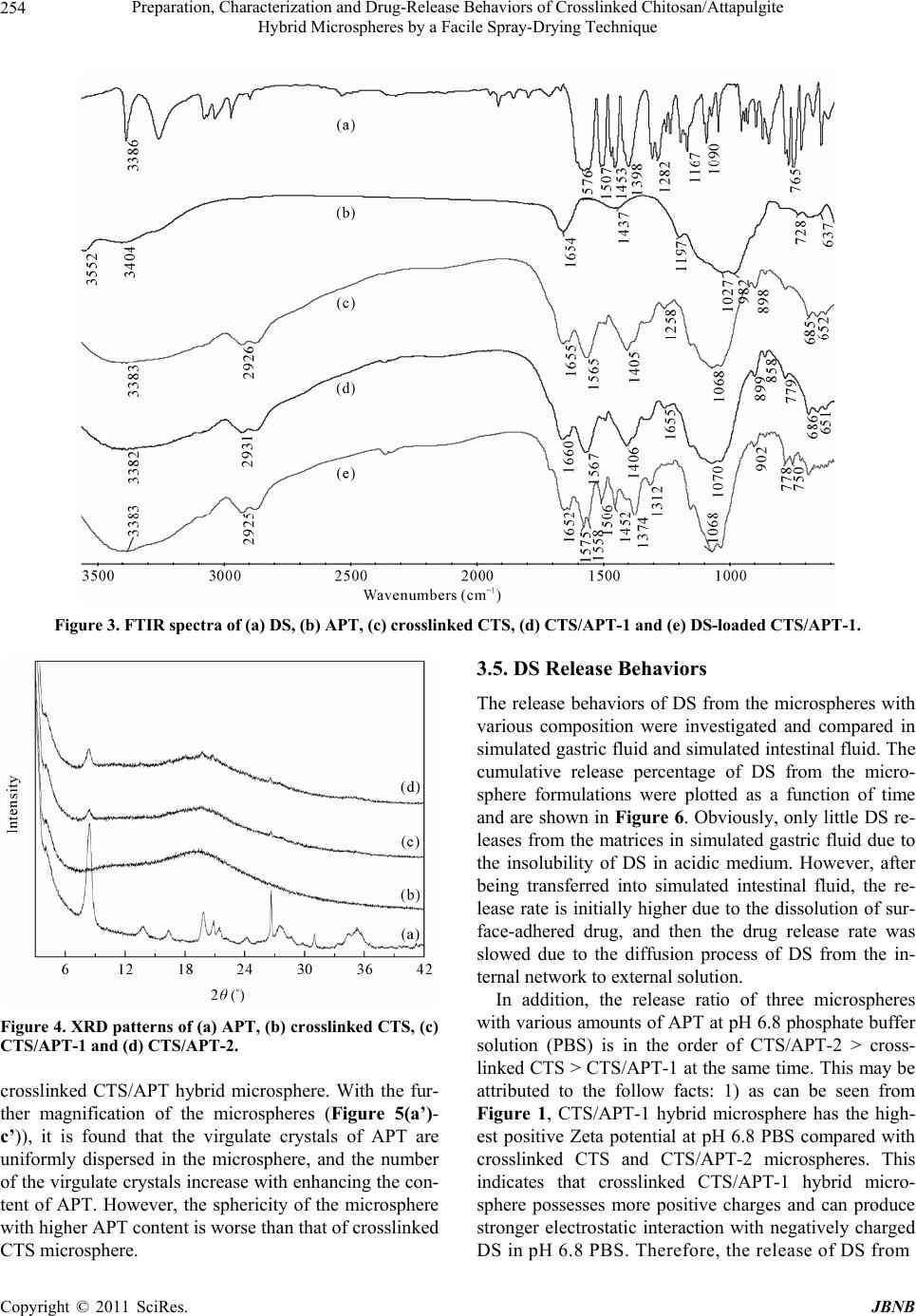 Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 254 Figure 3. FTIR spectra of (a) DS, (b) APT, (c) crosslinked CTS, (d) CTS/APT-1 and (e) DS-loaded CTS/APT-1. Figure 4. XRD patterns of (a) APT, (b) crosslinked CTS, (c) CTS/APT-1 and (d) CTS/APT-2. crosslinked CTS/APT hybrid microsphere. With the fur- ther magnification of the microspheres (Figure 5(a’)- c’)), it is found that the virgulate crystals of APT are uniformly dispersed in the microsphere, and the number of the virgulate crystals increase with enhancing the con- tent of APT. However, the sphericity of the microsphere with higher APT content is worse than that of crosslinked CTS microsphere. 3.5. DS Release Behaviors The release behaviors of DS from the microspheres with various composition were investigated and compared in simulated gastric fluid and simulated intestinal fluid. The cumulative release percentage of DS from the micro- sphere formulations were plotted as a function of time and are shown in Figure 6. Obviously, only little DS re- leases from the matrices in simulated gastric fluid due to the insolubility of DS in acidic medium. However, after being transferred into simulated intestinal fluid, the re- lease rate is initially higher due to the dissolution of sur- face-adhered drug, and then the drug release rate was slowed due to the diffusion process of DS from the in- ternal network to external solution. In addition, the release ratio of three microspheres with various amounts of APT at pH 6.8 phosphate buffer solution (PBS) is in the order of CTS/APT-2 > cross- linked CTS > CTS/APT-1 at the same time. This may be attributed to the follow facts: 1) as can be seen from Figure 1, CTS/APT-1 hybrid microsphere has the high- est positive Zeta potential at pH 6.8 PBS compared with crosslinked CTS and CTS/APT-2 microspheres. This indicates that crosslinked CTS/APT-1 hybrid micro- sphere possesses more positive charges and can produce stronger electrostatic interaction with negatively charged DS in pH 6.8 PBS. Therefore, the release of DS from 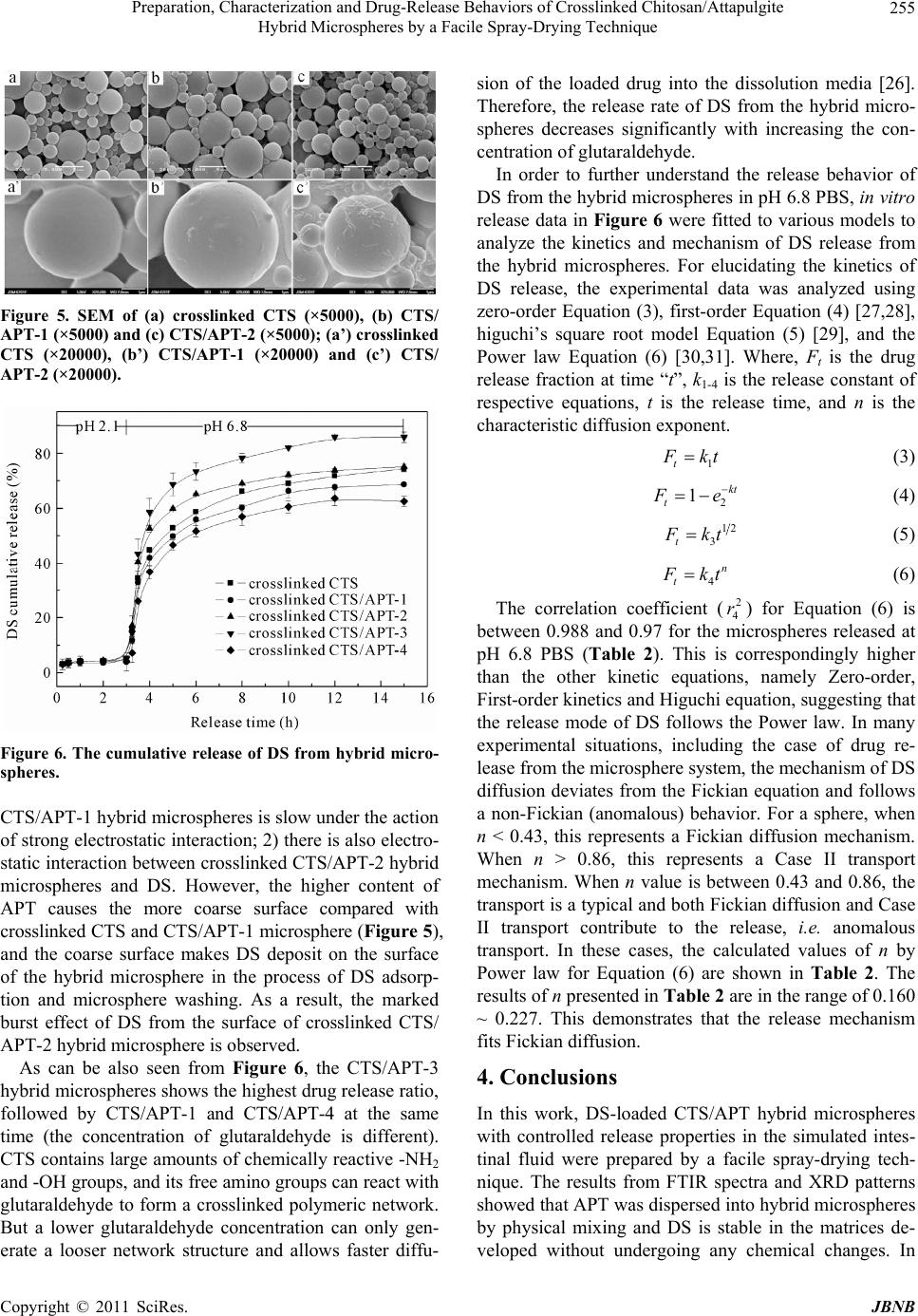 Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 255 Figure 5. SEM of (a) crosslinked CTS (×5000), (b) CTS/ APT-1 (×5000) and (c) CTS/APT-2 (×5000); (a’) crosslinked CTS (×20000), (b’) CTS/APT-1 (×20000) and (c’) CTS/ APT-2 (×20000). Figure 6. The cumulative release of DS from hybrid micro- spheres. CTS/APT-1 hybrid microspheres is slow under the action of strong electrostatic interaction; 2) there is also electro- static interaction between crosslinked CTS/APT-2 hybrid microspheres and DS. However, the higher content of APT causes the more coarse surface compared with crosslinked CTS and CTS/APT-1 microsphere (Figure 5), and the coarse surface makes DS deposit on the surface of the hybrid microsphere in the process of DS adsorp- tion and microsphere washing. As a result, the marked burst effect of DS from the surface of crosslinked CTS/ APT-2 hybrid microsphere is observed. As can be also seen from Figure 6, the CTS/APT-3 hybrid microspheres shows the highest drug release ratio, followed by CTS/APT-1 and CTS/APT-4 at the same time (the concentration of glutaraldehyde is different). CTS contains large amounts of chemically reactive -NH2 and -OH groups, and its free amino groups can react with glutaraldehyde to form a crosslinked polymeric network. But a lower glutaraldehyde concentration can only gen- erate a looser network structure and allows faster diffu- sion of the loaded drug into the dissolution media [26]. Therefore, the release rate of DS from the hybrid micro- spheres decreases significantly with increasing the con- centration of glutaraldehyde. In order to further understand the release behavior of DS from the hybrid microspheres in pH 6.8 PBS, in vitro release data in Figure 6 were fitted to various models to analyze the kinetics and mechanism of DS release from the hybrid microspheres. For elucidating the kinetics of DS release, the experimental data was analyzed using zero-order Equation (3), first-order Equation (4) [27,28], higuchi’s square root model Equation (5) [29], and the Power law Equation (6) [30,31]. Where, Ft is the drug release fraction at time “t”, k1-4 is the release constant of respective equations, t is the release time, and n is the characteristic diffusion exponent. 1t F kt (3) 2 1kt t F e (4) 12 3t F kt (5) 4 n t F kt (6) The correlation coefficient (2 4 r) for Equation (6) is between 0.988 and 0.97 for the microspheres released at pH 6.8 PBS (Table 2). This is correspondingly higher than the other kinetic equations, namely Zero-order, First-order kinetics and Higuchi equation, suggesting that the release mode of DS follows the Power law. In many experimental situations, including the case of drug re- lease from the microsphere system, the mechanism of DS diffusion deviates from the Fickian equation and follows a non-Fickian (anomalous) behavior. For a sphere, when n < 0.43, this represents a Fickian diffusion mechanism. When n > 0.86, this represents a Case II transport mechanism. When n value is between 0.43 and 0.86, the transport is a typical and both Fickian diffusion and Case II transport contribute to the release, i.e. anomalous transport. In these cases, the calculated values of n by Power law for Equation (6) are shown in Table 2. The results of n presented in Ta b le 2 are in the range of 0.160 ~ 0.227. This demonstrates that the release mechanism fits Fickian diffusion. 4. Conclusions In this work, DS-loaded CTS/APT hybrid microspheres with controlled release properties in the simulated intes- tinal fluid were prepared by a facile spray-drying tech- nique. The results from FTIR spectra and XRD patterns showed that APT was dispersed into hybrid microspheres by physical mixing and DS is stable in the matrices de- veloped without undergoing any chemical changes. In 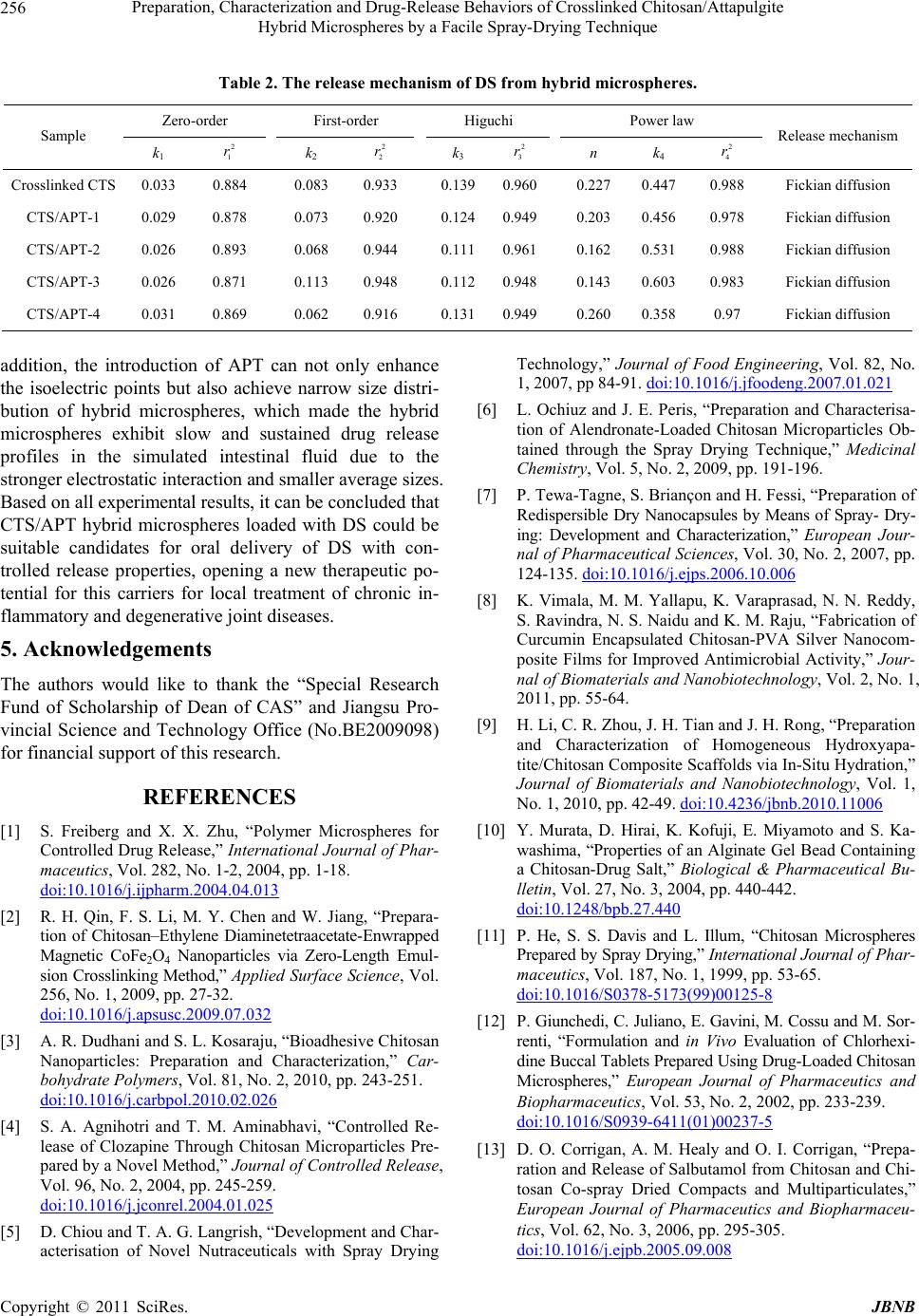 Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 256 Table 2. The release mechanism of DS from hybrid microspheres. Zero-order First-order Higuchi Power law Sample k1 2 1 r k2 2 2 r k3 2 3 r n k4 2 4 r Release mechanism Crosslinked CTS 0.033 0.884 0.083 0.933 0.1390.9600.227 0.447 0.988 Fickian diffusion CTS/APT-1 0.029 0.878 0.073 0.920 0.1240.9490.203 0.456 0.978 Fickian diffusion CTS/APT-2 0.026 0.893 0.068 0.944 0.1110.9610.162 0.531 0.988 Fickian diffusion CTS/APT-3 0.026 0.871 0.113 0.948 0.1120.9480.143 0.603 0.983 Fickian diffusion CTS/APT-4 0.031 0.869 0.062 0.916 0.1310.9490.260 0.358 0.97 Fickian diffusion addition, the introduction of APT can not only enhance the isoelectric points but also achieve narrow size distri- bution of hybrid microspheres, which made the hybrid microspheres exhibit slow and sustained drug release profiles in the simulated intestinal fluid due to the stronger electrostatic interaction and smaller average sizes. Based on all experimental results, it can be concluded that CTS/APT hybrid microspheres loaded with DS could be suitable candidates for oral delivery of DS with con- trolled release properties, opening a new therapeutic po- tential for this carriers for local treatment of chronic in- flammatory and degenerative joint diseases. 5. Acknowledgements The authors would like to thank the “Special Research Fund of Scholarship of Dean of CAS” and Jiangsu Pro- vincial Science and Technology Office (No.BE2009098) for financial support of this research. REFERENCES [1] S. Freiberg and X. X. Zhu, “Polymer Microspheres for Controlled Drug Release,” International Journal of Phar- maceutics, Vol. 282, No. 1-2, 2004, pp. 1-18. doi:10.1016/j.ijpharm.2004.04.013 [2] R. H. Qin, F. S. Li, M. Y. Chen and W. Jiang, “Prepara- tion of Chitosan–Ethylene Diaminetetraacetate-Enwrapped Magnetic CoFe2O4 Nanoparticles via Zero-Length Emul- sion Crosslinking Method,” Applied Surface Science, Vol. 256, No. 1, 2009, pp. 27-32. doi:10.1016/j.apsusc.2009.07.032 [3] A. R. Dudhani and S. L. Kosaraju, “Bioadhesive Chitosan Nanoparticles: Preparation and Characterization,” Car- bohydrate Polymers, Vol. 81, No. 2, 2010, pp. 243-251. doi:10.1016/j.carbpol.2010.02.026 [4] S. A. Agnihotri and T. M. Aminabhavi, “Controlled Re- lease of Clozapine Through Chitosan Microparticles Pre- pared by a Novel Method,” Journal of Controlled Release, Vol. 96, No. 2, 2004, pp. 245-259. doi:10.1016/j.jconrel.2004.01.025 [5] D. Chiou and T. A. G. Langrish, “Development and Char- acterisation of Novel Nutraceuticals with Spray Drying Technology,” Journal of Food Engineering, Vol. 82, No. 1, 2007, pp 84-91. doi:10.1016/j.jfoodeng.2007.01.021 [6] L. Ochiuz and J. E. Peris, “Preparation and Characterisa- tion of Alendronate-Loaded Chitosan Microparticles Ob- tained through the Spray Drying Technique,” Medicinal Chemistry, Vol. 5, No. 2, 2009, pp. 191-196. [7] P. Tewa-Tagne, S. Briançon and H. Fessi, “Preparation of Redispersible Dry Nanocapsules by Means of Spray- Dry- ing: Development and Characterization,” European Jour- nal of Pharmaceutical Sciences, Vol. 30, No. 2, 2007, pp. 124-135. doi:10.1016/j.ejps.2006.10.006 [8] K. Vimala, M. M. Yallapu, K. Varaprasad, N. N. Reddy, S. Ravindra, N. S. Naidu and K. M. Raju, “Fabrication of Curcumin Encapsulated Chitosan-PVA Silver Nanocom- posite Films for Improved Antimicrobial Activity,” Jour- nal of Biomaterials and Nanobiotechnology, Vol. 2, No. 1, 2011, pp. 55-64. [9] H. Li, C. R. Zhou, J. H. Tian and J. H. Rong, “Preparation and Characterization of Homogeneous Hydroxyapa- tite/Chitosan Composite Scaffolds via In-Situ Hydration,” Journal of Biomaterials and Nanobiotechnology, Vol. 1, No. 1, 2010, pp. 42-49. doi:10.4236/jbnb.2010.11006 [10] Y. Murata, D. Hirai, K. Kofuji, E. Miyamoto and S. Ka- washima, “Properties of an Alginate Gel Bead Containing a Chitosan-Drug Salt,” Biological & Pharmaceutical Bu- lletin, Vol. 27, No. 3, 2004, pp. 440-442. doi:10.1248/bpb.27.440 [11] P. He, S. S. Davis and L. Illum, “Chitosan Microspheres Prepared by Spray Drying,” International Journal of Phar- maceutics, Vol. 187, No. 1, 1999, pp. 53-65. doi:10.1016/S0378-5173(99)00125-8 [12] P. Giunchedi, C. Juliano, E. Gavini, M. Cossu and M. Sor- renti, “Formulation and in Vivo Evaluation of Chlorhexi- dine Buccal Tablets Prepared Using Drug-Loaded Chitosan Microspheres,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 53, No. 2, 2002, pp. 233-239. doi:10.1016/S0939-6411(01)00237-5 [13] D. O. Corrigan, A. M. Healy and O. I. Corrigan, “Prepa- ration and Release of Salbutamol from Chitosan and Chi- tosan Co-spray Dried Compacts and Multiparticulates,” European Journal of Pharmaceutics and Biopharmaceu- tics, Vol. 62, No. 3, 2006, pp. 295-305. doi:10.1016/j.ejpb.2005.09.008  Preparation, Characterization and Drug-Release Behaviors of Crosslinked Chitosan/Attapulgite Hybrid Microspheres by a Facile Spray-Drying Technique Copyright © 2011 SciRes. JBNB 257 [14] A. Alhalaweh, S. Andersson and S. P. Velaga, “Prepara- tion of Zolmitriptan–Chitosan Microparticles by Spray Drying for Nasal Delivery,” European Journal of Phar- maceutical Sciences, Vol. 38, No. 3, 2009, pp. 206-214. doi:10.1016/j.ejps.2009.07.003 [15] J. P. Zhang, Q. Wang and A. Q. Wang, “In situ Genera- tion of Sodium Alginate/ Hydroxyapatite Nanocomposite Beads as Drug-Controlled Release Matrices,” Acta Bio- materialia, Vol. 6, No. 2, 2010, pp. 445-454. doi:10.1016/j.actbio.2009.07.001 [16] M. D. Arco, E. Cebadera, S. Gutiérrez, C. Martín, M. J. Montero, V. Rives, J. Rocha and M. A. Sevilla, “Mg, Al Layered Double Hydroxides with Intercalated Indometha- cin: Synthesis, Characterization, and Pharmacological Study,” Journal of Pharmaceutical Science, Vol. 93, No. 6, 2004, pp. 1649-1658. doi:10.1002/jps.20054 [17] Q. Wang, X. L. Xie, X. W. Zhang, J. P. Zhang and A. Q. Wang, “Preparation and Swelling Properties of Ph Sensi- tive Composite Hydrogel Beads Based on Chitosan-G- Poly (Acrylic Acid)/Vermiculite and Sodium Alginate for Diclofenac Controlled Release,” International Journal of Biological Macromolecules, Vol. 46, No. 3, 2010, pp. 356-362. doi:10.1016/j.ijbiomac.2010.01.009 [18] M. P. S. Krekeler and S. Guggenheim, “Defects in Micro- structure in Palygorskite-Sepiolite Minerals: A Transmis- sion Electron Microscopy (TEM) Study,” Applied Clay Science, Vol. 39, No. 1-2, 2008, pp. 98-105. doi:10.1016/j.clay.2007.05.001 [19] M. J. Fernández-Hervás, M. A. Holgado, A. Fini and J. T. Fell, “In Vitro Evaluation of Alginate Beads of Diclofenac Salts”, International Journal of Pharmaceutics, Vol. 163, No. 1-2, 1998, pp. 23-34. doi:10.1016/S0378-5173(97)00333-5 [20] A. Lamprecht, N. Ubrich, H. Yamamoto, U. Schäfer, H. Takeuchi, P. Maincent, Y. Kawashima and C. M. Lehr, “Biodegradable Nanoparticles for Targeted Drug Deliv- ery in Treatment of Inflammatory Bowel Disease,” The Journal of Pharmacology and Experimental Therapeutics, Vol. 299, No. 2, pp. 775-781. [21] A. Lamprecht, U. Schafer and C. M. Lehr, “Size-Depend- ent Bioadhesion of Micro- and Nanoparticulate Carriers to the Inflamed Colonic Mucosa,” Pharmaceutical Re- search, Vol. 18, No. 6, pp. 788-793. doi:10.1023/A:1011032328064 [22] R. R. Dubey and R. H. Parikh, “Studies of PLGA Micro- spheres,” Pharmaceutical Technology Europe, Vol. 16, 2004, pp. 23-34. [23] N. Gottlieb and C. Schwartzbach, “Development of an Internal Mixing Two-Fluid Nozzle by Systematic Varia- tion of Internal Parts,” Proceedings of the Americas: In- stitute for Liquid Atomization and Spray Systems Confer- ence, Nottingham, 16-19 May 2004. Http://www.niro.com/niro/cmsresources.nsf/filenames/TF N_Niro_Ilass_2004_Final.pdf/$file/TFN_Niro_Ilass_200 4_Final.pdf [24] V. H. Kulkarni, P. V. Kulkarni and J. Keshavayya, “Glu- taraldehyde-Crosslinked Chitosan Beads for Controlled Release of Diclofenac Sodium,” Journal of Applied Polymer Science, Vol. 103, No. 1, 2007, pp. 211-217. doi:10.1002/app.25161 [25] T. Iliescu, M. Baia and W. Kiefer, “FT-Raman, Surface- Enhanced Raman Spectroscopy and Theoretical Investi- gations of Diclofenac Sodium,” Chemical Physics, Vol. 298, No. 1-3, 2004, pp. 167-174. doi:10.1016/j.chemphys.2003.11.018 [26] F. L. Mi, C. Y. Kuan, S. S. Shyu, S. T. Lee and S. F. Chang, “The Study of Gelation Kinetics and Chain-Re- laxation Properties of Glutaraldehyde-Cross-Linked Chi- tosan Gel and Their Effects on Microspheres Preparation and Drug Release,” Carbohydrate Polymers, Vol. 41, No. 4, 2000, pp. 389-396. doi:10.1016/S0144-8617(99)00104-6 [27] M. Gibaldi and S. Feldman, “Establishment of Sink Con- ditions in Dissolution Rate Determinations-Theoretical Considerations and Application to Nondisintegrating Do- sage Forms,” Journal of Pharmaceutical Sciences, Vol. 56, No. 10, 1967, pp. 1238-1242. doi:10.1002/jps.2600561005 [28] J. G. Wagner, “Interpretation of Percent Dissolved-Time Plots Derived from in Vitro Testing of Conventional Tab- lets and Capsules,” Journal of Pharmaceutical Sciences, Vol. 58, No. 10, 1969, pp. 1253-1257. doi:10.1002/jps.2600581021 [29] T. Higuchi, “Mechanism of Sustained—Action Medica- tion. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices,” Journal of Pharma- ceutical Sciences, Vol. 52, No. 12, 1963, pp. 1145-1149. doi:10.1002/jps.2600521210 [30] P. L. Ritger and N. A. Peppas, “A Simple Equation for Description of Solute Release. I. Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs,” Journal of Controlled Re- lease, Vol. 5, No. 1, 1987, pp. 23-36. doi:10.1016/0168-3659(87)90034-4 [31] J. Siepmann and N. A. Peppas, “Modeling of Drug Re- lease from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC),” Advanced Drug Delivery Re- views, Vol. 48, No. 2-3, 2001, pp. 139-157. doi:10.1016/S0169-409X(01)00112-0 |

