Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 281-292 doi:10.4236/jbnb.2011.23035 Published Online July 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 281 Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Yuri M. Yevdokimov1, Victor I. Salyanov1, Sergey V. Akulinichev2, Vladimir M. Skorkin2, Pavel V. Spirin1, Nataliya N. Orlova1,Vladimir I. Popenko1, Vladimir S. Prassolov1 1Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences, Moscow, Russia; 2Institute for Nuclear Research of the Russian Academy of Sciences, Moscow, Russia. Email: yevdokim@eimb.ru Received February 11th, 2011; revised March 22nd 2011; accepted April 21st, 2011. ABSTRACT The formation and physico-chemical properties of biomaterial, based on double-stranded (ds) DNA molecules and bearing high concentration of gadolinium, is described. This “rigid” biomaterial demonstrate a few unique properties: 1) the ds DNA molecules forming complexes with gadolinium are fixed in the spatial structure of “rigid” particles, 2) an abnormal negative band in the circular dichroism spectrum permits to follow the formation of this biomaterial; 3) local concentration gadolinium in the content of biomaterial can reach 40%. These properties show that we are dealing with a novel type of biomaterial strongly enriched by gadolinium. This opens a gateway for practical application of this biomaterial for neutron-capture reactions. A first attempt to apply this material for neutron-capture reaction in combi- nation with neutron generator of thermal neutron flux was performed. Positive result obtained at destruction of CHO cells allows one to state that the advantages of this biomaterial are a simple manipulation with it, a possibility to adjust its gadolinium content, long-term stability of its physico-chemical properties, as well as a reduced cost of neutron- capture experiment. Keywords: Liquid-Crystalline DNA Dispersions, Cholesterics, Circular Dichroism Spectrum, Gadolinium, Neutron-Capture Therapy 1. Introduction Biological molecules, in particular nucleic acid mole- cules, are becoming increasingly popular as polyfunc- tional object for nanobiotechnology [1]. Deliberate and controlled variation in the properties of these molecules provides possibilities for formation of various types of nanoconstructions (nanostructures, nano- biomaterials, etc.) allowing their wide application in bio- technology and medicine. For instance, nanoconstruc- tions, formed by double-stranded (ds) DNA molecules fixed in structure of cholesteric liquid-crystalline disper- sions (CLCDs) and cross-linked by nanobridges, were used as biosensing units for biosensor devices [1]. Be- sides, DNA nanoconstructions may be used as “carriers” for genetic material or as a “reservoir” for various bio- logically active compounds embedded in the composition of these structures. Among recently studied nanoconstructions, “rigid” nanoconstruction (“rigid” DNA particles) are of special practical importance and interest due to their unique phy- sicochemical properties [2]. Recently possibilities of application of the “rigid” par- ticles of (ds DNA-Gd) complexes for neutron-capture therapy (NCT) were hypotesized [3]. Neutron capture the- rapy (NCT) is a cancer cells treatment that utilizes nuclear neutron capture reaction (NCR) of radiation producing elements administrated in vivo by thermal neutron flux generated, as a rule, by nuclear reactor [4]. The current attention to radiation-producing elements is essentially focused on gadolinium for its favorable pro- perties, even though extensive studies have been reported on the other elements. It is known that naturally existing gadolinium consists of several stable isotopes. Among them 157Gd (15.6% in natural sample) has most broad medical application. Indeed, gadolinium neutron capture therapy (Gd-NCT) utilizes the following nuclear capture reaction (NCR) of 157Gd by thermal neutron irradiation [5]: 157Gd + nth→158Gd + γ-rays + internal conversion electrons→158Gd + γ-rays + Auger electrons + character-  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 282 istic X-rays. According to accepted point of view, the success of gadolinium neutron capture therapy depends, first, on a high accumulation of Gd in the tumor [6]. Therefore, the first problem here—a sufficient con- centration of gadolinium could be retained in the tumor tissue during neutron irradiation after intratumoral injec- tion. The second problem is the toxicity of free gadolin- ium, because gadolinium ion is strongly toxic even at low doses at its administration to tissues [7,8] For this reason, various kinds of “carriers” (ligands) capable of forming stable complexes with Gd3+ before it is administrated to patients have been constructed. Indeed, the chelate complexes of gadolinium with polymeric molecules (see, for instance [9]), nanoparticles of various origin [10,11] have been produced as potent biomaterials (bioconjugates) for targeting a site and con- trolled release of a drug. Biodegradable, biocompatible chitosan nanoparticles [4,11] have recently received con- siderable attention as systems capable of retaining gado- linium in the tumor tissue during a Gd-NCT trial. This facts show that the developers of NCT are inter- ested in particular molecular constructions (bioconjugates, biomaterials, etc.), which can retain a sufficient gadolin- ium concentration. Thus, a key factor in the success of the current Gd-NCT trial is the use of biomaterials by means of which Gd can be delivered efficiently and retained inside tumor tissues and/or cells during thermal neutron irradia- tion. The aim of this study was to investigate whether the “rigid” particles of the (ds DNA-Gd) complexes can be practically used as a biomaterial for Gd-NCT. For this purpose “rigid” ds DNA particles, highly loaded with ga- dolinium, were obtained and firstly tested in Gd-NCT for disintegration of the living cells. Our preliminary attempt showed that this biomaterial has a good potential as a gadolinium carrier for modern NCT. 2. Materials and Methods 2.1. Preparation of the “Rigid” Particles of the CLCD of (Double-Stranded DNA-Gadolinium) Complexes The formation of the CLCDs based on the phase exclu- sion of ds DNA from PEG-containing water-salt solu- tions was performed according to the Method that was described previously in detail [12]. In the physicochemi- cal sense, the system under investigation is particles of the CLCD of the ds DNA that are distributed isotropi- cally in the water-salt solution of PEG. These particles do not exist in the absence of high osmotic pressure of the solvent (induced by high PEG concentrations). The formed CLCD was treated with GdCl3 solutions to induce formation of (ds DNA-gadolinium) complexes. The efficiency of formation of these complexes was checked by the measuring the CD spectra. The gadolinium salts (99.99% of purity) were pur- chased from the Institute “Giredmet” (Moscow, Russia). The absorption spectra of all solutions were taken on a spectrophotometer (“Cary100”, Varian, USA) and the CD spectra were recorded by a portable dichrometer SKD-2 (manufactured by the Institute of Spectroscopy of the RAS, Troitzk, Moscow Region). In all cases, the quartz cells with 1 cm optical path were used. The morphology of the dsDNA CLCD particles treated by GdCl3 was examined using Atomic Force Microscope P47-SPM-MDT (produced by NT-MDT, Russia). To iso- late these particles, the solution in which they were formed was filtered through a poly(ethyleneterephtalate) (PETP) nuclear membrane filter with size of pores of 150 nm (produced by the Institute of Crystallography of the RAS), that allowed us to immobilize particles; filters were dried in air for no less than 1 h. To estimate the number of Gd3+ ions in the content ds DNA СLCD particles treated by GdCl3, their magnetic properties have been measured and total magnetic mo- ment of Gd3+ ions was determined. The magnetic proper- ties of the samples were measured at magnetic field of 71.29 mT (712.9 Oe) at sample position by the super- conducting interferometer device (SQUID-magnetometer) produced by D. Mendeleev University (Moscow, Russia) [13]. The concentration of the gadolinium in the content of ds DNA СLCD particles treated with GdCl3 was addi- tionally checked by the neutron activation analysis [14]. The fluorescence of SYBR Green (an intercalating fluorescent dye, “Invitrogen” USA, Lot 666062) was measured by the fluorescence spectrophotometer (“Cary Eclipse”, Varian, USA). The particles of CLCD formed by (ds DNA-Gd) com- plexes were sedimented as a result of low speed centri- fugation, the pellet was re-suspended in 0.3 ml of H2O and added to 5 ml of the standard Dulbecco’s MEM me- dium. 2.2. Gd-NCT with “Rigid” Particles of CLCD Formed by (ds DNA-Gd) Complexes 2.2.1. Cell Line Cultivation Chinese hamster ovary (CHO) adherent cells were main- tained in plastic flasks containing standard Dulbecco’s MEM medium supplemented with 10% fetal bovine se- rum (FBS), 4 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mg/ml streptomycin and 100 units/ml penicillin in a humidified atmosphere containing 5% carbon dioxide at 37˚C.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 283 For cell line passage cell monolayer was washed with PBS (10 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4), standard solution of trypsine- EDTA (Sigma) was added, then plates with cells were placed in a 5% CO2 atmosphere at 37˚C for 3-5 minutes, and then medium DMEM with FCS was added, cells were suspended and were plated in flasks at necessary concentrations. CHO cells, forming monolayers, were seed in 4 cul- tural flasks (set A - N1 and N2; set B- N3 and N4; see Figure 1) with 25 cm2 square 500000 cells per flask. (Monolayers formed by these cells were in their original state within whole time of experiment). In 20 hours me- dium in two flasks (set A - N2 and set B- N4) was changed with fresh one, and in two flasks (set A - N1 and set B - N3) with DMEM medium containing “rigid” par- ticles of CLCD formed by (ds DNA-Gd) complexes (the amount of the “rigid” particles per 1 CHO cell in these flasks was equal to about 5 × 104, that provides “close enough contacts” between the cells and “rigid” particles). Then, in 2 hours all four flasks were closed hermetically and two of them (one with CHO cells plus “rigid” parti- cles of CLCD formed by (ds DNA-Gd) complexes (in set A - N1), and the other one - CHO cells only with DMEM medium (in set A - N2) were irradiated with thermal neutrons (see Figure 1). Two flasks (set B-N3 and N4), which were not irradi- ated (see below), were incubated during 1h in thermo- stated (37˚C) polymeric box located nearby neutron gen- erator. 2.2.2. Gd-NCT with “Rigid” Particles of CLCD In contrast to classical neutron source such as huge nu- clear reactors, we first attempted to use more smaller in size, more cheaper neutron generator NG-400 (France) with the neutron energy about 14 MeV and the total in- tensity of the order of 1011 neutrons/sec for the thermal neutron capture inside “rigid” particles the (ds DNA-Gd) complex and generation of the secondary irradiation out- side of these particles. The thermal neutrons were produced by the moderator system consisting of a tungsten converter and poly (eth- ylene) block (20 × 20 × 20 cm, called as “phantom”) assembled in neutron generator. The earlier estimations showed that the conversion electrons, X-rays and gamma rays (range in tissue about 5 × 104 nm), which are gener- ated as a result on the gadolinium thermal neutron cap- ture reaction, can cause the ds DNA double-strand breaks, inducing their killing. Two flasks - one with “rigid” particles and CHO cells (set A - N1, see above) and the other one - CHO cells with DMEM medium (set A - N2) were placed in the rectangular hole (10 × 5 × 5 cm) inside a “phantom”. Figure 1. Principal scheme of the Gd-NCT experimental set-up with the use of the “rigid” DNA particles as carriers for gadolinium. Set B was used as a control. The numbers from 1 to 4 in sets A and B denote the monolayers of initial CHO cells. These flasks (samples) were fixed at the depth of 5 cm inside the “phantom” and exposed at 37˚C to thermal neutrons generated by NG-400. The irradiation time was about 1 h and the thermal neutron fluence was about 51011 neutron/сm2. The ther- mal and fast neutron fluxes were estimated by method of activation analysis [15]. 3. Results 3.1. Formation and Properties of “Rigid” Particles of CLCD of (ds DNA-Gd) Complexes Figure 2 compares the CD spectrum of the CLCD (curve 1) formed by initial ds DNA molecules in water-salt PEG-containing solution to the CD spectra for CLCDs (curves 2-5) treated with GdCl3 solution. The formation of ds DNA dispersion is clearly ac- companied by an appearance of an intense negative band in the CD spectrum in the region of the spectra, where the DNA nitrogen bases absorb. One can remind that every particle of CLCD contains about 104 ds DNA molecules fixed on distances within 2.5 - 5.0 nm (depending on osmotic pressure of the sol- vent [12]. Note that the particles of the low-molecular mass ds DNA dispersions are “microscopic droplets of concentrated DNA solution”, which cannot be “taken in hand” or “directly seen”. According to theoretical calculations, the appearance 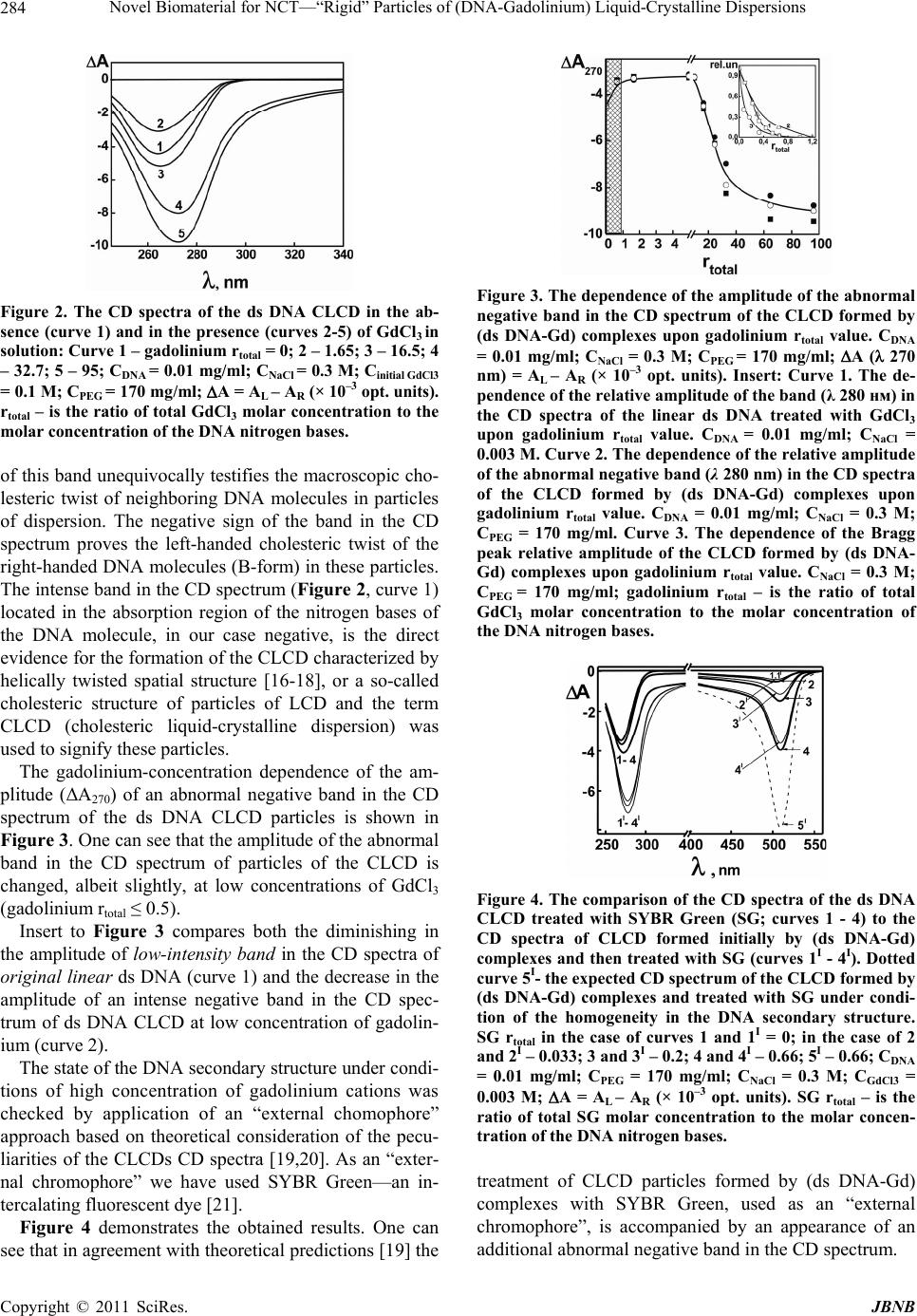 Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 284 Figure 2. The CD spectra of the ds DNA CLCD in the ab- sence (curve 1) and in the presence (curves 2-5) of GdCl3 in solution: Curve 1 – gadolinium rtotal = 0; 2 – 1.65; 3 – 16.5; 4 – 32.7; 5 – 95; CDNA = 0.01 mg/ml; CNaCl = 0.3 M; Cinitial GdCl3 = 0.1 M; CPEG = 170 mg/ml; A = AL – AR (× 10–3 opt. units). rtotal – is the ratio of total GdCl3 molar concentration to the molar concentration of the DNA nitrogen bases. of this band unequivocally testifies the macroscopic cho- lesteric twist of neighboring DNA molecules in particles of dispersion. The negative sign of the band in the CD spectrum proves the left-handed cholesteric twist of the right-handed DNA molecules (B-form) in these particles. The intense band in the CD spectrum (Figure 2, curve 1) located in the absorption region of the nitrogen bases of the DNA molecule, in our case negative, is the direct evidence for the formation of the CLCD characterized by helically twisted spatial structure [16-18], or a so-called cholesteric structure of particles of LCD and the term CLCD (cholesteric liquid-crystalline dispersion) was used to signify these particles. The gadolinium-concentration dependence of the am- plitude (ΔA270) of an abnormal negative band in the CD spectrum of the ds DNA CLCD particles is shown in Figure 3. One can see that the amplitude of the abnormal band in the CD spectrum of particles of the CLCD is changed, albeit slightly, at low concentrations of GdCl3 (gadolinium rtotal ≤ 0.5). Insert to Figure 3 compares both the diminishing in the amplitude of low-intensity band in the CD spectra of original linear ds DNA (curve 1) and the decrease in the amplitude of an intense negative band in the CD spec- trum of ds DNA CLCD at low concentration of gadolin- ium (curve 2). The state of the DNA secondary structure under condi- tions of high concentration of gadolinium cations was checked by application of an “external chomophore” approach based on theoretical consideration of the pecu- liarities of the CLCDs CD spectra [19,20]. As an “exter- nal chromophore” we have used SYBR Green—an in- tercalating fluorescent dye [21]. Figure 4 demonstrates the obtained results. One can see that in agreement with theoretical predictions [19] the Figure 3. The dependence of the amplitude of the abnormal negative band in the CD spectrum of the CLCD formed by (ds DNA-Gd) complexes upon gadolinium rtotal value. CDNA = 0.01 mg/ml; CNaCl = 0.3 M; CPEG = 170 mg/ml; A ( 270 nm) = AL – AR (× 10–3 opt. units). Insert: Curve 1. The de- pendence of the relative amplitude of the band (λ 280 нм) in the CD spectra of the linear ds DNA treated with GdCl3 upon gadolinium rtotal value. CDNA = 0.01 mg/ml; CNaCl = 0.003 M. Curve 2. The dependence of the relative amplitude of the abnormal negative band (λ 280 nm) in the CD spectra of the CLCD formed by (ds DNA-Gd) complexes upon gadolinium rtotal value. CDNA = 0.01 mg/ml; CNaCl = 0.3 M; CPEG = 170 mg/ml. Curve 3. The dependence of the Bragg peak relative amplitude of the CLCD formed by (ds DNA- Gd) complexes upon gadolinium rtotal value. CNaCl = 0.3 M; CPEG = 170 mg/ml; gadolinium rtotal – is the ratio of total GdCl3 molar concentration to the molar concentration of the DNA nitrogen bases. Figure 4. The comparison of the CD spectra of the ds DNA CLCD treated with SYBR Green (SG; curves 1 - 4) to the CD spectra of CLCD formed initially by (ds DNA-Gd) complexes and then treated with SG (curves 1I - 4I). Dotted curve 5I- the expected CD spectrum of the CLCD formed by (ds DNA-Gd) complexes and treated with SG under condi- tion of the homogeneity in the DNA secondary structure. SG rtotal in the case of curves 1 and 1I = 0; in the case of 2 and 2I – 0.033; 3 and 3I – 0.2; 4 and 4I – 0.66; 5I – 0.66; CDNA = 0.01 mg/ml; CPEG = 170 mg/ml; CNaCl = 0.3 M; CGdCl3 = 0.003 M; A = AL – AR (× 10–3 opt. units). SG rtotal – is the ratio of total SG molar concentration to the molar concen- tration of the DNA nitrogen bases. treatment of CLCD particles formed by (ds DNA-Gd) complexes with SYBR Green, used as an “external chromophore”, is accompanied by an appearance of an additional abnormal negative band in the CD spectrum.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 285 Figure 5 compares the changes in the emission inten- sity of SYBR Green intercalated between nitrogen bases of initial, linear ds DNA with homogeneous secondary structure (curve 1), to ds DNA in the content of CLCD (curve 2) as well as ds DNA in the content of CLCD treated with gadolinium (curve 3). Figure 6(a) displays the 2D-AFM images of “rigid” CLCD particles formed by (ds DNA - Gd) complexes immobilized onto the surface of a nuclear membrane filter. It is evident that these particles exist as independent individual objects, which are easy to visualize. Figure 6(b) demonstrates the size distribution of these particles as well as the pores in the filter. 3.2. Gd-NCT with “Rigid” Particles of CLCD Formed by (ds DNA-Gd) Complexes We used biomaterial, i.e., “rigid”(ds DNA-Gd) particles as a potential platform for Gd-NCT. For this purpose effect of radiation as a result of Gd-NCR on culturated CHO cells was measured. These cells were exposed to thermal neutrons flow from neutron generator in the presence and in the absence of “rigid” (ds DNA-Gd) par- ticles (see Figure 1). After irradiation of the cells in the flasks in set A (see Figure 1) all four flasks from set A and set B were maintained in a 5% CO2 atmosphere at 37˚C within 16 hours. Then medium in all flasks was changed with fresh one without “rigid” particles, and the images of CHO cells in monolayers were analyzed by light microscope (Leica DMI4000). The images were taken in 20, 60 and 120 hours after irradiation One can remind that the time of experiment with Gd-NCT was about 3 hours. Hence, the first important question to be answered is the penetration of “rigid” par- ticles containing Gd into CHO cells within this time. Figure 5. The fluorescence spectra of the initial, linear ds DNA and CLCD formed by ds DNA as well as the spectrum of CLCD of (ds DNA-Gd) complexes treated by SYBR Green (SG, curves 1, 2, and 3, respectively). CDNA = 0.01 mg/ml; CNaCl = 0.3 M; CPEG = 170 mg/ml; CSG = 9.73 × 10–6 M; CGdCl3 = 0.003 M. (a) (b) Figure 6. (a) 2-D AFM image of the “rigid” particles immo- bilized onto the surface of the nuclear membrane filter (PETP). CDNA = 0.001 mg/ml; CNaCl = 0.03 M; CPEG = 17 mg/ml; CGdCl3 = 0.0023 M. (The dark spots are “pores” in the nuclear membrane filter); (b) Size distribution of the ds DNA CLCD particles treated by GdCl3 (1) and the pores (2) in the membrane filter. CDNA = 0.001 mg/ml; CNaCl = 0.03 M; CPEG = 17 mg/ml; CGdCl 3 = 0.0023 M. Control experiment showed, that before irradiation, the penetration of “rigid” (ds DNA-Gd) particles into CHO cell was practically absent. This means that in our case the “rigid” particles, which are located outside the CHO cells, were used for NCT. Figure 7(a) shows the image typical of initial CHO cells without irradiation (set B N4). Figure 7(b) represents the image of CHO cells in monolayers corresponding to flask N2 (set A), i.e., the image for CHO cells irradiated by thermal neutrons in the absence gadolinium carrier. Figure 7(c) shows the image for CHO cells irradiated by thermal neutrons in the presence of “rigid” gadolin- ium carrier (set A N1). 4. Discussion 4.1. Formation and Morphology of “Rigid” Particles of CLCD of (ds DNA-Gd) Complexes Figure 2 shows that a quite sharp increase in the ampli-  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 286 (a) (b) (c) Figure 7. The images of CHO cells monolayers taken after 120 hours of cell processing by light microscope (Leica DMI4000). (a) the image without the thermal neutron irra- diation and without gadolinium carrier; (b) the image with thermal neutron irradiation without gadolinium carrier; (c) the image after 1 hour of the thermal neutron irradiation with gadolinium carrier. tude of the intense negative band in the CD spectrum of the CLCD of the (ds DNA-Gd) complexes occurs at a large concentration of gadolinium cations in the solution (rtotal > 20) and its maximum is shifted by 10 nm toward long wavelengths (inset to Figure 2, curves 1-5). As it was shown previously [3,22-24] when rare earth cations, and in particular, Gd cations, are bonded to lin- ear ds DNA, noticeable alterations in the CD spectra of these molecules were observed at gadolinium rtotal values close to 0.5 [22,24]. This effect was explained as a de- formation (alteration) of the ds DNA secondary structure [25,26]. This deformation can be associated with the conformational transition of B Z type [25,27]. Besides, the junctions between B-DNA and Z-DNA fragments contain extruded bases providing the sites with modified local properties. Combination of these two effects is ac- companied by breaking of the regular, homogeneous character of the ds DNA secondary structure. The “modi- fied” ds DNA molecules can be separated into alternating fragments differing in conformations, for instance, of B Z – Z B – B Z B –Z– type. Hence, even low concentrations of gadolinium cations are capable of inducing the breaking of regular character of the ds DNA secondary structure of initial linear ds DNA molecules. Because formation of ds DNA CLCD does not result in change in the parameters of the DNA secondary structure, one can expect that the breaking process happens in the case of ds DNA molecules packed in CLCD particles. To check the character of the secondary structure of ds DNA molecules ordered in the particles of CLCD treated with various concentrations of gadolinium salt, the small- angle X-ray scattering (SAXS) curves of these objects were recently obtained [28]. The most important observation detected by SAXS is that structural changes in the ds DNA molecules in the content of CLCD particles occur while the concentrations of the gadolinium salt are very low. At gadolinium rtotal = 0.66 the characteristic Bragg peak in X-ray scattering curves has completely disappeared (Figure 3, curve 3). Therefore, if the fragments of the neighboring molecules of the complexes of the DNA with gadolinium or even all molecules packed in particles of CLCD acquire an inhomogeneous secondary structure, the translational order of these fragments (molecules) is broken and the small-angle reflection on X-ray scattering curves must disappear and this disappearance is observed experimen- tally [28]. Hence, the analysis of the SAXS spectra. con- firms the assumption that the treatment of particles of the CLCD by gadolinium cations leads to the appearance of the modified secondary structure of the DNA molecules. Taking into account the suggestion above that “modi- fied” DNA molecules are separated into alternating fragments differing in conformations (e.g., B – Z – B – B – Z – Z etc.), the existence of ds DNA fragments with the B-form was checked by the method of “external chro- mophore”. As an external chromophore we have used intercalating drug—SYBR Green, which is highly selec- tive for native B-form of ds DNA molecules with regular secondary structure. Figure 4 shows that in the CD spectrum of (ds DNA- Gd) complexes there are two bands. One occurs in the absorption region of the DNA nitrogen bases ( ~ 270 nm) and the other lies in the absorption region of SYBR Green chromophores ( ~510 nm). Under binding of SYBR Green with ds DNA molecules in content of CLCD particles formed by (ds DNA-Gd) complexes both bands have negative signs despite of SYBR Green con- centration. The identical signs of two bands in the CD spectra unequivocally mean that SYBR Green molecules are fixed in quasinematic DNA layers. The amplitude of the band in the CD spectrum in the region of SYBR Green absorption grows with increasing number of its molecules bound to DNA, although the amplitude of the band in the region of DNA absorption remains practi- cally constant. The shown CD spectra mean that the ori- entation of SYBR Green molecules coincides with the orientation of the nitrogen base about the DNA axis and SYBR Green molecules intercalate into DNA so that the angle between SYBR molecule and the long axes of the DNA is ~90˚.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 287 However, the experimentally measured amplitudes (compare, for instance, curve 4’ to theoretically calcu- lated curve 5’) are 2 times smaller then expected ones (if one can take into account the correlation between the amplitudes of abnormal bands in the DNA and “external chromophores” absorption regions [19]. The detected difference shows that, indeed, that at high gadolinium concentration the breaking of the ds DNA secondary structure in the CLCD particles of (ds DNA-Gd) com- plexes takes place. The measurements of the SYBR Green fluorescence in the content of CLCD particles formed by (ds DNA-Gd) complexes speak in favor of this point of view. Figure 5 demonstrates that the fluorescence intensity of SYBR Green is decreased in the case of both ds DNA CLCD and (ds DNA-Gd) CLCD. The most important facts con- sist in the following: 1) condensation of ds DNA mole- cule and formation of the ds DNA CLCDs is accompa- nied by drop in the intensity of fluorescence of SYBR, and 2) there is difference in fluorescence of SYBR Green intercalated between nitrogen bases pairs in the content of ds DNA molecules ordered in initial CLCD and in the content of CLCD treated with gadolinium. Since the sol- vent used for measurements of curves (2) and (3) was not changed and because SYBR Green molecules bind with regular B-form of ds DNA, the difference between curves (2) and (3) confirms ones more the statement that neighboring ds DNA molecules, packed in particles of the CLCD (ds DNA-Gd) complexes, acquire an inhomo- geneous secondary structure. Figure 3 shows that at high gadolinium concentration in solution (rtotal > 20) the CD spectrum of CLCD formed by ds DNA changes dramatically. The observed increase in the amplitude of the negative band and the change in the shape of the CD spectrum of the DNA CLCD are similar to changes in the CD spectra of this CLCD upon cross-linking of neighboring DNA molecules due to the formation of nanobridges between them [29]. The forma- tion of such nanobridges leads both to decrease in solu- bility of initial CLCD structure and to the disappearance of the “liquid” character in the location of DNA mole- cules in the particles of the CLCD [1]. This allows one to suppose, that interaction of gado- linium with ds DNA molecules is accompanied by de- crease in solubility of these molecules as well. In addi- tion, the inhomogeneous chemical nature of the nitrogen bases in ds DNA molecules leads to the fact that the in- teraction between gadolinium cations and ds DNA mo- lecules is accompanied by nanoscale conformational changes (similar to the changes that are appeared at B Z transition) only in the fragments of these molecules. Because separation of chains of the ds DNA molecules in the content of particles of the LCD is impossible due to the sterical reasons [30,31], the alteration of the ds DNA secondary structure, induced by gadolinium treatment, can be “transformed” into the change in the mode of spa- tial packing of the neighboring DNA molecules in these particles. Because ds DNA molecules cannot “leave” the physical volume of CLCD particles, due to the fixed os- motic pressure of PEG-containing solution, the loss of solubility of individual neighboring ds DNA molecules combined with an increase in the interaction between their fragments with different conformations initiates the transition of the overall structure of CLCD particles from a “liquid” to a “rigid” state [32]. It is known, that a “liquid” mode of spatial location of ds DNA molecules in the particles of CLCD dispersions prevents their immobilization on the surface of mem- brane filters. However, if poorly soluble CLCD particles consisting of molecules of the (ds DNA–Gd) complexes are formed, the immobilization of particles on the surface of the nuclear membrane filter becomes possible and the size and shape of these particles can be investigated. Figure 6(a) shows the AFM image of ds DNA CLCD particles after their treatment with GdCl3 and immobili- zation on nuclear membrane filter. Figure 6(b) demon- strates the size distribution of these particles as well as the pores in the filter. One can see that these particles exist as independent, individual objects. The presence of single particles (Figure 6(a)) testifies that at treatment of particles of ds DNA CLCD by GdCl3, the “liquid” character of the DNA packing in these parti- cles is disappeared and the particles have a rigid spatial structure. Therefore, particles of the CLCD of the ds DNAs whose phosphate groups are neutralized by gadolinium ions become poorly soluble and can exist in the absence of osmotic pressure of the PEG-containing solution and the osmotic pressure of the water-salt PEG-containing solution is not required for supporting the spatial struc- ture of CLCD particles formed by (DNA-gadolinium) complexes. The mean size of particles makes 4500 - 5000 A˚, i.e. the mean diameter of the ds DNA CLCD particles after gadolinium treatment coincides with the mean diameter of initial ds DNA CLCD particles [12,33]. The particles have shape of the spherocylinders and the diameter of particles is close to their height. The obtained result is important, because it allows one to suggest that the av- erage packing density of the DNA molecules in particles of the CLCD of the (ds DNA–Gd) complex is quite close to the packing density of the DNA molecules in particles of the CLCD formed from initial DNA molecules. In this case, the mean concentration of chromophores (nitrogen bases) of the DNA in particles of the CLCD of the  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 288 DNA–gadolinium complex must also retain not only high [34], but sufficient for holding the abnormal optical ac- tivity of these particles. The visualization of single particles indicates that, when particles of the CLCD of ds DNA are treated by the GdCl3 solution, the liquid character of packing of DNA molecules in these particles is indeed disappeared and particles acquire a rigid spatial structure. Such a structure is presented not only the decrease in the solubility of DNA molecules, but also the presence of strong interac- tion between the fragments of neighboring DNA mole- cules, because gadolinium ions can be nonuniformly dis- tributed. Hence, the treatment of the ds DNA CLCD by GdCl3, is accompanied not only by neutralization of phosphate groups of the DNA molecules by Gd3+ ions, but by a sig- nificant attraction between the neighboring DNA mole- cules. Disappearance of the fluidity of the ds DNA CLCD particles proves a short-range attractive interac- tion between the charged DNA molecules arising from interlocking Gd3+ ions, sometimes called as “counterion cross-links”. An existence of independent particles speaks in favor of an appearance of noncompensated positive surface charge on the CLCD particles. This, in turn, pro- hibits the coalescence of these particles. In addition, nonuniform distribution of gadolinium ions over the surface of DNA molecules having the in- homogeneous secondary structure is accompanied by irregular interaction between the fragments of the neigh- boring DNA molecules in quasinematic layers. Because each phosphate group of the DNA molecules, carrying one “effective” negative charge, is neutralized by Gd3+ ion [35], that carries three positive charges, this means that the altered surface charge distribution makes an ad- ditional contribution to the chiral interaction between adjacent (ds DNA-Gd) complexes in the particles [22,19]. Under these conditions, the interaction between the modified DNA molecules can induce change in the twisting of the cholesteric helical structure of the DNA molecules. In this case one can expect that the pitch (P) of the spatial twist of cholesteric structure formed by the ds DNA is changed as a result of interaction of gadolin- ium ions with these molecules. Indeed, the change in the twist angle between neighbor- ing quasinematic layers of CLCD is supported by results of the theoretical calculations [3,32,36] according to which an increase in the twist angle (decrease in P value) of the spatial structure of (ds DNA-Gd) CLCD particles [36] determines a drastic increase in the amplitude of an abnormal negative CD band in the absorption region of DNA nitrogen bases, when the CLCD particles are transformed from “liquid” to into “rigid” state. The results above allows one to suggest the scheme of “liquid-rigid” structural transition of ds DNA CLCD shown in Figure 8. One can see, that here the spatial ordering of neigh- boring DNA molecules in quasi-nematic layers is practi- cally absent; besides, under these conditions the twist angle between neighboring DNA quasi-nematic layers is increased (the P value is decreased, right structure). Ac- cording to [32,37] in the presence of large excess of gadolinium cations, these cations can displace the so- dium ions, initially bounded to the phosphate groups of the ds DNA. The gadolinium ions neutralizing the nega- tive charges of the phosphate groups of the ds DNAs make particles of the CLCD of (ds DNA-Gd) insoluble in PEG-salt-aqueous solutions. It is worth noting that, when Gd3+ ions are bonded to polyphosphates, poorly soluble Gd-polyphosphate is formed (solubility constant is equal to about 10–12 M) [38,39]. Since ds DNA molecules have polyphosphate nature, these molecules in the presence of saturating gadolinium concentrations become poorly soluble in poly(ethyleneglycol)-water salt solutions. Un- der high concentration of gadolinium, the stable spatial structure of dispersion particles is formed and the pres- ence of poly(ethyleneglycol) is not required to stabilize the structure of particles of the CLCD. Moreover, gado- linium ions, neutralizing the charges of the phosphate groups of the DNAs, create an excess positive surface charge on particles of the CLCD and aggregation of these particles. This behavior is corroborated by the atomic force microscopic data according to which these gado- linium-ion-treated particles of the CLCD of the DNAs are existing as single independent objects (see Figure 6). According to this scheme the amplification of the ab- normal negative band (λ ~ 270 nm) in the CD spectrum of CLCD particles formed by ds DNA molecules treated with high concentration of gadolinium cations confirms the formation of “rigid” CLCD particles of (ds DNA-Gd) Figure 8. Sheme of transition from “liquid” to “rigid” structure of the particle of the CLCD induced by high Gd3+ concentration.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 289 complex (Figures 2 and 3) under used conditions. In addition, the results of low-temperature magne- tometric study and neutron activation analyses showed [3, 15] that in the case of “rigid” particles formed at rtotal > 20, one gadolinium cation is bounded approximately to one DNA phosphate group. The evaluations showed that local concentration gadolinium in the content of these particles can reach 40%, i.e., we are dealing with a novel type of biomaterial containing a very high local concen- tration of gadolinium. The obtained “rigid” CLCD particles strongly enriched by gadolinium open a possibility for various manipula- tions with them, for instance, their application as “Gd- carriers” at neutron capture therapy (NCT). 4.2. Gd-NCT with “Rigid” Particles of CLCD Formed by (ds DNA-Gd) Complexes A potential of the “rigid” (ds DNA-Gd) particles as bio- material for Gd-NCT, is based on a few facts: 1) the formation of these particles can be easily checked by the CD spectroscopy or by the AFM; 2) a long-term stability of the physicochemical proper- ties of these particles allows one to manipulate with these particles; 3) this biomaterial has a higher concentration of 157Gd compared to other known Gd-carriers such as, for in- stance, Gd-chelate complexes [4,6,9,11]. Comparison of Figure 7(b) to Figure 7(a) shows that irradiation of CHO cells by the thermal neutron fluence (5 × 1011 neutron/сm2) within 1 h results only in minor (if any!) changes in the CHO cells and does not influence their ability to grow. Even after 120 hours of cell proc- essing, these cells, irradiated in absence of (ds DNA-Gd) particles grow as initial cells and form monolayer. This signifies that thermal neutron irradiation of intact CHO cells under our conditions does not influence strongly the proliferation ability of these cells. Figure 7(c) shows the image for CHO cells irradiated by thermal neutrons in the presence of “rigid” gadolin- ium carrier (set A N1). It can be seen, that after 120 hours of cell processing, the image of CHO cells differs from that of cells irradiated without “rigid” gadolinium carrier. The efficiency of cells proliferation is reduced and cells don’t form colonies. The growth of CHO cells administrated with the “rigid” (ds DNA-Gd) particles and then irradiated with thermal neutrons was significantly suppressed compared to that in control cells. Besides, the cell debris in cultural medium begins to appear after 60 hours of cell processing (data not shown). The amount of cell debris after 120 hours shows that significant part of cells was disintegrated. Finally, the irradiation of CHO cells in presence of “rigid” particles results in full ab- sence of alive CHO cells. Taking into account that flasks N1 and N2 (set A) were irradiated by thermal neutrons simultaneously the difference in the cell killing efficacy for these samples (Figure 1, set A) might be due to the thermal NCR in- duced only by gadolinium in the content of “rigid” parti- cles containing (ds DNA-Gd) complexes. Hence, CHO cells in the sample with gadolinium were killed, while the cells in control samples survived under the same conditions of irradiation [40]. The presence of strongly deformed cells (collapsed cells) Figure 7(c) allows one to suppose, that although concrete reasons for an appearance of these cells were not investigated carefully, that irradiation of CHO cells in presence of (ds DNA-Gd) “rigid” particles is accom- panied by a few processes: 1) disintegration of genetic material of these cells; 2) deformation and destruction of lipoprotein mem- brane as a result of possible retention of Gd-containing particles in lipoprotein membrane of CHO cells and 3) penetration of small fraction of (ds DNA-Gd) “rigid” particles into the CHO cells inducing additional destruc- tion of these cells. It is necessary to stress that according to known data [5,7,8,40] in the case of other Gd-carries the cell growth was inhibited until to 10 days after the neutron irradia- tion. Considering the reasons for the effects shown in Fig- ure 7(c), one can remind the following. When bom- barded with thermal neutrons, 157Gd releases photons and electrons with energies up to 7.9 MeV [5,41]. Previous studies on CHO cells have shown a significant enhance- ment of lethal effects induced by Gd-NCR [5]. During the Gd-NCR, the emission of γ-rays is followed by the internal conversion and subsequent emission of Auger electrons [41] and these electrons play an important role in cell killing. As the range of these electrons is ex- tremely limited in tissue, gadolinium distribution in the cells, particularly with respect to the genetic material, is crucial in determining the extent of biological effects induced by Gd-NCT. Indeed, it was shown in [42,43] that in the thermal neutron irradiation of plasmid DNA/ gadolinium mixture the extent of double-strand breaks to be considerably reduced by sequestering the gadolinium from DNA, suggesting the effect to have been mainly due to Auger electrons. However, the ranges of high-energy electrons and photons produced in the gadolinium NCR [41] are suffi- ciently long within tissue for cell inactivation to occur even if Gd is present in the vicinity of the cell. Indeed, it was demonstrated that mouse ascites cells in the perito- neal cavity to be inactivated by the radiation released from 157Gd contained in microcapsules, suggesting the proximity of 157Gd to cellular genome not to be critical.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 290 Taking into account the fact that gadolinium in micro- capsules was effective in suppressing murine ascites tu- mor, most of the effects observed apparently resulted from the radiation of high-energy internal-conversion electrons having adequate ranges and photons from Gd. This result permits to suppose that the intracellular pres- ence of Gd is, indeed, not critical in Gd-NCT. It is known as well that the energy of conversion elec- trons is equivalent to the path inside biological tissue more then 50 m [44]. It was demonstrated earlier [45], that the conversion electrons (the energy 7 KeV) and -rays are not significantly absorbed inside the initial “rigid” (ds DNA-Gd) CLCD particles. Besides, the cal- culations showed that more than 70% of the conversion electrons can penetrate into the CHO cells and create the radiation dose, requested for cell destruction. Taking into account that the (ds DNA-Gd) CLCD particle density was about of 5·104 particles per cell and the thermal neu- tron fluence was of the order of 5 × 1011 neutron/сm2, this results in 100 neutron captures i in n each particle. In that case the secondary electrons and photons, with the probability of about 100%, can induce the DNA dou- ble-strand breaks in the cell nucleus if the (ds DNA-Gd) CLCD particles located nearby the surface of CHO cells. This means that we can expect that the secondary irradia- tion from “rigid” (ds DNA-Gd) particles can hit the ma- terial of cell nucleus, inducing its disintegration. Indeed, comparison of the images shown in Figure 7 in combi- nation with the value of neutron fluence equals to 5 × 1011 neutron/cm2 and high Gd-concentration in “rigid” particles used in our study permits one to state, that highly-gadolinium-loaded DNA “rigid” particles have potential usefulness for Gd-NCT. We are clearly understanding that for practical medical application, the optimization of physico-chemical pa- rameters of “rigid” (ds DNA-Gd) particles, including con- centration of Gd, as well as the time before irradiation and the time of irradiation is required. One can suppose that the efficiency of application of these particles in Gd-NCT can be even increased in the case of forced penetration of DNA “rigid” particles inside CHO cells. Besides, it is necessary to perform “the size of “rigid” particles—the dose” calculations [46,47] because, as it was shown in [46,47], the doses from neutrons, prompt γ-rays and internal conversion electrons, and the dose distribution to be a function of strongly-interrelated pa- rameters such as tumor size, gadolinium concentration and its spatial distribution through the tumor, tumor-to- normal tissue concentration ration and tumor site. In conclusion, one can say that the obtained results (despite of their noncomplete character) showed that “rigid” (ds DNA-Gd) particles have a potential as a novel biomaterial with high concentration of gadolinium for NCT. In this case appears a possibility to use simpler (in comparison to nuclear reactors) devices for NCT realiza- tion. Our results demonstrate as well that the intracellular presence of Gd-carrier is not an obligatory condition for effective disintegration of CHO cells at Gd-NCT. REFERENCES [1] Yu. M., Yevdokimov and V. V Sythev, “Nanotechnology and Nucleic Acids,” Open Nanoscience Journal, Vol. 1, No. 1, 2007, pp. 19-31. [2] Yu. M. Yevdokimov, V. I. Salyanov and S. G. Skuridin, “From Liquid Crystals to DNA Nanoconstructions,” Mo- lecular Biology, Vol. 43, No. 2, 2009, pp. 284-300. doi:10.1134/S0026893309020113 [3] Yu. M. Yevdokimov, V. I. Salyanov, O. V. Kondrashina, V. I. Borshevsky, S. V. Semenov, A. A. Gasanov, I. V. Re- shetov, V. D. Kuznetsov, V. N. Nikiforov, S. V. Akuli- nichev, M. V. Mordovskoi, S. I. Potashev and V. M. Skor- kin, “Particles of Liquid-Crystalline Dispersions Formed by (Nucleic Acid–Rare Earth Element) Complexes as a Potential Platform for Neutron Capture Therapy,” Inter- national Journal of Biological Macromolecules, Vol. 37, No. 4, 2005, pp. 165-173. doi:10.1016/j.ijbiomac.2005.10.001 [4] H. Tokumitsu, H. Ichikawa, T. K. Saha, Y. Fukumori and L. H. Block, “Design and Preparation of Gadolinium- Loaded Chitosan Particles for Cancer Neutron Capture Therapy,” S.T.P. Pharma Sciences, Vol. 10, No. 1, 2000, pp. 39-49. [5] R. C. Greenwood, C. W. Reich, H. A. Baader, H. R. Koch, D. Breitig, O. W. B. Schult, B. Fogelberg, A. Backlin, W. Mampe, T. von Egidy and K. Schreckenbach, “Collective and Two-Quasiparticle States in 158Gd Observed through Study of Radiation Neutron Capture in 157Gd,” Nuclear Physics, Vol. A 304, No. 2, 1978, pp. 327-428. [6] T. K Saha, K. Jono, H. Ichikawa and Y. Fukumori, “Pre- paration and Evaluation of Glutaraldehyde Cross-Linked Chitosan Microspheres as a Gadolinium Reservoir for Neutron-Capture Therapy,” Chemical & Pharmaceutical Bulletin, Vol. 46, 1988, pp. 537-539. [7] J. Greiberg, J. M. Wolf, J. Wyman, L. Zou and R. M. Terek, “Gadolinium Inhibits Thymidine Incorporation and Induces Apoptosis in Chondrocytes,” Journal of Ortho- paedic Research, Vol. 19, No. 5, 2001, pp. 797-801. doi:10.1016/S0736-0266(01)00025-0 [8] J. P. Mizgerd, R. M. Molina, R. C. Stearns, J. D. Brain and A. E. Warner, “Gadolinium Induces Marcophage Apop- tosis,” Journal of Leukocyte Biology, Vol. 59, No. 2, 1996, pp. 189-195. [9] Q. F. Leclercq, M. Cohen-Ohana, N. Mignet, A. Sharbati, J. Herscovici, D. Sherman and G. Byk, “Design, Synthe- sis, and Evaluation of Gadolinium Cationic Lipids as Tools for Biodistribution Studies of Gene Delivery Com- plexes,” Bioconjugate Chemistry, Vol. 14, No. 1, 2003, pp. 119-119. doi:10.1021/bc025567e [10] H. Tokumitsu, J. Hiratsuka, Y. Sakurai, T. Kobayashi, H.  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 291 Ichikawa and Y. Fukumori, “Gadolinium Neutron-Capture Therapy Using Novel Gadopentetic Acid-Chitosan Com- plex Nanoparticles: In Vivo Growth Suppression of Ex- perimental Melanoma Solid Tumor,” Cancer Letters, Vol. 150, No. 2, pp. 177-182. doi:10.1016/S0304-3835(99)00388-2 [11] H. Tokumitsu, H. Ichikawa, Y. Fukumori, “Chitosan- Ga- dopentetic Acid Complex Nanoparticles for Gadolinium Neutron-Capture Therapy of Cancer: Preparation of Novel Emulsion-Droplet Coalescence Technique and Charac- terization,” Pharmaceutical Research, Vol. 16, No. 12, 1999, pp. 1830-1835. doi:10.1023/A:1018995124527 [12] Yu. M. Yevdokimov, S. G Skuridin and G. B. Lortki- panidze, “Liquid-Crystalline Dispersions of Nucleic Ac- ids,” Liquid Crystals, Vol. 12, No. 1, 1992, pp. 1-16. doi:10.1080/02678299208029034 [13] V. N. Nikiforov, V. D. Kuznetsov, Yu. D. Nechipurenko, V. I. Salyanov and Yu. M. Yevdokimov, “Magnetic Prop- erties of Copper as a Constituent of Nanobridges Formed between Spatially Fixed Deoxyribonucleic Acid Mole- cules,” JETP Letters, Vol. 81, No. 6, 2005, pp. 327-329. doi:10.1134/1.1931012 [14] D, De Soete, R. Gijbelts and J, Hoste, “Neutron Activa- tion Analysis,” John Wiley and Sons, New York, 1972. [15] S. V. Akulinichev, V. M. Skorkin, V. N. Nikiforov, V. I. Salyanov, A. I. Evseev, O. V. Kondrashina and Yu. M. Yevdokimov, “A New Biomaterial Based on the (DNA- Gd) Complex: 1. Determination of Gadolinium Concen- tration in Particles,” Med. Fizika, Vol. 31, No. 4, 2006, pp. 64- 69. [16] D. Keller and C. J. Bustamante, “Theory of the Interac- tion of Light with Large Inhomogeneous Molecular Ag- gregates,” Journal of Chemical Physics, Vol. 84, 1986, pp. 2972-2979.doi:10.1063/1.450278 [17] M.-H. Kim, L. Ulibarri, D. Keller and C. Bustamante, “The Psi-Type Dichroism of Large Molecular Aggegates. III. Calculations,” Journal of Chemical Physics, Vol. 84, 1986, pp. 2981-2989.doi:10.1063/1.450279 [18] Y. Bouligand, “Cholesteric Order in Biopolymers,” Poly- mer Preprints (A.C.S.), Vol. 18, 1977, pp. 33-38. [19] V. A. Belyakov, V. P Orlov, S. V Semenov, S. G Sku- ridin and Yu. M. Yevdokimov, “Comparison of Calcu- lated and Observed CD spectra of Liquid Crystalline Dis- persions Formed from Double-Stranded DNA and from DNA Complexes with Coloured Compounds,” Liquid Crystals, Vol. 20, No. 6, 1996, pp. 777-784. doi:10.1080/02678299608033172 [20] F. D. Saeva, P. E. Sharpe and G. R Olin, “Сholesteric liquid Crystal Induced Circular Dichroism (LCID) V. Mechanic Aspects of LCID,” Journal of the American Chemical Society, Vol. 95, No. 23, 1973, pp. 7656-7659. doi:10.1021/ja00804a019 [21] H. Zipper, H. Brunner, J. Bernhagen and F. Vitzthum, “Investigation on DNA Intercalation and Surface Binding by SYBR Green I, Its Structure Determination and Meth- dological Implications,” Nucleic Acids Research, Vol. 32, No. 12, 2004, pp. 3-10. doi:10.1093/nar/gnh101 [22] T. Haertle, J. Augustyniak and W. Guschlbauer, “Is Tb3+ Fluorescence Enhancement Only Due to Binding to Sin- gle Stranded Polynucleotides?” Nucleic Acids Research, Vol. 9, No. 22, 1981, pp. 6191-6197. doi:10.1093/nar/9.22.6191 [23] F. E. Rosetto and E. Nieboer, “The Interaction of Metal Ions with Synthetic DNA: Induction of Conformational and Structural Transitions,” Journal of Inorganic Bioche- mistry, Vol. 54, No. 3, 1994, pp. 167-186. doi:10.1016/0162-0134(94)80011-1 [24] V. I. Salyanov, A. I. Evseev, V. I. Popenko, A. A. Gasanov, K. A. Dembo, O. V. Kondrashina, E. V. Shtykova and Yu. M. Yevdokimov, “Gadolinium Complexes of Linear and Liquid-Crystalline DNA,” Biophysics, Vol. 52, No. 3, 2007, pp. 288-292. doi:10.1134/S0006350907030050 [25] D. Gersanovsky, P. Colson, C. Houssier and E. Fredericq, “Terbium (3+) as a Probe of Nucleic Acid Structure: Does It Alter the DNA Conformation in Solution?” Bio- chimica et Biophysica Acta, Vol. 824, No. 4, 1985, pp. 313-325. [26] D. S. Gross and H. Simpkins, “Evidence for Two-Site Binding in the Terbium (III)-Nucleic Acid Interaction,” The Journal of Biological Chemistry, Vol. 256, No. 18, 1981, pp. 9593-9598. [27] S. C. Ha, K. Lowenhaupt, A. Rich, Y. Kim and K. K. Kim, “Crystal Structure of a Junction between B-DNA and Z-DNA Reveals Two Extruded Bases,” Nature, Vol. 437, No. 7062, 2005, pp. 1183-1186. doi:10.1038/nature04088 [28] E. V. Shtykova, V. V. Volkov, V. I. Salyanov and Yu. M. Yevdokimov, “SAXS-Data-Based Structural Modeling of DNA–Gadolinium Complexes Fixed in Particles of Cho- lesteric Liquid-Crystalline Dispersions,” European Bio- physics Journal, Vol. 39, No. 9, 2010, pp. 1313-1322. doi:10.1007/s00249-010-0584-0 [29] Yu. M. Yevdokimov, S. G. Skuridin, Yu. D. Ne- chi- purenko, M. A. Zakharov, V. I. Salyanov, A. A. Kurnosov, V. D. Kuznetsov and V. N. Nikiforov, “Nanoconstructions Based on Double-Stranded Nucleic Acids,” International Journal of Biological Macromolecules, Vol. 36, No. 1-2, 2005, pp. 103-115. doi:10.1016/j.ijbiomac.2005.04.004 [30] D. Grasso, S. Fasone, C. La Rosa and V. Salyanov, “A Calorimetric Study of the Different Thermal Behaviour of DNA in the Isotropic and Liquid-Crystalline States,” Liq- uid Crystals, Vol. 9, No. 2, 1991, pp. 299-305. doi:10.1080/02678299108035507 [31] D. Grasso and R. Gabriele-Campisi, “A DSC Study of The Liquid Crystalline Phases of Salmon Sperm DNA,” Liquid Crystals, Vol. 15, No. 5, 1993, pp. 701-708. doi:10.1080/02678299308036488 [32] Yu. M. Yevdokimov, V. I. Salyanov, E. V. Shtykova, K. A. Dembo, V. V. V. Olkov, P. V. Spirin, A. S. Slusheva and V. S. Prassolov, “A Transition in DNA Molecule’s Spatial Ordering Due to Nano-Scale Structures Changes,” The Open Nanoscience Journal, Vol. 2, 2008, pp. 17-28. doi:10.2174/1874140100802010017 [33] Yu. M. Yevdokimov, “Liquid-Crystalline Forms of DNA  Novel Biomaterial for NCT—“Rigid” Particles of (DNA-Gadolinium) Liquid-Crystalline Dispersions Copyright © 2011 SciRes. JBNB 292 and Their Biological Function,” Liquid Crystals and Their Practical Application, Vol. 3, No. 1, 2003, pp. 10- 47. [34] Yu. M. Yevdokimov, V. I. Salyanov, K. A. Dembo and F. Spener, “Recognition of DNA Molecules and Their Package in Liquid Crystals,” Sensory Systems, Vol. 13, No. 2, 1999, pp. 158-169. [35] L. Li, J. Yang, X. Wu, C. Sun and G. Zhou, “Study on Co-Luminescence Effect of Terbium-Gadolinium-Nucleic Acids-Cetylpyridine Bromide System,” Journal of Lumi- nescence, Vol. 101, No. 1-2, 2003, pp. 141-146. doi:10.1016/S0022-2313(02)00406-4 [36] Yu. M. Yevdokimov, V. I. Salyanov, O. V. Kondrashina, A. A. Gasanov, E. V. Shtykova and K. A. Dembo, “Rare- Earth- Cation-Induced Change in the Cholesteric Twist- ing of Neighboring Nucleic Acid Molecules,” Journal of Experimental and Theoretical Physics, Vol. 104, No. 3, 2007, pp. 499-507.doi:10.1134/S1063776107030168 [37] P. A. Lessing and A. W. Erickson, “Synthesis and Char- acterization of Gadolinium Phosphate Neutron Absorber,” Journal of the European Ceramic Society, Vol. 23, No. 16, 2003, pp. 3049-3057. doi:10.1016/S0955-2219(03)00100-6 [38] R. J. Mumper and M. Jay, “Formation and Stability of Lanthanide Complexes and Their Encapsulation Into Poly- meric Microspheres,” The Journal of Physical Chemistry, Vol. 96, No. 21, 1992, pp. 8626-8631. doi:10.1021/j100200a076 [39] Y-H. Qi, Q.-Y. Zhang and L. Xu, “Correlation Analysis of the Structure and Stability Constants of Gadolinium (III) Complexes,” Journal of Chemical Information and Computer Sciences, Vol. 42, No. 6, 2002, pp. 1471-1475. doi:10.1021/ci020027x [40] Y. Akine, N .Tokita, K. Tokuuye, M. Satoh, H. Churei, C. Le Pechoux, T. Kobayashi and K. Kanda, “Suppression of Rabbit VX-2 Subcutaneous Tumor Growth by Gado- linium Neutron Capture Therapy,” Japanese Journal of Cancer Research, Vol. 84, No. 8, 1993, pp. 841-843. [41] R. M. Brugger and J.-L. A. Shin, “Evaluation of Gado- linium–157 as a Neutron Capture Therapy Agent,” Strahlentherapie und Onkologie, Vol. 165, No. 2-3, 1989, pp. 153-156. [42] R. F. Martin, G. D’Cunha, M. Pardee and B. J. Allen, “Induction of Double-Strand Breaks Following Neutron Capture by DNA-Bound 157Gd,” International Journal of Radiation Biology, Vol. 54, No. 2, 1988, pp. 205-208. doi:10.1080/09553008814551641 [43] R. F. Martin, G. D’Cunha, M. Pardee and B. J Allen, “Induction of DNA Double-Strand Breaks by 157Gd Neu- tron Capture,” Pigment Cell Research, Vol. 2, No. 4, 1989, pp. 330-332. doi:10.1111/j.1600-0749.1989.tb00213.x [44] T. Goortley, R. Zamenhof and H. Nikjoo, “Calculated DNA Damage from Gadolinium Auger Electron and Re- lation to Dose Distribution in a Head Phantom,” Interna- tional Journal of Radiation Biology. Vol. 80, No. 11-12, 2004, pp. 933-940. doi:10.1080/09553000400017564 [45] S. V. Akulinichev, Yu. M. Yevdokimov, D. B. Labeznik, M. L. Plyaschkyavich, A. I. Evseev, V. I. Salyanov and B. M. Skorkin, “Biomaterial Based on Particles of the Cho- lesteric Liquid-Crystalline Dispersions of the (DNA-Gd) Complexes. II. Secondary Irradiation after Neutron Cap- ture in Particles,” Med. Fizika, (in Russian), Vol. 32, No. 3, 2006, pp. 54-58. [46] J. T. Masiakowski, J. L. Horton and L. J. Peters, “Gado- linium Neutron Capture Therapy for Brain Tumors: A Computer Study,” Medical Physics, Vol. 19, No. 5, 1992, pp. 1277-1284. doi:10.1118/1.596761 [47] T. Matsumoto, “Transport Calculations of Depth-Dose Distributions for Gadolinium Neutron Capture Therapy,” Physics in Medicine & Biology, Vol. 37, No. 1, 1992, pp. 155-162. doi:10.1088/0031-9155/37/1/010 |

