Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 311-317 doi:10.4236/jbnb.2011.23038 Published Online July 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 311 Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Hossein Hosseinzadeh1*, Mina Sadeghzadeh2, Mirzaagha Babazadeh2 1Chemistry Department, Payame Noor University, Tehran, Iran; 2Department of Chemistry, Science Faculty, Islamic Azad Univer- sity, Tabriz Branch, Tabriz, Iran. Email: *h_hoseinzadeh@pnu.ac.ir Received December 3rd, 2010; revised March 20th 2011; accepted April 21st, 2011. ABSTRACT A novel biopolymer-based superabsorbent hydrogel composite based on kappa-carrageenan (κC) have been prepared via graft copolymerization of acrylic acid (AA) in the presence of bentonite powder using methylenebisacrylamide (MBA) as a crosslinking agent and ammonium persulfate (APS) as an initiator. The hydrogel structure was confirmed using FTIR spectroscopy and the morphology of the samples was examined by scanning electron microscopy (SEM). The affecting variables onto graft polymerization (i.e. AA, MBA and APS concentration, as well as the bentonite amount) were systematically optimized to achieve a hydrogel with swelling capacity as high as possible. The results of Brun- auer-Emmett-Teller (BET) analysis showed that the average pore diameter of the synthesized hydrogel was 11.5 nm. The effect of various salt media and solutions with different pHs on the swelling of the superabsorbent was also studied. Keywords: Carrageenan, Bentonite, Poly(Acrylic Acid), Hydrogel, Composite 1. Introduction Polymer hydrogels, as a sort of unique soft materials, have attracted more and more attention because of their academic importance and special applications [1,2]. Su- perabsorbent polymers (SAPs) are special soft and pli- able polymeric materials that can be absorb large quanti- ties of water, saline or physiological solutions while the absorbed solutions are not removable even under pres- sure [3,4]. Because of their superior properties, they have found extensive applications such as disposable diapers, feminine napkins, drug delivery systems, and soil for agriculture and horticulture [5-7]. For the majority of applications, the hydrogels have to possess high absorption capacity and elevated swelling rate and show a strong swollen gel. Hydrogels with high mechanical strength are required in some applications such as artificial cartilage [8,9], controlled drug delivery [10], hygiene and agricultural uses [1]. The gel strength can be improved through different methods such as sur- face crosslinking [1,11-13] and creating composite [14- 16] and nanocomposite structures [17-20]. Because of their exceptional properties, i.e. biocom- patibility, biodegradability, renewability, and non-toxicity, polysaccharides are the main part of the natural-based superabsorbent hydrogels [21-24]. The higher production cost and low gel strength of these superabsorbents, how- ever, restrict their application widely. To improve these limitations, inorganic compounds with low cost can be used. The introduction of inorganic fillers to a polymer matrix increases its strength and stiffness properties. Among inorganic compounds, special attention has been paid to clay minerals in the field of nanocomposites be- cause of their small particle size and intercalation proper- ties. Mineral powders are hydrated layered aluminosili- cate with reactive –OH groups on the surface. The inter- action of mineral powders, reactive site of natural poly- mers and monomers results in a superabsorbent compos- ite. Superabsorbent composites based on synthetic poly- mers [15,25] or natural polymers [26,27] have been re- ported. Carrageenan is a collective term for linear sulfated polysaccharides that are obtained commercially by alka- line extraction of certain species of red seaweeds [28]. Schematic diagram of the idealized structure of the re- peat units for the most well-known and most important type of carrageenan family, kappa-carrageenan (κC), are  Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 312 presented in Figure 1. The presence of hydrophilic sul- fate groups with high ionization tendency and less sensi- tivity to salt solution was our main idea for synthesis of carrageenan-based superabsorbent hydrogels. Moreover high viscosity of carrageenan solutions de- creased diffusion of molecular oxygen in the reaction mix- ture that consequently decreased the inhibiting effect of the oxygen in free radical polymerization process. This fact has already been observed in the case of other poly- saccharides [29,30]. Therefore we were able to apply crosslinking graft copolymerization reaction under at- mospheric conditions which causes brevity and simplic- ity in industrial processes. In this work, we attempt to synthesize and characterize new superabsorbent composites based on kappa-carra- geenan (κC) in the presence of bentonite particles. The preparation of the biopolymer-based superabsorbent composites can also improve the mechanical properties of materials and can lower the cost of the finished prod- uct compared with the synthetic counterparts as well as providing biodegradable characteristics. The reaction variables affecting the water absorbency of the κC-g- poly(acrylic acid)/bentonite as well as the salt- and pH- sensitivity of the hydrogels were investigated. 2. Experimental The polysaccharide, κC (chemical grade, MW 50000, from Condinson Co., Denmark) was used without further purification. Acrylic acid (from Merck) was used after vacuum distillation. MBA (from Fluka), APS (from Merck) and bentonite (from Aldrich, particle size < 5 µm) were used as received. All other chemicals were of ana- lytical grade. 2.1. Superabsorbent Composite Synthesis A pre-weighed amount of of κC (0.30 - 0.70 g) was added to 40 mL degassed distilled water in a 1-L reactor equipped with a mechanical stirrer (RZR 2021, a three- blade propeller type, Heidolph, Schwabach, Germany) and stirred for 10 min. The reactor was placed in a ther- mostated water bath to control the reaction temperature at 80˚C. After complete dissolution of κC, various amounts of bentonite powder (0.70 - 0.30 g) were added to the κC solution and allowed to stir for 10 min. Then, APS ini- tiator (0.05 - 0.50 g, dissolved in 5 mL water) was added to the reaction mixture and the mixture was stirred for 10 min. MBA (0.05 - 0.25 g, dissolved in 5 mL water) and AA (1.0 - 5.0 g, completely neutralized with NaOH) were poured into the reactor. All of the reactions were carried out at 80˚C under an argon gas atmosphere. At the end of the propagation reaction, the gelly product was poured in ethanol (300 ml) and allowed to dewater for 24 h. Then, the product was filtered and washed with 100 O O OH OOH O OH O O3SO - Figure 1. Repeating disaccharide units of kappa-carrageenan (κC). mL ethanol. The filtered product was dried in an oven at 50˚C for 10 h. After grinding, the powdered superabsor- bent composite was stored away from moisture, heat and light. 2.2. Swelling Measurements An accurately weighed sample (0.2 ± 0.001 g) of the powdered superabsorbent with average particle sizes between 40 - 60 mesh (250 - 350 μm) was immersed in distilled water (200 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capac- ity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method and using the following formula: Weight of swollen gel Weight of dried gel Weight of dried gel gg (1) 3. Results and Discussion 3.1. Synthesis and Characterization The hydrogel composite was prepared by graft copoly- merization of acrylic acid onto κC in the presence of a crosslinking agent and powdery bentonite (Scheme 1). Ammonium persulfate was used as an initiator. The per- sulfate is decomposed under heating and produced sul- fate anion-radicals that abstract hydrogen from –OH groups of κC backbones. So, this persulfate-saccharide redox system results in active centers capable to radically initiate polymerization of acrylic acid led to a graft co- polymer. Since a crosslinking agent, e.g. MBA, is pre- sented in the system, the copolymer comprises a cross- linked structure. FTIR spectroscopy was used for identification of the hydrogel. In Figure 2, (a) represents the spectrum of the physical mixture of κC and bentonite. In the layer silicate structure of bentonite, the hydroxyl groups show absorp- tion bands at 3630 - 3680 cm–1. The broad band at 3200 - 3400 cm–1 is due to stretching of –OH groups of the poly- saccharide. In the spectrum of the composite ((b) in Fig- ure 2), two new absorption peaks at 1579 and 1722 cm–1 are appeared. The characteristic band at 1572 cm–1 is due to C=O asymmetric stretching in carboxylate anion that is reconfirmed by another peak at 1410 cm–1 which is re- lated to the symmetric stretching mode of the carboxylate groups. The absorption band at 1722 cm–1 can be corres- 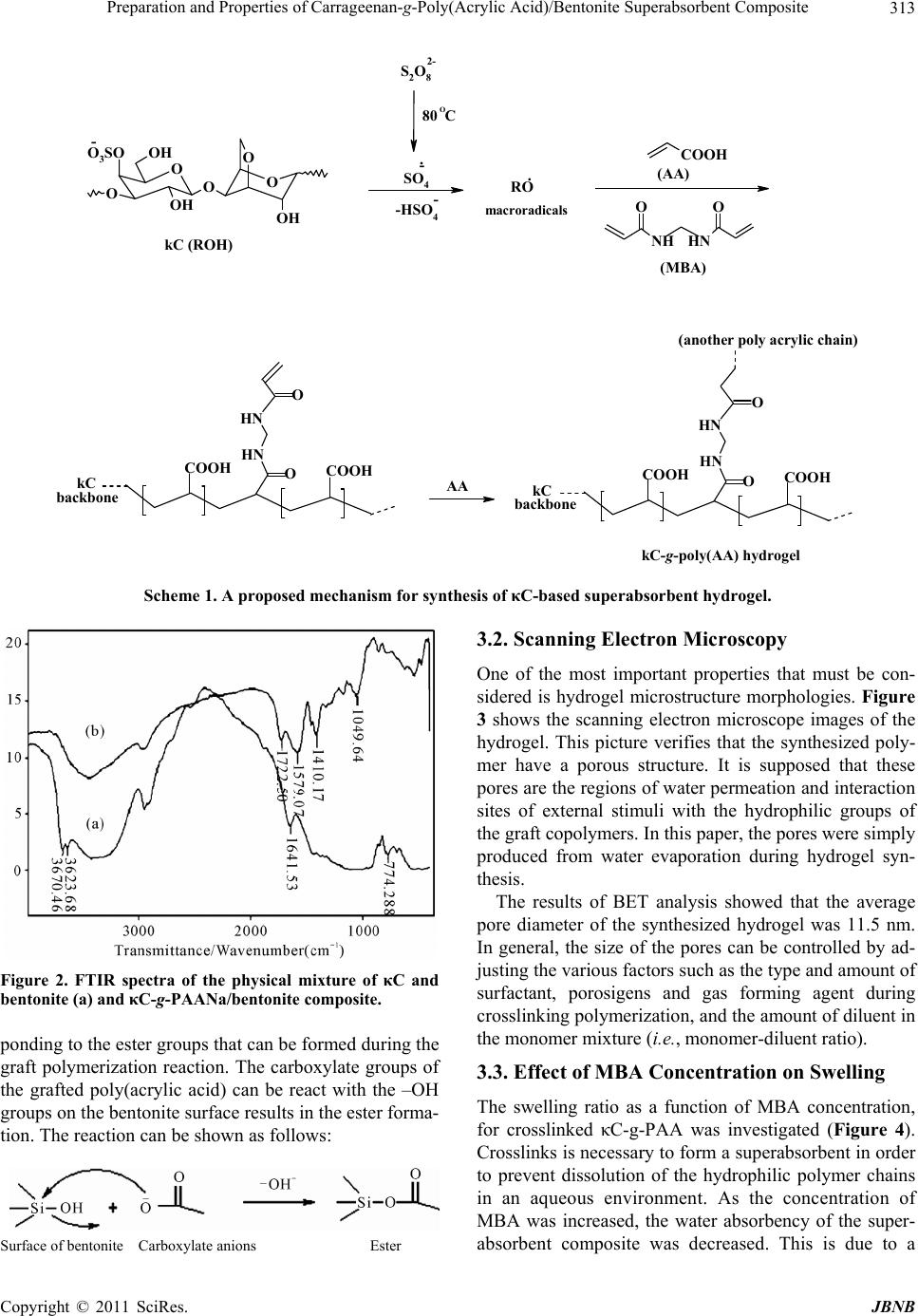 Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 313 NH OO HN (MBA) COOH (AA) COOH O NH HN O NH HN (another poly acrylic chain) OO backbone kC backbon e AA COOH kC O O OH OOH O OH O O3SO - SO4 . - S2O8 -HSO4 . - kC (ROH) RO OC COOH COOH macroradicals kC-g-poly(AA) hydrogel 2- 80 Scheme 1. A proposed mechanism for synthesis of κC-based superabsorbent hydrogel. Figure 2. FTIR spectra of the physical mixture of κC and bentonite (a) and κC-g-PAANa/bentonite composite. ponding to the ester groups that can be formed during the graft polymerization reaction. The carboxylate groups of the grafted poly(acrylic acid) can be react with the –OH groups on the bentonite surface results in the ester forma- tion. The reaction can be shown as follows: Surface of bentonite Carboxylate anions Ester 3.2. Scanning Electron Microscopy One of the most important properties that must be con- sidered is hydrogel microstructure morphologies. Figure 3 shows the scanning electron microscope images of the hydrogel. This picture verifies that the synthesized poly- mer have a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers. In this paper, the pores were simply produced from water evaporation during hydrogel syn- thesis. The results of BET analysis showed that the average pore diameter of the synthesized hydrogel was 11.5 nm. In general, the size of the pores can be controlled by ad- justing the various factors such as the type and amount of surfactant, porosigens and gas forming agent during crosslinking polymerization, and the amount of diluent in the monomer mixture (i.e., monomer-diluent ratio). 3.3. Effect of MBA Concentration on Swelling The swelling ratio as a function of MBA concentration, for crosslinked κC-g-PAA was investigated (Figure 4). Crosslinks is necessary to form a superabsorbent in order to prevent dissolution of the hydrophilic polymer chains in an aqueous environment. As the concentration of MBA was increased, the water absorbency of the super- absorbent composite was decreased. This is due to a 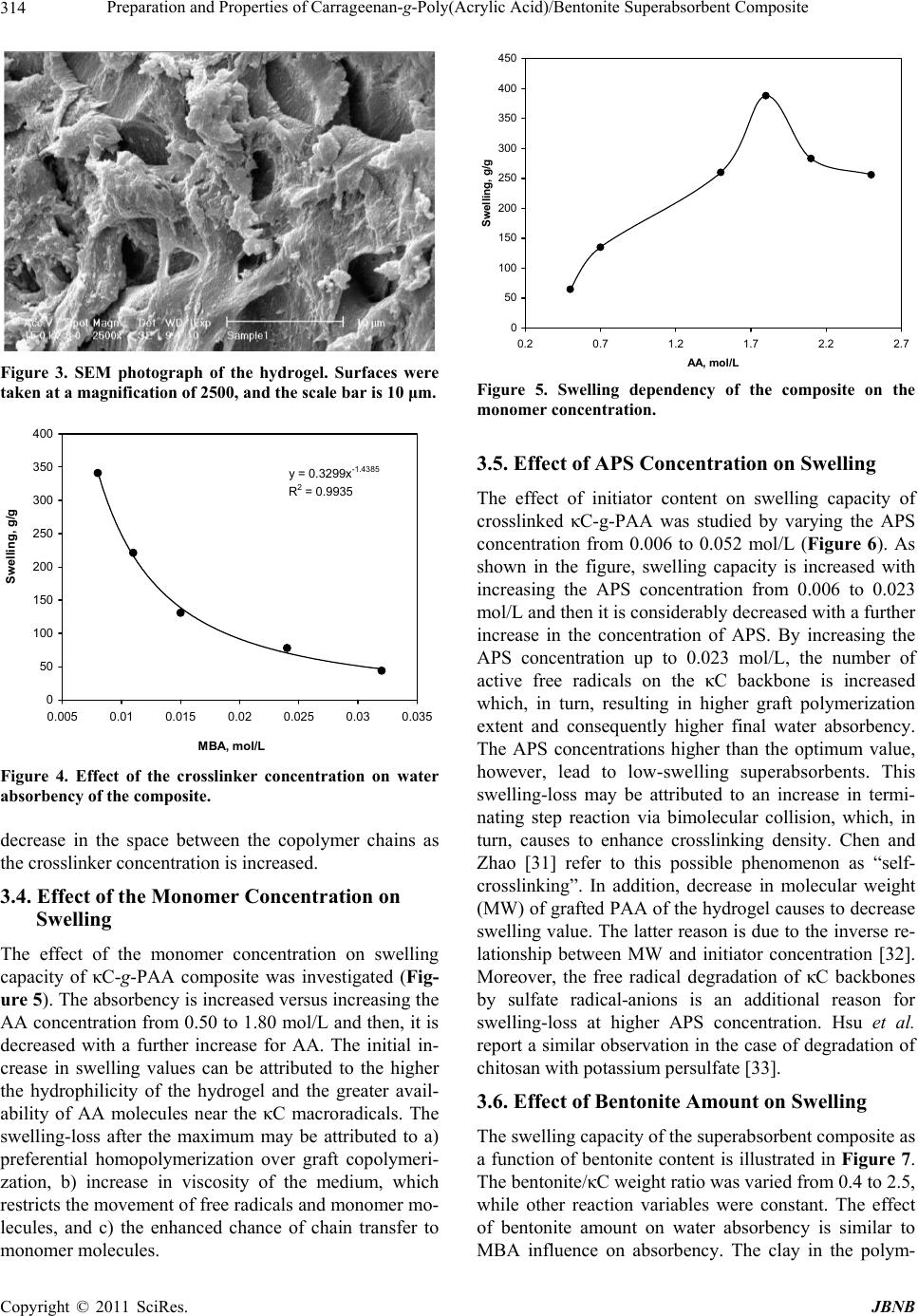 Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 314 Figure 3. SEM photograph of the hydrogel. Surfaces were taken at a magnification of 2500, and the scale bar is 10 μm. y = 0.3299x -1.4385 R 2 = 0.9935 0 50 100 150 200 250 300 350 400 0.005 0.01 0.015 0.02 0.025 0.03 0.035 MBA, mol/L Swelling, g/g Figure 4. Effect of the crosslinker concentration on water absorbency of the composite. decrease in the space between the copolymer chains as the crosslinker concentration is increased. 3.4. Effect of the Monomer Concentration on Swelling The effect of the monomer concentration on swelling capacity of κC-g-PAA composite was investigated (Fig- ure 5). The absorbency is increased versus increasing the AA concentration from 0.50 to 1.80 mol/L and then, it is decreased with a further increase for AA. The initial in- crease in swelling values can be attributed to the higher the hydrophilicity of the hydrogel and the greater avail- ability of AA molecules near the κC macroradicals. The swelling-loss after the maximum may be attributed to a) preferential homopolymerization over graft copolymeri- zation, b) increase in viscosity of the medium, which restricts the movement of free radicals and monomer mo- lecules, and c) the enhanced chance of chain transfer to monomer molecules. 0 50 100 150 200 250 300 350 400 450 0.2 0.7 1.2 1.7 2.2 2.7 A A , mol/L Swelling, g/g Figure 5. Swelling dependency of the composite on the monomer concentration. 3.5. Effect of APS Concentration on Swelling The effect of initiator content on swelling capacity of crosslinked κC-g-PAA was studied by varying the APS concentration from 0.006 to 0.052 mol/L (Figure 6). As shown in the figure, swelling capacity is increased with increasing the APS concentration from 0.006 to 0.023 mol/L and then it is considerably decreased with a further increase in the concentration of APS. By increasing the APS concentration up to 0.023 mol/L, the number of active free radicals on the κC backbone is increased which, in turn, resulting in higher graft polymerization extent and consequently higher final water absorbency. The APS concentrations higher than the optimum value, however, lead to low-swelling superabsorbents. This swelling-loss may be attributed to an increase in termi- nating step reaction via bimolecular collision, which, in turn, causes to enhance crosslinking density. Chen and Zhao [31] refer to this possible phenomenon as “self- crosslinking”. In addition, decrease in molecular weight (MW) of grafted PAA of the hydrogel causes to decrease swelling value. The latter reason is due to the inverse re- lationship between MW and initiator concentration [32]. Moreover, the free radical degradation of κC backbones by sulfate radical-anions is an additional reason for swelling-loss at higher APS concentration. Hsu et al. report a similar observation in the case of degradation of chitosan with potassium persulfate [33]. 3.6. Effect of Bentonite Amount on Swelling The swelling capacity of the superabsorbent composite as a function of bentonite content is illustrated in Figure 7. The bentonite/κC weight ratio was varied from 0.4 to 2.5, while other reaction variables were constant. The effect of bentonite amount on water absorbency is similar to MBA influence on absorbency. The clay in the polym- 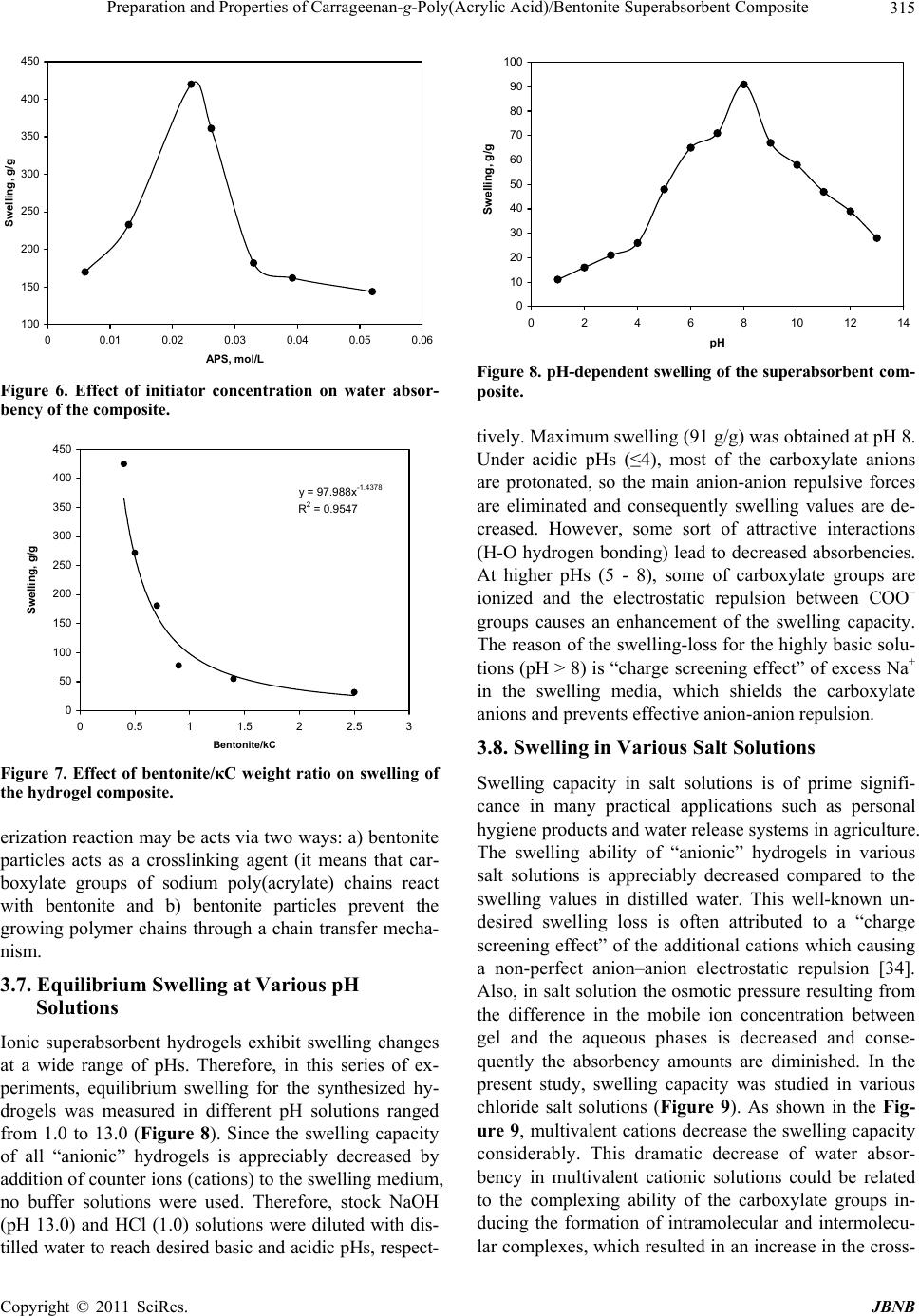 Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 315 100 150 200 250 300 350 400 450 00.01 0.02 0.03 0.04 0.05 0.06 APS, mol/L Swelling, g/g Figure 6. Effect of initiator concentration on water absor- bency of the composite. y = 97.988x -1.4378 R 2 = 0.9547 0 50 100 150 200 250 300 350 400 450 00.511.522.53 Bentonite/kC Swelling, g/g Figure 7. Effect of bentonite/κC weight ratio on swelling of the hydrogel composite. erization reaction may be acts via two ways: a) bentonite particles acts as a crosslinking agent (it means that car- boxylate groups of sodium poly(acrylate) chains react with bentonite and b) bentonite particles prevent the growing polymer chains through a chain transfer mecha- nism. 3.7. Equilibrium Swelling at Various pH Solutions Ionic superabsorbent hydrogels exhibit swelling changes at a wide range of pHs. Therefore, in this series of ex- periments, equilibrium swelling for the synthesized hy- drogels was measured in different pH solutions ranged from 1.0 to 13.0 (Figure 8). Since the swelling capacity of all “anionic” hydrogels is appreciably decreased by addition of counter ions (cations) to the swelling medium, no buffer solutions were used. Therefore, stock NaOH (pH 13.0) and HCl (1.0) solutions were diluted with dis- tilled water to reach desired basic and acidic pHs, respect- 0 10 20 30 40 50 60 70 80 90 100 02468101214 pH Swelling, g/g Figure 8. pH-dependent swelling of the superabsorbent com- posite. tively. Maximum swelling (91 g/g) was obtained at pH 8. Under acidic pHs (≤4), most of the carboxylate anions are protonated, so the main anion-anion repulsive forces are eliminated and consequently swelling values are de- creased. However, some sort of attractive interactions (H-O hydrogen bonding) lead to decreased absorbencies. At higher pHs (5 - 8), some of carboxylate groups are ionized and the electrostatic repulsion between COO– groups causes an enhancement of the swelling capacity. The reason of the swelling-loss for the highly basic solu- tions (pH > 8) is “charge screening effect” of excess Na+ in the swelling media, which shields the carboxylate anions and prevents effective anion-anion repulsion. 3.8. Swelling in Various Salt Solutions Swelling capacity in salt solutions is of prime signifi- cance in many practical applications such as personal hygiene products and water release systems in agriculture. The swelling ability of “anionic” hydrogels in various salt solutions is appreciably decreased compared to the swelling values in distilled water. This well-known un- desired swelling loss is often attributed to a “charge screening effect” of the additional cations which causing a non-perfect anion–anion electrostatic repulsion [34]. Also, in salt solution the osmotic pressure resulting from the difference in the mobile ion concentration between gel and the aqueous phases is decreased and conse- quently the absorbency amounts are diminished. In the present study, swelling capacity was studied in various chloride salt solutions (Figure 9). As shown in the Fig- ure 9, multivalent cations decrease the swelling capacity considerably. This dramatic decrease of water absor- bency in multivalent cationic solutions could be related to the complexing ability of the carboxylate groups in- ducing the formation of intramolecular and intermolecu- lar complexes, which resulted in an increase in the cross-  Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 316 0 10 20 30 40 50 60 70 80 NaClCaCl2 AlCl3 Swelling, g/g Figure 9. Swelling capacity of the hydrogel in different chloride salt solutions (0.15 M). linking density of the network [35]. 4. Conclusions In the present study, a novel superabsorbent composite based on κC was prepared by graft copolymerization of AA in the presence of a crosslinking agent. The resultant superabsorbent composite had a large degree of water absorbency. The study of FTIR spectra shows that in the composite spectrum a new absorption band at 1722 cm–1 was appeared that attributed to the ester formation from replacement of hydroxyl groups of bentonite with grafted carboxylate anions onto polysaccharide backbones. The effect of the bentonite amount and MBA concentration showed that with increasing of these parameters, the wa- ter absorbency of the superabsorbent composite are de- creased. The swelling of hydrogel exhibited high sensitivity to pH. Study of effect of H+/OH– concentration carried out at various pHs shows that the swelling of hydrogel causes several large volume changes. So, we investigated the pH-sensitivity of the hydrogel. Ionic repulsion between charge groups incorporated in the gel matrix by an ex- ternal pH modulation could be assumed as the main driv- ing force responsible for such abrupt swelling changes. This superabsorbent network intelligently responding to pH may be considered as an excellent candidate to design novel drug delivery systems. Also swelling measurement of the synthesized com- posites in different salt solutions showed appreciable swelling capacity, especially in NaCl solution. This be- havior is may be due to anti-salt characteristics of the carrageenan part sulfate groups of the superabsorbing networks. Overall, we report a crosslinking polymerization to achieve superabsorbing composite materials with lower cost and lower salt-sensitivity. The hydrogel composites will most probably posses higher biodegradability (due to the κC part) and higher swollen gel strength (due to the inorganic parts). The latter properties are of the subjects under consideration in our laboratory. REFERENCES [1] O. V. Khutoryanskaya, Z. A. Mayeva, G. A. Mun and V. V. Khutoryanskiy, “Designing Temperature-Responsive Biocompatible Copolymers and Hydrogels Based on 2- Hydroxyethyl(meth)acrylates,” Biomacromolecules, Vol. 9, No. 12, 2008, pp. 3353-3361. doi:10.1021/bm8006242 [2] W. E. Hennink and C. F. van Nostrum, “Novel Crosslink- ing Methods to Design Hydrogels,” Advanced Drug De- livery Reviews, Vol. 54, No. 1, 2002, pp. 13-36. doi:10.1016/S0169-409X(01)00240-X [3] F. L. Buchholz and A. T. Graham, “Modern Superabsor- bent Polymer Technology,” Wiley, New York, 1997. [4] L. B. Peppas and R. S. Harland, “Absorbent Polymer Technology,” Elsevier, Amsterdam, 1990. [5] R. Po, “Water-Absorbent Polymers: A Patent Survey,” Journal of Macromolecular Science—Reviews in Macro- molecular, Vol. 34, No. 4, 1994, pp. 607-662. [6] J. Kost, “Encyclopedia of Controlled Drug Delivery,” E. Mathiowitz, Ed., Wiley, New York, 1999, p. 445. [7] N. A. Peppas and A. G. Mikes, “Hydrogels in Medicine and Pharmacy,” CRC Press, Boca Raton, Vol. 1, 1986. [8] J. P. Fisher, S. Jo, A. G. Mikos and A. H. Reddi, “Ther- moreversible Hydrogel Scaffolds for Articular Cartilage Engineering,” Journal of Biomedical Materials Research, Vol. 71A, No. 2, 2004, pp. 268-274. doi:10.1002/jbm.a.30148 [9] S. J. Bryant, K. L. Durnad and K. S. Anseth, “Manipula- tions in Hydrogel Chemistry Control Photoencapsulated Chondrocyte Behavior and Their Extracellular Matrix Production,” Journal of Biomedical Materials Research, Vol. 67A, No. 4, 2003, pp. 1430-1438. doi:10.1002/jbm.a.20003 [10] H. Omidian, J. G. Rocca and K. Park, “Advances in Su- perporous Hydrogels,” Journal of Controlled Release, Vol. 102, No. 2, 2005, pp. 3-12. doi:10.1016/j.jconrel.2004.09.028 [11] S. J. Smit, “Superabsorbent Polymer Having Improved Absorption Rate and Absorption Under Pressure,” US Pa- tent 5399591, 1995. [12] R. Chambers, “Superabsorbent Polymers and Products Therefrom,” US Patent 5597873, 1997. [13] A. Mitchell, “Method of Manufacturing a Semiconductor Device,” US Patent 6376318, 2002. [14] K. Kabiri and M. J. Zohuriaan-Mehr, “Superabsorbent Hydrogel Composites,” Polymers for Advanced Technolo- gies, Vol. 14, No. 6, 2003, pp. 438-444. doi:10.1002/pat.356 [15] J. Lin, J. Wu, Z. Yang and M. Pu, “Synthesis and Proper- ties of Poly(acrylic acid)/Mica Superabsorbent Nano- composite,” Macromolecular Rapid Communications, Vol.  Preparation and Properties of Carrageenan-g-Poly(Acrylic Acid)/Bentonite Superabsorbent Composite Copyright © 2011 SciRes. JBNB 317 22, No. 6, 2001, pp. 422-424. doi:10.1002/1521-3927(20010301)22:6<422::AID-MAR C422>3.0.CO;2-R [16] J. Wu, J. Lin, G. Lin and C. Wei, “Influence of the COOH and COONa Groups and Crosslink Density of Poly(Acrylic Acid)/Montmorillonite Superabsorbent Composite on Water Absorbency,” Polymer International, Vol. 50, No. 9, 2001, pp. 1050-1053.doi:10.1002/pi.728 [17] K. Haraguchi, T. Takenisa and S, Fan S, “Effects of Clay Content on the Properties of Nanocomposite Hydrogels Composed of Poly(N-isopropylacrylamide) and Clay,” Macromolecules, Vol. 35, No. 27, 2002, pp. 10162-10171. doi:10.1021/ma021301r [18] K. Haraguchi and T. Takenisa, “Nanocomposite Hy- drogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/ Deswelling Properties,” Advanced Materials, Vol. 14, No. 16, 2002, pp. 1120-1128. doi:10.1002/1521-4095(20020816)14:16<1120::AID-AD MA1120>3.0.CO;2-9 [19] Y. Liu, M. Zhu, X. Liu, W. Zhang, B. Sun and Y. Chen, “Synthesis and Characterization of Novel Monomers and Polymers Containing Chiral (−)-Menthyl Groups,” Poly- mer, Vol. 47, No. 14, 2006, pp. 4901-4908. doi:10.1016/j.polymer.2006.05.043 [20] K. Xu, J. Wang, S. Xiang, Q. Chen, Y. Yue and X. Su, “Poly-Ampholytes Superabsorbent Nanocomposites with Excellent Gel Strength,” Composites Science and Tech- nology, Vol. 67, No. 15, 2007, pp. 3480-3486. doi:10.1016/j.compscitech.2007.02.009 [21] V. D. Athawale and V. Lele, “Graft Copolymerization Onto Starch. 3: Grafting of Acrylamide Using Ceric Ion Initiation and Preparation of Its Hydrogels,” Starch/ Starke, Vol. 50, No. 10, 1998, pp. 426-432. doi:10.1002/(SICI)1521-379X(199810)50:10<426::AID- STAR426>3.0.CO;2-# [22] D. W. Lim, H. S. Whang and K. J. Yoon, “Synthesis and Absorbency of a Superabsorbent from Sodium Starch Sulfate-g-Polyacrylonitrile,” Journal of Applied Polymer Science, Vol. 79, No. 8, 2003, pp. 1423-1430. doi:10.1002/1097-4628(20010222)79:8<1423::AID-APP 90>3.0.CO;2-V [23] Y. Sugahara and O. Takahisa, “Synthesis of Starch-Graft- Polyacrylonitrile Hydrolyzate and Its Characterization,” Journal of Applied Polymer Science, Vol. 82, No. 6, pp. 1437-1443. doi:10.1002/app.1981 [24] J. M. Joshi and S.V. Kumar, “Graft Copolymerization of 2-Hydroxyethylmethacrylate onto Carboxymethyl Chito- san Using CAN as an Initiator,” Polymer, Vol. 47, No. 6, 2006, pp. 2198-2204. doi:10.1016/j.polymer.2005.11.050 [25] J. Lin, J. Wu, Z. Yang and M. Pu, “Synthesis and Proper- ties of Poly(Acrylic Acid)/Montmorilonite Superabsorbent Composites,” Polymers and Polymer Composites, Vol. 9, No. 7, 2001, pp. 469-471. [26] J. Wu, J. Lin, M. Zhou and C. Wei, “Synthesis and Prop- erties of Starch-Graft-Polyacrylamide/Clay Superabsor- bent Composite,” Macromolecular Rapid Communica- tions, Vol. 21, No. 15, 2000, pp. 1032-1034. doi:10.1002/1521-3927(20001001)21:15<1032::AID-MA RC1032>3.0.CO;2-N [27] J. Wu, Y. Wei, J. Lin and S. Lin, “Study on Starch-g- Acrylamide/Mineral Powder Superabsorbent Composite,” Polymer, Vol. 44, No. 21, 2003, pp. 6513- 6520. doi:10.1016/S0032-3861(03)00728-6 [28] R. E. Kirk and D. F. Othmer, “Encyclopedia of Chemical Technology,” 4th Edition, John Wiley & Sons, New York, 1992, Vol. 4, p. 942. [29] A. Pourjavadi and M. J. Zohuriaan-Mehr, “Modification of Carbohydrate Polymers via Grafting in Air. 1. Ceric-Induced Synthesis of Starch-g-Polyacrylonitrile in Presence and Absence of Oxygen,” Starch/Starke, Vol. 54, No. 3-4, 2002, pp. 140-147. doi:10.1002/1521-379X(200204)54:3/4<140::AID-STAR 140>3.0.CO;2-I [30] A. Pourjavadi and M. J. Zohuriaan-Mehr, “Modification of Carbohydrate Polymers via Grafting in Air. 2. Ceric- Initiated Graft Copolymerization of Acrylonitrile onto Natural and Modified Polysaccharides,” Starch/ Starke, Vol. 54, No. 10, 2002, pp. 482-488. doi:10.1002/1521-379X(200210)54:10<482::AID-STAR 482>3.0.CO;2-I [31] J. Chen and Y. Zhao, “Relationship between Water Ab- sorbency and Reaction Conditions in Aqueous Solution Polymerization of Polyacrylate Superabsorbents,” Jour- nal of Applied Polymer Science, Vol. 75, No. 6, 2000, pp. 808-814. doi:10.1002/(SICI)1097-4628(20000207)75:6<808::AID- APP10>3.0.CO;2-3 [32] J. Branrup and E. H. Immergut, “Polymer Handbook,” 3rd Edition, New York, Wiley, 1989. [33] S. C. Hsu, T. M. Don and W. Y. Chiu, “Free Radical Degradation of Chitosan with Potassium Persulfate,” Polymer Degradation and Stability, Vol. 75, No. 1, 2002, pp. 73-83.doi:10.1016/S0141-3910(01)00205-1 [34] P. J. Flory, “Principles of Polymer Chemistry,” Cornell University Press, New York, Ithaca, 1953. [35] D. Castal, A. Ricard and R. Audebert, “Swelling of Ani- onic and Cationic Starch-Based Superabsorbents In Water and Saline Solution,” Journal of Applied Polymer Science, Vol. 39, No. 1, 1990, pp. 11-29. doi:10.1002/app.1990.070390102 |

