Journal of Water Resource and Protection
Vol.07 No.09(2015), Article ID:57897,10 pages
10.4236/jwarp.2015.79060
Potential of Magnesium Chloride for Nutrient Rejection in Forward Osmosis
Yatnanta Padma Devia1,2*, Tsuyoshi Imai1, Takaya Higuchi1, Ariyo Kanno3, Koichi Yamamoto3, Masahiko Sekine3, Tuan Van Le4
1Division of Environmental Science and Engineering, Graduate School of Science and Engineering, Yamaguchi University, Ube-shi, Japan
2Department of Civil Engineering, Brawijaya University, Malang, Indonesia
3Division of Civil and Environmental Engineering, Graduate School of Science and Engineering, Yamaguchi University, Ube-shi, Japan
4Department of Environmental Science, Hue University of Science, Hue City, Vietnam
Email: *yatnanta@yahoo.com, imai@yamaguchi-u.ac.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 3 June 2015; accepted 10 July 2015; published 13 July 2015
ABSTRACT
Wastewater may contain high levels of the nutrients: nitrogen and phosphorus. Excessive release of nutrients to the environment can cause severe environmental problem such as eutrophication leading to algal blooms, oxygen deficiency, and fish kills. The forward osmosis (FO) could be a choice of treatment. FO process presents the results of using four kinds of variation in concentration of magnesium chloride (MgCl2) as draw solution and the two kinds of commercial membranes for nutrient rejection in the same cross flow velocity at 0.25 m/s and temperature at 25˚C. Nutrients consisting of nitrogen (nitrite, nitrate, and ammonium) and phosphorus (phosphate) in feed solution were successfully rejected with an efficiency of mostly more 95%. The water flux in membrane HTI-NW achieved lower 7.55 - 9.61 L/m2・hr than in membrane HTI-ES that exceeds until 13.58 - 15.10 L/m2・hr. The reverse solute in membrane HTI-NW is seemly constant along all concentration of DS MgCl2 that the chloride diffusion is slightly higher than magnesium. In membrane HTI-ES, the reverse solute of chloride was almost three times than that of magnesium. The concentration of MgCl2 plays a significant role in rejecting nutrients by the Donnan’s potential and the diffusion constant in low and high concentration of DS, respectively.
Keywords:
Forward Osmosis, Magnesium Chloride, Nutrient

1. Introduction
Forward osmosis (FO) membrane technology research has grown remarkably in the last decade. In FO, solutions of lower and higher osmotic pressure potential are named feed solution (FS) and draw solution (DS), respectively. Natural osmotic difference drives water from FS to DS through a membrane. FO uses a semi-permeable membrane to separate water from feed solute effectively. A selectively or semi-permeable membrane allows passage of water, but rejects solute molecules or ions [1] . The DS and membrane should be optimized to increase the efficiency and to decrease concentration polarization [2] . The concentration polarization is caused by the concentration difference between the FS and DS troughs across an FO membrane [3] . The concentration polarization arises as the water flux in FO has an opposite direction to the reverse solute flux [4] . According to Ge et al. (2013), the resolution of high efficiency FO membranes and suitable DS, related to the molecular solution, is required [5] .
This research utilized MgCl2 as a molecular solution DS for the following reasons. MgCl2 has a relatively high osmotic pressure that has been tested for the prediction of the properties of solutions over a wide range of concentrations and temperatures [1] . Achilli et al. (2010), in their experiments, concluded that MgCl2 may be the best DS for most water and wastewater applications, and suggested that it warranted further investigation to be used in environmental engineering applications [6] . The role of MgCl2 in the FO process of wastewater treat- ment application, especially for nutrient from secondary treated effluent and its rejection mechanism, has been rarely investigated. Excessive release of nutrient to the environment can cause severe environmental problem such as eutrophication leading to algal blooms, oxygen deficiency, and fish kills [7] . Some previous experiments have been conducted for membrane test by using MgCl2 as DS and deionized (DI) water as FS [8] - [11] . According to Lay et al. (2010) and Lee et al. (2010) multivalent ions (e.g. Ca2+ and Mg2+) solution with lower diffusion coefficients may be preferable in some specific applications in which high removal is desired [12] [13] .
Mostly the previous studies used sodium chloride (NaCl) and sea salt as DS. A study by Cath et al. (2010), investigating the rejection of ammonia and nitrate by FO membrane, showed that rejections of 74% and 78%, respectively, were achieved with sea salt as DS and secondary effluent as FS [14] . In their study, combined FO and RO membrane was also used that resulted in higher rejection of 94% for ammonia and 97% for nitrate. Holloway et al. (2007) investigated the rejection of ammonia, total kjeldahl nitrogen (TKN), and orthophosphate by a cellulose triacetate membrane that uses NaCl as DS [15] . Their study indicated that 82.9%, 91.6% and 99.8% rejection of ammonia, TKN, and orthophosphate were achieved, respectively, for FO-treated centrate in the increasing FS concentration. Xue et al. (2015) investigated enriching nitrogen and phosphorus with synthetic seawater as DS [16] . They concluded, at water reduction 50%, dissolved organic carbon and phosphate were 2.3-fold concentrated, ammonia 2.1-fold concentrated, while nitrite and nitrate were 1.9-fold and 1.3-fold, respectively. Retention of ammonia by cellulose triacetate (CTA) membrane was approximately 90% and negative retention by thin film composite (TFC) membrane in active layer-feed solution orientation.
Based on the aforementioned reasons, this study aims to investigate MgCl2 potential for nutrient rejection using the FO process. This study established an understanding of the mechanism and relation of MgCl2 as DS, nutrient on FS and membrane to provide further insight into the rejection of nutrients by the FO process. This can be potentially useful for future application in wastewater treatment plants.
2. Material and Methods
2.1. Feed and Draw Solution
A sample of secondary treated effluent from the Eastern Municipal Wastewater Treatment Plant in Ube City, Yamaguchi, Japan was collected and its nutrient concentration, i.e., nitrogen content (nitrite, nitrate, and ammonium) and phosphorus (phosphate) content, was measured as shown in Table 1.
In our FO process, an artificial secondary treated effluent was used as FS, which was prepared by referring to the actual concentrations of secondary treated effluent. The sources of nitrite, nitrate, ammonium, and phosphate were 0.03 mM sodium nitrite (NaNO2), 2.8 mM potassium nitrate (KNO3), 0.85 mM ammonium chloride (NH4Cl), and 0.3 mM potassium hydrogen phosphate (KH2PO4), respectively. The DS was prepared by dissolving magnesium chloride hexahydrate (MgCl2.6H2O) in DI water (SA 2100E Eyela Japan) at concentrations of 0.5 M, 1 M, 1.5 M, and 2 M.
Standard solution was prepared in the desired concentration range using stock standard and dilute DI water.
Table 1. Concentration of nutrients in an actual secondary treated effluent sample.
Samples were diluted to facilitate the measurement within the standard calibration range. The nitrogen (NO2-N, NO3-N, and NH4-N) and phosphorus (PO4-P) contents were determined by referring to the standard methods [17] using a UV-Vis spectrophotometer principle (Hitachi U-1800). The viscosity of FS and DS was measured by a viscometer (TVC-5 Toki Sangyo Japan).
2.2. Membrane
Two of the FO membranes used in this study were acquired from Hydration Technology Innovations (HTI, Albany, OR). The membrane chemistry are proprietary, though it is believed that the membranes were asymmetric CTA nonwoven support layer (HTI-NW) and TFC with embedded polyester screen support (HTI-ES), and negatively charge surface [4] . The surface-active layer, support layer, and cross section of the membranes were observed using Scanning Electron Microscopy (SEM) (Keyence VE 8800).
2.3. Forward Osmosis Cross Flow Set-Up
The membrane was installed in a membrane module consisting of two rectangular sides with the dimensions 135 mm long, 90 mm wide, and 4 mm deep and an effective membrane area of 0.012 m2 that permitted the FS and DS to flow to the membrane. In FO application mode, the active and support layers of the membrane were facing the FS and DS, respectively [5] . Two peristaltic pumps equipped with a speed controller (Eyela, RP-2100) were used to recirculate the FS and DS. Cross flow velocities of 0.25 m/s were applied. Two proportional flasks were used to store 3.5 L artificial FS and 1 L DS. The weight of both of these flasks and their contents were measured (PB5001-5 Mettler Toledo USA) at initial and final stages of the FO process to calculate reverse solute flux. In the reverse solute flux calculation, not only initial and final volume of FS but also their conductivities (Horiba ES-14) were measured. The permeate water from FS through the membrane into the DS was allowed to overflow into a beaker placed on a balance meter (PJ3000 Mettler-Toledo USA). The change in weight on the balance was recorded for the measurement of the water flux through the membrane. The FO cross flow apparatus was completed with conductivity meter and balance meter. This apparatus showed in Figure 1. A single cross flow experiment was carried out in 8 hours [15] [18] . During the experiment, the room temperature was maintained at 25˚C ± 1.0˚C. The pH of FS and DS were 7.2 ± 0.2 and 6.3 ± 0.2, respectively. The temperature and pH were monitored intermittently with a thermometer and pH-meter (Horiba D-13), respectively. At the end of the cross-flow process, permeates were collected and analyzed for nitrogen (NO2-N, NO3-N, and NH4-N), and phosphorus (PO4-P) content. The calculation for each rejection was done by subtracting the initial concentration from the final concentration.
2.4. Nutrient Rejection
According to McCutcheon et al. (2006) the determination of nutrient rejection in the FS is performed by collecting a sample of diluted DS after a complete FO run [19] . Based on the final concentration of the nutrients in the diluted DS and the initial concentration of the nutrients in the FS before the FO cross flow process, the percentage of rejection, R is calculated [8] [15] [19] [20] . This rejection is showed the actual retention of nutrients that separated/concentrated from permeate water/water recovery. The equation is:
Figure 1. FO cross flow apparatus.
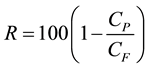 (1)
(1)
where CP and CF are final nutrients concentration in diluted DS after FO process and initial nutrients concentration in FS before FO process, respectively. The nitrogen (NO2 -N, NO3 -N, and NH4 -N), and phosphorus (PO4 -P) were determined by referring to the Standard Methods [17] using a UV-Vis spectrophotometer (Hitachi U-1800). All samples were diluted to allow for measurement within the standard calibration range.
2.5. Water Flux Calculation
Osmosis leads to water flux from FS to DS across the FO membrane, resulting in an increase in the weight of the DS. The water flux can be calculated using Equation (2), where the change in weight of DS was converted to volume and was divided by the membrane area and time duration.
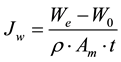 (2)
(2)
where Jw is the water flux (L/m2×hr), We the final weight of DS at the end of the FO process (g), W0 the initial weight of DS (g), ρ density of fluid (kg/m3), Am the membrane area (m2), and t the time duration (hour).
The relation between the water flux and osmotic pressure difference is expressed by the following Equation (3):
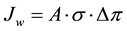 (3)
(3)
where A is the water permeability coefficient (L/(m2×hr×bar)), σ is the reflection coefficient, and △π is the osmotic pressure difference (bar). More specifically, the relation between the osmotic pressure and concentration is explained by the Van’t Hoff equation derived from the Morse equation. The osmotic pressure is linearly related to the concentration of the solution that is determined by Equation (4):
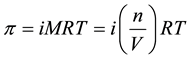 (4)
(4)
where p is the osmotic pressure, i is the Van’t Hoff factor, M is the molar concentration of solute particles, which is equal to the ratio of the number of solute moles (n) to the volume of the solution (V), R is the gas constant of 8.3145 J∙K−1∙mol−1, and T is the absolute temperature.
2.6. Reverse Solute Calculation
The transported solute from the DS to the FS is named reverse solute. A conductivity meter and a balance meter were used to determine the concentration and volume of FS, respectively, before and after the FO process. Thus, the characteristics of the reverse solute were measured. The concentration, which is measured in mS/cm, was converted to g/L TDS and then divided by the membrane area (m2) and the operation time (hours). The reverse solute flux was determined using mass balance calculation as seen on Equation (5):
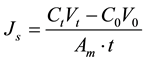 (5)
(5)
where Js is the reverse solute flux (g/m2×hr); C0 and Ct are the concentration of solute in the FS before and after the FO process, respectively; V0 and Vt are the volume of the FS before and after the FO process, respectively.
To investigate each diffusion constant of ion Mg2+ and Cl−, analysis of these ions were conducted using Optical Emission Spectrometry Inductively Coupled Plasma (OES ICP Optima 3300) for Mg2+ and argentometric method for Cl−.
The reverse solute flux was affected by concentration difference between FS and DS. This phenomenon can be described by Fick’s Law in Equation (6) [6] :
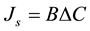 (6)
(6)
where B is the solute permeability coefficient (L/m2×hr) and DC is the concentration difference across the membrane (g/L).
The fluid in different concentration has a viscosity value that is considered to the diffusion constant calculation. The viscosity was measured by a viscometer (TVC-5 Toki Sangyo Japan). The diffusion constant can be expressed as
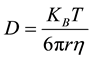 (7)
(7)
where D is the diffusion constant, KB is the Boltzmann constant (1.381 ´ 10−23 m2×kg/s2×K), T is the absolute temperature, h is the viscosity, and r represents the radius of spherical particles.
3. Result and Discussion
3.1. Nutrient Rejection
To determine whether the induced nutrient rejection by MgCl2 concentration variation was related to the membrane characteristics, data of percentage rejection from the four kinds of variation in concentration of MgCl2 and the two kinds of membranes were generated (Figure 2). When low concentration 0.5 M MgCl2 was used as DS, the rejection of NO2, NO3, and PO4 in membrane HTI-NW were 40.7%, 72.9% and 75.7%, respectively. Whereas, NH4 rejection reached 99.4%. Using membrane HTI-ES, the experiment yielded lower rejections than that of HTI-NW 15.9%, 67.7%, 44.5% and 35.8% of NO2, NO3, PO4 and NH4, respectively.
Figure 2. Rejection nutrients based on variation of DS MgCl2 concentration at 0.5 M, 1 M, 1.5 M and 2 M using membrane HTI-NW and HTI-ES. Experiment conditions: cross flow velocity 0.25 m/s, temperature 25˚C ± 1.0˚C.
In both of membranes, a low concentration of MgCl2 mostly does not achieve high nutrient rejection except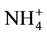 . The nutrient sources in the FS and MgCl2 in the DS include polyatomic ions that could dissociate in water. In the ionized water, the monovalent, negatively charge, low molecular weight ions, such as
. The nutrient sources in the FS and MgCl2 in the DS include polyatomic ions that could dissociate in water. In the ionized water, the monovalent, negatively charge, low molecular weight ions, such as 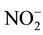 and
and 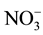 anions, were rejected less than both the
anions, were rejected less than both the 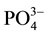 anion (multivalent, negatively charge, large molecular weight) and
anion (multivalent, negatively charge, large molecular weight) and 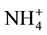 cation (monovalent, positively charge, and low molecular weight). The presence of multiple ions with varying charges in the FS in which electrical potential at the interfaces of the FS active layer membrane and the DS support layer membrane resulted the adsorption difference of cations and anions. It caused by the Donnan potential. The Donnan potential prevents some of the ions to pass through the FO membrane and is identical to the rejection process.
cation (monovalent, positively charge, and low molecular weight). The presence of multiple ions with varying charges in the FS in which electrical potential at the interfaces of the FS active layer membrane and the DS support layer membrane resulted the adsorption difference of cations and anions. It caused by the Donnan potential. The Donnan potential prevents some of the ions to pass through the FO membrane and is identical to the rejection process.
Furthermore, since the FS does not contain MgCl2, the Mg2+ and Cl− ions try to diffuse along the concentration gradient. To determine the difference diffusion constant between Mg2+ and Cl− ions, it can be calculated by using Equation (7) with T = 25˚C or 298.15 K, h = 1.0 mPa.s or 1.10−3 kg/m.s, 0.5 M MgCl2, and r as the hydrated ionic radius. The value of the hydrated diameter ion of Mg2+ and Cl− are 0.8 nm and 0.3 nm, respectively [6] . The respective diffusion constants are 0.55 × 10−9 m2/s for Mg2+ ion and 1.5 × 10−9 m2/s for Cl− ion. It seems that Cl− ion controlled the diffusion from DS to FS along concentration gradient. The development of negatively charge in FS force the anion nutrient such as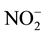 ,
, 














The rejection increased with increasing concentrations of MgCl2. Thus, by using 2 M MgCl2, the rejection of NO2, NO3, NH4, and PO4 in membrane HTI-NW were measured as 89.0%, 98.7%, 96.2%, and 99.6%, respectively; whereas in membrane HTI-ES, the nutrient rejections were 25.8%, 96.7%, 96.6%, and 86.4%, respectively. A high concentration of the DS will increase the osmotic pressure difference, thereby increasing the driving force, which in turn results in the enhancement of water flux from the FS to DS, and the reverse solute from the DS to FS by the diffusion constant. This diffusion constant is produced by the imbalance in the concentration between the anion and cation nutrient concentrations in the FS and the DS. This affects to electrical double layer into porous membrane. The Cl− ion diffusion controlled into the negatively charge membrane, creating a layer of Cl− into the membrane. The electrostatic repulsion of the negatively charge nutrient ions, 



The commonly used DS in FO are NaCl and seawater. The diameter of the hydrated Na+ ion is 0.45 nm, resulting in a higher diffusion constant than Mg2+. In comparison, Na+ and Cl− ions have very similar high diffusion constants, because of their some similar hydrated diameters or nearly equimolar [24] . Therefore, difference in diffusion constants between both of them is small, causing varied performance of anion or cation rejection. The seawater seemly has more various diffusion constant due to the complexity of ions. It worth noting that in this study, the Cl− ion seemly higher diffuse than that of Mg2+ ion due to an indication of high NH4+ cation rejection. The further explanation of individual Mg2+ and Cl− ions was explained in sub chapter 3.2.
The percentage of rejection varies for membrane HTI-NW and HTI-ES, and the difference between the two could be explained by membrane morphologies. The surface of the active layer, support layer, and a cross-sectional area of the membrane HTI-NW are shown in Figures 3(a)-(c), respectively, and those for membrane HTI-ES are shown in Figures 4(a)-(c), with the help of SEM test. The active layer membrane HTI-NW

Figure 3. SEM images of the HTI-NW membrane produced by HTI: (a) Surface layer of active layer (±100×); (b) Surface layer of support layer (100×); (c) Cross-sectional area (±200×).

Figure 4. SEM images of the HTI-ES membrane by HTI: (a) Surface layer of active layer (±100×); (b) Surface layer of support layer (100×); (c) Cross-sectional area (±200×).
appears smoother and less porous (Figure 3(a)) resulting in a good nutrient rejection efficiency (Figure 2) than that of membrane HTI-ES (Figure 4(a)).
3.2. Water Flux and Reverse Solute
To determine whether the MgCl2 concentration and membrane morphologies were related to water flux and reverse solute, the data of Figure 5 were generated. When the experiment using the same FS concentration and higher DS concentration, the concentration difference between FS and DS increases, the difference in osmotic pressure increases, and generates a higher water flux through the membrane due to the driving force that was verified by Equation (3). Moreover, the importance of MgCl2 in driving the water flux was confirmed by Equation (4), according to which a DS with a higher Van’t Hoff factor could produce a higher osmotic pressure. Thus, the osmotic pressure difference would be increased, and this would result in an increase in the water flux. For example, MgCl2 has a higher Van’t Hoff factor (i = 3) than NaCl (i = 2), which is commonly used as the DS solute. The higher Van’t Hoff factor would increase the osmotic pressure, which is the driving force, and finally increase the water flux. The water flux in membrane HTI-NW achieved 7.55 - 9.61 L/m2・hr and in membrane HTI-ES, it exceeds 13.58 - 15.10 L/m2・hr. These findings indicate that the membrane morphologies affect the water flux. Membrane HTI-ES resulted higher water flux than membrane HTI-NW, which were likely to be affected by the porous condition of active layer and support layer. The active layer of membrane HTI-NW appears to be thicker, smooth and less porous (Figure 3(a) and Figure 3(c)), resulting in lower water flux than in case of membrane HTI-ES (Figure 4(a) and Figure 4(c)). The lower water flux as indicated in HTI-NW membrane is likely related to higher possibility of the occurrence of concentration polarization. The nonlinear dependence of flux on osmotic pressure investigated in FO mode is primarily a result of internal concentration polarization [24] . The concentrated FS coupled rapid permeation to DS caused diluted internal concentration polarization at the membrane interface in the support layer [25] . This occurs when the difference in concentration across the active layer of the membrane varies from the difference in concentration in the DS [18] . The porous support layer contributes to the internal concentration polarization [19] , along with thickness, porosity, and tortuosity. The internal concentration polarization depends on the diffusion coefficient and on the membrane support layer [18] .
Figure 5. Water flux and reverse solute based on variation of DS MgCl2 concentration at 0.5 M, 1 M, 1.5 M and 2 M using membrane HTI-NW and HTI-ES. Experiment conditions: cross flow velocity 0.25 m/s, temperature 25˚C ± 1.0˚C.
When the concentration difference between FS and DS increases, the water flux, along with the reverse solute flux, increases as explained in Equation (6). In the FO process, the reverse movement of the solute from the DS to the FS through the membrane is unavoidable. This is because of the difference of concentrations [2] . In this study, the reverse solute flux rate reached 3.38 - 5.26 g/m2-hr in membrane HTI-NW and increased considerably to 13.84 - 25.29 g/m2-hr in membrane HTI-ES. The morphologies of the support layer of the membrane that faces the DS affected the reverse solute flux. The support layer with high resistance of the solute to diffusion will result in low reverse solute flux. Figure 3(b) is the SEM image of the support layer of membrane HTI-NW, in which the tortuosity seems higher and less porous, which makes it more capable of restraining the reverse solute flux, as compared to membrane HTI-ES (Figure 4(b)). The resistance of the solute to the diffusion within the membrane support layer explained the lower reverse solute flux in case of high tortuosity and less porous of membrane HTI-NW as compared to HTI-ES, which is characterized by lower tortuosity and higher porosity.
The investigation of reverse solute (Figure 6) was measured by monitoring increasing FS conductivity for MgCl2 and through ICP for individual Mg2+ ion and argentometric method for individual Cl− ion. The result indicated that concentration of DS MgCl2 less affect to reverse solute of membrane HTI-NW than membrane HTI-ES. The reverse solute in membrane HTI-NW seemly constant along all concentration of DS MgCl2. The Cl− ion diffusion slightly higher than Mg2+ion. In membrane HTI-ES, the reverse solute of Cl− ion was almost three times that of Mg2+ ion.
Coday et al. (2013), in their research using NaCl as DS, resulted reverse salt of Na+ ion was higher than that of Cl− ion in TFC membrane and in the contrary, Cl− ion higher diffuse than Na+ ion in CTA membrane [23] . This phenomenon possible to occurred due to Na+ and Cl− ions were nearly equimolar [24] resulted similar diffusion constant, thereby difficult to differentiate the dominant effect, whether Na+ or Cl− ions. Xue et al. (2015) using synthetic seawater as DS, resulted nutrient retention mostly achieved high retention 60% - 90% and only TFC membrane give negative result for ammonium retention. The hypothesis to explain the negative result are greater ammonium permeability to TFC membrane and high negative zeta potential of TFC membrane that similar level to that of a cation exchange membrane [16] [26] . The other possibility is complex composition of seawater that not as effective as a NaCl solution as DS [27] . The use single DS such as MgCl2 with high osmotic pressure potential seemly promising especially to reject charge ion such nutrient ions



4. Conclusions
The rejection of nitrogen (NO2, NO3, and NH4) and phosphorus (PO4) nutrients can be achieved mostly more 95% by using MgCl2 2 M in the FO process. The concentration difference between the dissociated ions of MgCl2 in the DS plays a significant role in rejecting ion nutrients in the FS by the Donnan potential effect in low


Figure 6. Reverse solute based on investigation MgCl2 (conductivity meter), ion Mg2+ (ICP), and ion Cl− (argentometric method) on HTI-NW and HTI-ES membranes. Experiment conditions: cross flow velocity 0.25 m/s, temperature 25 ± 1.0. (a) Membrane HTI-NW; (b) Membrane HTI-ES.
concentrations DS and by diffusion constant in high concentrations. Interestingly, using MgCl2 as DS, the Cl− ion is more dominantly to diffuse from DS to FS. The Cl− ion diffusion is slightly higher than Mg2+ ion diffusion in membrane HTI-NW, and almost three times than that in membrane HTI-ES. These conditions resulted in high rejection for anion nutrients due to electrostatic repulsion and cation nutrient due to attractions of water and Cl− ion.
The increasing concentration difference between FS and DS generates higher water flux and reverse solute flux, and lower water flux and reverse solute flux on membrane HTI-NW as compared to HTI-ES. However, these studies are limited by the operation time that not only investigates further about the efficiency of FO in long periods. Future experiments should assess the long-time operation or compare nutrient rejection for other dissociated ionic DS.
Acknowledgements
We would like to acknowledge the Yamaguchi University for supporting and providing the facilities for our study. The first author is grateful to the Directorate General of Higher Education, Ministry of Education and Culture, Indonesia for the financial grant.
Cite this paper
Yatnanta PadmaDevia,TsuyoshiImai,TakayaHiguchi,AriyoKanno,KoichiYamamoto,MasahikoSekine,Tuan VanLe, (2015) Potential of Magnesium Chloride for Nutrient Rejection in Forward Osmosis. Journal of Water Resource and Protection,07,730-740. doi: 10.4236/jwarp.2015.79060
References
- 1. Cath, T.Y., Childress, A.E. and Elimelech, M. (2006) Forward Osmosis: Principles, Applications, and Recent Developments. Journal of Membrane Science, 281, 70-87.
http://dx.doi.org/10.1016/j.memsci.2006.05.048 - 2. Lutchmiah, K., Verliefde, A.R.D., Roest, K., Rietveld, L.C. and Cornelissen, E.R. (2014) Forward Osmosis for Application in Wastewater Treatment: A Review. Water Research, 58, 179-197.
http://dx.doi.org/10.1016/j.watres.2014.03.045 - 3. Zhao, S., Zou, L., Tang, C.Y. and Mulcahy, D. (2012) Recent Development in Forward Osmosis: Opportunities and Challenges. Journal of Membrane Science, 396, 1-21.
http://dx.doi.org/10.1016/j.memsci.2011.12.023 - 4. Wei, J., Qiu, C., Tang, C.Y., Wang, R. and Fane, A.G. (2011) Synthesis and Characterization of Flat-Sheet Thin Film Composite Forward Osmosis Membranes. Journal of Membrane Science, 372, 292-302.
http://dx.doi.org/10.1016/j.memsci.2011.02.013 - 5. Ge, Q., Ling, M. and Chung, T.-S. (2013) Draw Solutions for Forward Osmosis Processes: Development, Challenges, and Prospects for the Future. Journal of Membrane Science, 47, 2386-2393.
http://dx.doi.org/10.1016/j.memsci.2013.03.046 - 6. Achilli, A., Cath, T.Y. and Childress, A.E. (2010) Selection of Inorganic-Based Draw Solution for Forward Osmosis Applications. Journal of Membrane Science, 364, 233-241.
http://dx.doi.org/10.1016/j.memsci.2010.08.010 - 7. Ji, D., Xi, B., Su, J., Huo, S., He, L., Liu, H. and Yang, Q. (2013) A Model to Determine the Lake Nutrient Standards for Drinking Water Sources in Yunnan-Guizhou Plateu Ecoregion, China. Journal of Environmental Sciences, 25, 1773-1783. http://dx.doi.org/10.1016/S1001-0742(12)60184-3
- 8. Yang, Q., Wang, K.Y. and Chung, T.S. (2009) Dual Layer Hollow Fibers with Enhanced Flux as Novel Forward Osmosis Membranes for Water Production. Environmental Science Technology, 43, 2800-2805.
http://dx.doi.org/10.1021/es803360t - 9. Saren, Q., Qiu, C.Q. and Tang, C.Y. (2011) Synthesis and Characterization of Novel Forward Osmosis Membrane Based on Layer-by-Layer Assembly. Environmental Science Technology, 45, 5201-5208.
http://dx.doi.org/10.1021/es200115w - 10. Qiu, C., Qi, S. and Tang, C.Y. (2011) Synthesis of High Flux Forward Osmosis Membranes by Chemically Cross-linked Layer-by-Layer Polyelectrolytes. Journal of Membrane Science, 381, 74-80.
http://dx.doi.org/10.1016/j.memsci.2011.07.013 - 11. Qiu, C.Q., Setiawan, L., Wang, R., Tang, C.Y. and Fane, A.G. (2012) High Performance Flat Sheet Forward Osmosis Membrane with an NF-Like Selective Layer on a Woven Fabric Embedded Substrate. Desalination, 287, 266-270. http://dx.doi.org/10.1016/j.desal.2011.06.047
- 12. Lee, S., Boo, C., Elimelech, M. and Hong, S. (2008) Comparison of Fouling Behavior in Forward Osmosis (FO) and Reverse Osmosis (RO). Journal of Membrane Science, 365, 34-39.
- 13. Lay, W.C.L., Chong, T.H., Tang, C.Y., Fane, A.G., Zhang, J. and Liu, Y. (2010) Fouling Propensity of Forward Osmosis: Investigation of the Slower Flux Decline Phenomenon. Water Science & Technology, 61, 927-936. http://dx.doi.org/10.2166/wst.2010.835
- 14. Cath, T.Y., Hancock, N.T., Lundin, C.D., Jones, C.H. and Drewes, J.E. (2010) A Multi-Barrier Osmotic Dilution Process for Simultaneous Desalination and Purification of Impaired Water. Journal of Membrane Science, 362, 417-426. http://dx.doi.org/10.1016/j.memsci.2010.06.056
- 15. Holloway, R.W., Childress, A.E., Dennett, K.E. and Cath, T.Y. (2007) Forward Osmosis for Concentration of Anaerobic Digester Centrate. Water Research, 41, 4005-4014.
http://dx.doi.org/10.1016/j.watres.2007.05.054 - 16. Xue, W., Tobino, T., Nakajima, F. and Yamamoto, K. (2015) Seawater-Driven Forward Osmosis for Enriching Nitrogen and Phosphorous in Treated Municipal Wastewater: Effect of Membrane Properties and Feed Solution Chemistry. Water Research, 69, 120-130.
http://dx.doi.org/10.1016/j.watres.2014.11.007 - 17. American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF) (1998) Standard Method for the Examination of Water and Wastewater. 20th Edition, American Public Health Association, Washington DC.
- 18. Cath, T.Y., Drewes, J.E. and Lundin, C.D. (2009) A Novel Hybrid Forward Osmosis Process for Drinking Water Augmentation Using Impaired Water and Saline Water Sources. WERC Consortium for Environmental Education and Technology Development at New Mexico State University and Water Research Foundation.
- 19. McCutcheon, J.R., McGinnis, R.L. and Elimelech, M. (2006) Desalination by Ammonia-Carbon Dioxide Forward Osmosis: Influence of Draw and Feed Solution Concentrations on Process Performance. Journal of Membrane Science, 278, 114-123. http://dx.doi.org/10.1016/j.memsci.2005.10.048
- 20. Yip, N.Y., Tiraferri, A., Phillip, W.A., Schiffman, J.D. and Elimelech, M. (2010) High Performance Thin-Film Composite Forward Osmosis Membrane. Environmental Science & Technology, 44, 3812-3818.
http://dx.doi.org/10.1021/es1002555 - 21. Nguyen, T.P.N., Yun, E.T., Kim, I.C. and Kwon, Y.N. (2013) Preparation of Cellulose Triacetate/Cellulose Acetate (CTA/CA)-Based Membranes for Forward Osmosis. Journal of Membrane Science, 433, 49-49.
http://dx.doi.org/10.1016/j.memsci.2013.01.027 - 22. Bian, L.X., Fang, Y.Y. and Wang, X.L. (2014) Experimental Investigation into the Transmembrane Electrical Potential of the Forward Osmosis Membrane Process in Electrolyte Solutions. Membrane, 4, 275-286. http://dx.doi.org/10.3390/membranes4020275
- 23. Coday, B.D., Heil, D.M., Xu, P. and Cath, T.Y. (2013) Effects of Transmembrane Hydraulic Pressure on Performance Forward Osmosis Membranes. Environmental Science & Technology, 47, 2386-2393.
http://dx.doi.org/10.1021/es304519p - 24. Phillip, W.A., Yong, J.S. and Elimelech, M. (2010) Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environmental Science & Technology, 44, 5170-5176.
http://dx.doi.org/10.1021/es100901n - 25. Wang, Z.W., Tang, J.X., Zhu, C.W., Dong, Y., Wang, Q.Y. and Wu, Z.C. (2015) Chemical Cleaning Protocols for Thin Film Composite (TFC) Polyamide Forward Osmosis Membranes Used for Municipal Wastewater Treatment. Journal of Membrane Science, 475, 184-192.
http://dx.doi.org/10.1016/j.memsci.2014.10.032 - 26. Xie, H., Saito, T. and Hickner, M.A. (2011) Zeta Potential of Ion-Conductive Membranes by Streaming Current Measurement. Langmuir, 27, 4721-4727.
- 27. Zhang, F., Brastad, K.S. and He, Z. (2011) Integrating Forward Osmosis into Microbial Fuel Cells for Wastewater Treatment, Water Extraction and Bioelectricity Generation. Environmental Science & Technology, 45, 6690-6696. http://dx.doi.org/10.1021/es201505t
NOTES
*Corresponding author.










