Journal of Water Resource and Protection
Vol.5 No.12(2013), Article ID:40548,10 pages DOI:10.4236/jwarp.2013.512122
Abatement of Helminth Eggs and Bacterial and Viral Indicators in Soil after Land Application of Treated Sludges
1Department of Microbiology, Pontificia Universidad Javeriana, Bogotá, Colombia
2Department of Microbiology, University of Barcelona, Barcelona, Spain
3The Water Research Institute, University of Barcelona, Barcelona, Spain
Email: *campos@javeriana.edu.co
Copyright © 2013 Claudia Campos et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 12, 2013; revised October 15, 2013; accepted November 9, 2013
Keywords: Fecal Indicators; Land Application; Sludge; Soil; Treatment
ABSTRACT
The reduction of helminth eggs, fecal coliforms and somatic coliphages present in sewage sludge after treatments and abatement by application to soil was determined. Traditional stabilization processes produced small changes in the concentrations of the parameters studied. In contrast, thermal treatments and liming produced dramatic reductions. Fecal coliforms were the most affected by both types of treatments; somatic coliphages showed some persistence after 30 minutes at 60˚C; and both somatic coliphages and helminth eggs showed some persistence to storage in quick lime. However, both treatments supplied biosolid suitable for unrestricted application in agriculture. Abatement in soils in the climatic conditions tested (mild to cold temperatures and high relative humidity) was slow and took several months to reach the background levels. These results suggest that environmental conditions (temperature and humidity) play the main role in inactivating the microorganisms, since abatement was similar in different soil types. The extended permanence of pathogens and microbial indicators in soil after the application of treated sludges indicates that, in the normal weather conditions of the areas where the study was performed and the amounts of sludges applied, contaminant microbes are not easily mobilized from the complex matrixes that constitute the treated sludges and that consequently in normal conditions their release as diffuse pollution is of lesser importance.
1. Introduction
Large amounts of biowastes are produced worldwide by agriculture and forestry, municipalities and industries. Traditionally biowastes have been landfilled, incinerated or landspread. Recycling biodegradable waste in agriculture is considered a way of maintaining or restoring the quality of soils, because of the fertilizing capacity and the improvement properties of the organic matter contained in these materials. It is considered the most economic, practical and environmentally beneficial management option. But recycling wastes by land spreading is not free of hazards because of their potential for soil and water contamination [1]. These hazards, besides contamination of food or directly of persons in contact with soil after land application of biosolids, embrace their potential contribution to diffuse pollution that afterwards can reach both surface and subsurface water bodies. Regarding diffuse pollution, increasing the knowledge about the mobilization and fate of pollutants from fields where sludges have been applied is a challenging aim. Some biowastes such as raw and treated sewage sludge that are frequently named as biosolids, septic tank sludge and slurries and manure, may contain enteric pathogens hazardous for humans, animals or both (zoonotic infections), besides other hazards. Commonly, these biosolids need some additional treatments to ensure certain abatement level of pathogens. Different regulations set the microbiological quality parameters of sludges to be applied to soil [2-4]. In these regulations, different kinds of biosolids are considered regarding chemical and microbiological quality and authorized uses. As an example, class B biosolids as defined by USEPA [4] require a microbiological quality that can be achieved with little or no additional treatment of biosolids produced in common sewage sludge plants, and can be used in agriculture with some temporal restrictions regarding crop harvesting, animal grazing and public contact. During these restriction time periods, materials receive further indirect treatment when exposed to the natural conditions of the environment such as air humidity, UV radiation of the sun, heat, wind, and soil microbes, which contribute to the abatement of microbial contaminants. Because of environmental variability, mostly due to differences in climatic conditions, it is essential to know the abatement of pathogens and indicators in various geographical areas with very different environmental conditions and to develop practices and regulations according to the characteristics of each region. In contrast, no appropriate management practices or regulations exist yet to minimize the risk of pathogen transmission by septic-tank sludge and slurries and manure application to soil. Thus, more broad information of abatement in soil of all types of pathogens and indicators in different regions of the world is needed.
Conventional treatments of sewage sludge conceived to reach the quality required for certain uses include stabilization/digestion (meso and thermophilic, aerobic and anaerobic), thermal treatments, liming and storage, among other less common treatments. Fairly abundant information on abatement of pathogens and indicators by conventional treatments is available mostly for bacterial pathogens [5-7]. Information about the abatement in soil of bacterial pathogens of human and animal origin is also available but to a lesser extent [8,9]. Taking into consideration that viruses and parasites are more resistant than bacteria to both natural and anthropogenic stressors [10- 12], obtaining information on the abatement in soil of these more resistant faecal-bounded pathogens is viewed as necessary. However, it has to be considered that there is a large variety of pathogens that respond differently to natural (irradiation, heat, desiccation, etc.) or anthropogenic (disinfecting procedures) stressors. Many different groups of bacteria, viruses, protozoa and helminths are present in different concentrations in human faeces, and hence in domestic wastewater and sludges as well as in slurries and manure. Consequently, the direct monitoring and quantification of all pathogens associated to solids are not feasible tasks. The surrogate indicator organisms are those that are easier to measure than specific pathogens. Nowadays, the faecal coliform bacteria are standard indicators in biosolids in the USA [4] and E. coli in the European Union standards (European Community, 2001). However, hygiene standards based on the removal of the fecal coliform bacteria or E. coli as indicators can easily be achieved by treatments that do not remove other pathogens and indicators as efficiently as those indicators. In fact, faecal coliforms and E. coli have been described to be more vulnerable to stressors than most viral, protozoan and parasite pathogens [10,11,13]. Bacteriophages [10] and the spores of sulphite-reducing clostridia (SSRC) [11] are being studied as alternative surrogates for virus and parasites. Due to the very great resistance and persistence described for SSRC in sewage sludge treatments [5,7,14] and their persistence in soil [9], they may be less appropriate than bacteriophages as indicators of pathogen abatement in sludge treatments and after their deposition in soil. Different groups of bacteriophages have been proposed as indicators of pathogen inactivation through different sludge treatment processes [7,14,15]. Somatic coliphages seem to excel the other phages for this purpose because they are fairly resistant and persistent under natural conditions and are easier to detect and more abundant in sludge than the other phages. The limited information available regarding the abatement in soil of fecal contaminants provided by spraying sewage of liquid slurries indicates that somatic coliphages present good concentrations and can be tracked long time after application [16,17]. It also points out that somatic coliphages may be a better indicator of the risk of enteric viruses in the environment than traditional bacterial indicators like fecal coliforms. In contrast, no information on phages provided to the soil by biosolids is available.
The investigation reported herein had two main purposes. The first purpose was to provide information regarding the microbiological quality of sludges in Colombia, in order to determine which sort of biosolid application can be envisioned and what sustainable and feasible treatments are required. According to WHO, Colombia is a low medium income country, still with an intermediate burden of diarrheic diseases [18] and where soil-transmitted helminth infection are a public health problem [19]. The second purpose was to study the abatement of three parameters: fecal coliforms, somatic coliphages and viable helminth eggs with low, intermediate, and high resistance respectively, by conventional treatments of sludges and in soil. Persistence in soil was expected to provide information not only about the persistence but also about the mobilization, and hence the potential contribution to diffuse pollution, of these contaminant microbes once applied to soil. The parameters were determined by standard procedures in sludges before and after different treatments in a wastewater treatment plan. Their persistence in soil was monitored after the land application of the biosolid in the Andean altiplano of Colombia, a zone with high rainfall, mild temperatures and high relative humidity located at 2200 - 2700 m above sea level.
2. Material and Methods
2.1. Sampling
Samples were taken at the plot corners and at the center by digging a small hole with a shovel to a depth of 10 cm. The samples from each plot were placed in a plastic bag with a hermetic seal and thoroughly mixed.
Sludge and soil samples were transported to the laboratory, kept at 4˚C and tested within the next 12 hours.
2.2. Samples and Experimental Settings
2.2.1. Sludge
Biosolids used in our study were obtained from two municipal wastewater treatment plants, one in Bogotá and the other one in Medellin (Colombia). In the treatment plant in Bogotá organic matter and suspended solids are removed by primary chemical treatment; the resulting sludge enters a thickening process for 20 h; then the thickened sludge (TS) undergoes anaerobic digestion in mesophilic conditions at 35˚C for 20 to 22 days (MADs); finally the sludge is dehydrated through band filters rendering the biosolid (BS) with 30.9% - 32.5% dry matter (DM). The plant in Medellin is a typical activated sludge plant with the resulting sludge treated with the same stabilization processes as described for the plant of Bogota.
2.2.2. Thermal Treatment
The MADS and the BS, as indicated in Table 1 of results, were exposed to different temperatures and times.
1) Liming Biosolids were treated with quick lime (CaO) to reach a final concentration of 25% and then they were exposed to air but sheltered from the rain for up to 43 days in the full scale treatments, and to 98 days in the pilot treatment.
2) Land-Spreading The survival of enteric micro-organisms added to soil by application of biosolids was monitored in five experimental settings.
Experimental setting 1. The soil was characterized as sandy inceptisol and the biosolid was from the plant of Bogotá. The following treatments were applied: plots type 1, 1 part of soil:1 part of biosolid; plots type 2, 3 parts of soil:1 part of biosolid; plots type 3, biosolid alone; and plots type 4, soil alone (control samples). Each application was done by triplicate. All the plots, including the control plot, were tested at days 0, 15 and 43. Nine samples were tested in the control and biosolid alone plots and 18 in the experimental plots.
Experimental setting 2. The soil was characterized as clayey inceptisol and the biosolid was from the plant of Bogotá. The following treatments were applied: plots type
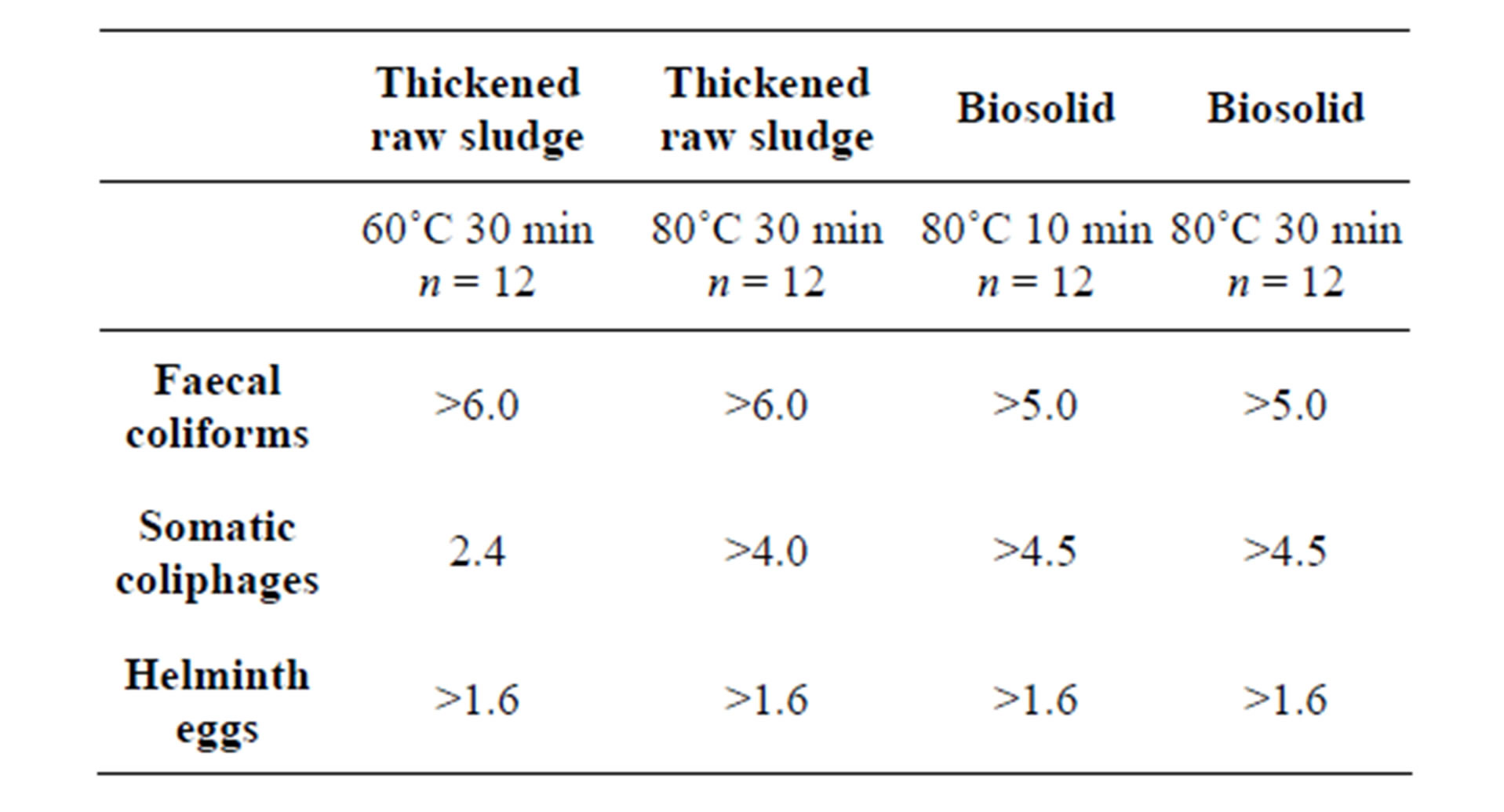
Table 1. Log10 reductions after different thermal treatments of sludge; n is the numbers of samples tested.
1, 3 parts soil:1 part biosolid; plots type 2, 2 parts soil:1 part biosolid; plots type 3, 1 part soil:1 part biosolid; plots type 4, biosolid alone; and plots type 5:soil alone (control samples). Each application was done by triplicate. All the plots, including the control plot were tested at days 0, 30, 60, 120 and 240. Fifteen samples were tested in the control and biosolid alone plots and 45 in the experimental plots.
Experimental setting 3. The soil was characterized as andisol and the biosolid was from the plant of Medellín. The following treatments were applied: plots type 1: biosolid diluted in water (3012 kg/ha) and sprayed on the soil; plot type 2, soil alone (control samples). Each application was done by quadruplicate and the experiment was repeated three times. The control plots were tested at days 0 and 60, and the experimental plot at days 0, 15, 30 and 60. Twenty-four samples were tested in the control plots and 48 in the experimental plots.
Experimental setting 4. The experiment was carried out using an arid quarry soil after the removal of all the topsoil and organic layers. The following treatments were applied: plots type 1, 8 parts of soil:1 part of biosolid; plots type 2, 4 parts of soil:1 part of biosolid; plots type 3, 2 parts of soil:1 part of biosolid; plot type 4, soil alone (control samples). Each application was done by triplicate. All the plots, including the control plot, were tested at days 0, 15, 30 and 60. Fifteen samples were tested in the control plots and 45 in the experimental plots.
Experimental setting 5. The experiment was carried out using an arid quarry soil after the removal of all the topsoil and organic layers. The following treatments were applied: plots type 1, 1part of soil:1 part of biosolid; plots type 2, 1 part of soil: 2 parts of biosolid; plots type 3, 1 part of soil: 4 parts of biosolid; plots type 4, 1 part of soil:8 parts of biosolid; plots type 5, biosolid alone; and plots type 5, soil alone (control samples). Each application was done by triplicate. All the plots, including the control plot, were tested at days 0, 30 and 60. Nine samples were tested in the control and biosolid alone plots and 36 in the experimental plots. Environmental conditions in the different experimental settings during the monitoring periods were relatively similar with average minimum and maximum temperatures ranging from 12˚C to 21˚C, high relative humidity ranging from 75% to 83%, and a monthly rainfall ranging from 70 to 110 mm.
2.3. Quantification of Microorganisms
Fecal coliforms, somatic coliphages and helminth eggs were recorded in experimental settings 1, 2 and 3. In experimental settings 4 and 5, only fecal coliforms and somatic coliphages were recorded.
2.3.1. Fecal Coliforms (FC)
To quantify fecal coliforms, 30 ml or g of sludges, biosolids and soil were mixed with 270 ml of sterile phosphate buffer and suspended by magnetic stirring at room temperature for 15 min. This suspension was used to prepare 10-fold dilutions as described in USEPA standard procedures. Then fecal coliforms were quantified by the membrane filter procedure, according to the USEPA standard. Results are expressed as colony forming units per 10 grams of dry matter (CFU/10 g DM).
2.3.2. Somatic Coliphages (SOMCPH)
Bacteriophages were first extracted from sludges, biosolid and soil as described previously by Lasobras et al. [20] with minor modifications. Briefly, samples were mixed with 10% beef extract pH 7.2 in a 1:10 (w⁄v) ratio and homogenized by magnetic stirring for 30 min at room temperature. The suspension was then centrifuged at 4000 g for 30 min at 4˚C. The supernatant was subsequently filtered through a 0.22 µm pore size polyethersulfone non-protein binding membrane filter (Millipore, Bedford, MA, USA). The permeate was then tested for the presence of bacteriophages. Plaque forming units (PFU) of somatic coliphages were counted by the double agar layer technique on Escherichia coli strain WG5 following the ISO 10705-2 protocol [21]. Results are expressed as plaque-forming units per 10 grams of dry mater (PFU/10 g DM).
2.3.3. Helminth Eggs (HE)
Viable helminth eggs were detected and quantified according to [22]. Briefly, this technique consisted of mixing and shaking the equivalent of 10 g of sample of total solids with Tween 80 solution (0.1%). Tween 80 is a detergent that adheres to the eggs and thus separates them from solid particles. The first sedimentation stage lasts 3 h, after which the supernatant is discarded and the sediment is filtered in a 160-μm sieve to remove the larger particles. The filtered and retained solids were washed with 1 to 2 L of distilled water and pooled together. Afterwards the sample was subjected to a second sedimentation for 3 h, the supernatant was discarded and the sediment mixed with 10 volumes of ZnSO4 solution (density 1.3) and centrifuged at 660 g for 5 min. The sediment was discarded and 1 L of water was added to the supernatant. Finally, a 3-h sedimentation was performed, in which the supernatant was discarded and the sediment mixed with 15 mL aceto-acetic buffer and 10 mL of diethyl ether to remove the lipophilic ether-soluble material. The resulting sediment was mixed with 5 mL of 0.1 N H2SO4 and then incubated at 26˚C for 4 weeks with a loose lid to allow air exchange. Finally, the sample was examined under a light microscope, eggs were counted and viability determined based on the formation of developed larvae. Results are expressed as the number of viable helminth eggs per 10 grams of dry mater.
2.3.4. Salmonella
The Most Probable Number of Salmonella sp. was determined according to USEPA [4]. Briefly, a given volume of sample was inoculated into the enrichment medium tryptic soy broth (TSB), and incubated for 24 hours at 37˚C. After incubation, an aliquot of the enrichment culture was inoculated onto modified semisolid RappaportVassiliadis (MSRV). The modified MSRV medium contains novobiocin and malachite green to inhibit non-Salmonella species, while allowing most Salmonella species to grow. Presumptive Salmonella colonies were isolated on xylose-lysine desoxycholate agar (XLD), and confirmed using lysine-iron agar (LIA), triple sugar iron agar (TSI), and urea broth.
2.4. Data Analysis
Statgraphics Plus software version 5.1 (Statistical Graphics Corporation, Herndon, VA, USA) was used for data treatment and statistical analysis. A P-value < 0.5 was considered statistically significant. Some data were plotted as boxes and whiskers. This plotting provides summary statistics using five numbers: the minimum, the maximum, the median, the 25th and the 75th percentiles. The outliers (▫) and extreme outliers (▪) values are also plotted.
Log10 reductions in treatments and soil have been calculated as the differences between the average (geometric mean; or arithmetic mean of the logarithm) of the values before and after the treatments or the lapse of time passed since deposition in soil.
3. Results and Discussion
3.1. Indicators and Pathogens in Sludges
The concentrations of fecal coliforms, somatic coliphages and helminth eggs, expressed as log10 units per 10 g of sludge, in thickened sludge (TS), sludge after their mesophilic anaerobic digestion (MAD) and biosolid (BS), are plotted in Figure 1. The average values of fecal coliforms (7.6 ± 0.5 CFU per 10 g) and somatic coliphages (6.2 ± 0.3 PFU per 10 g) in the thickened sludges are within the range of values reported elsewhere [7,20]. Significant (P < 0.5), although small decreases averaging 1.3 and 0.9 log10 for fecal coliforms and somatic coliphages, respectively, have been achieved with the mesophilic anaerobic digestion. Reductions of similar extents have been reported elsewhere for fecal coliforms, E. coli and somatic coliphages [7,23] indicating that the hygienizing effect of MAD has little effect. However, the numbers of both indicators in biosolids after dewatering the MADs showed significant increases in number of both fecal coliforms and somatic coliphages that reached average values of 6.7 ± 0.6 CFU and 6.4 ± 0.4 PFU per 10 g respectively. These values were midway and significantly different (P < 0.5) from those in TS and MADs. Increases of similar range had been described for fecal coliforms [24,25] in some dewatering processes, but no data are available for somatic coliphages. Regrowth and reactivation have been pointed as potential causes for this increase in numbers of fecal coliforms. However, knowing the unlikely replication of somatic coliphages under the conditions occurring in sludges [26], differences in the extraction efficiencies from the different matrixes, which are quite different in texture, cannot be excluded as the cause of these changes.
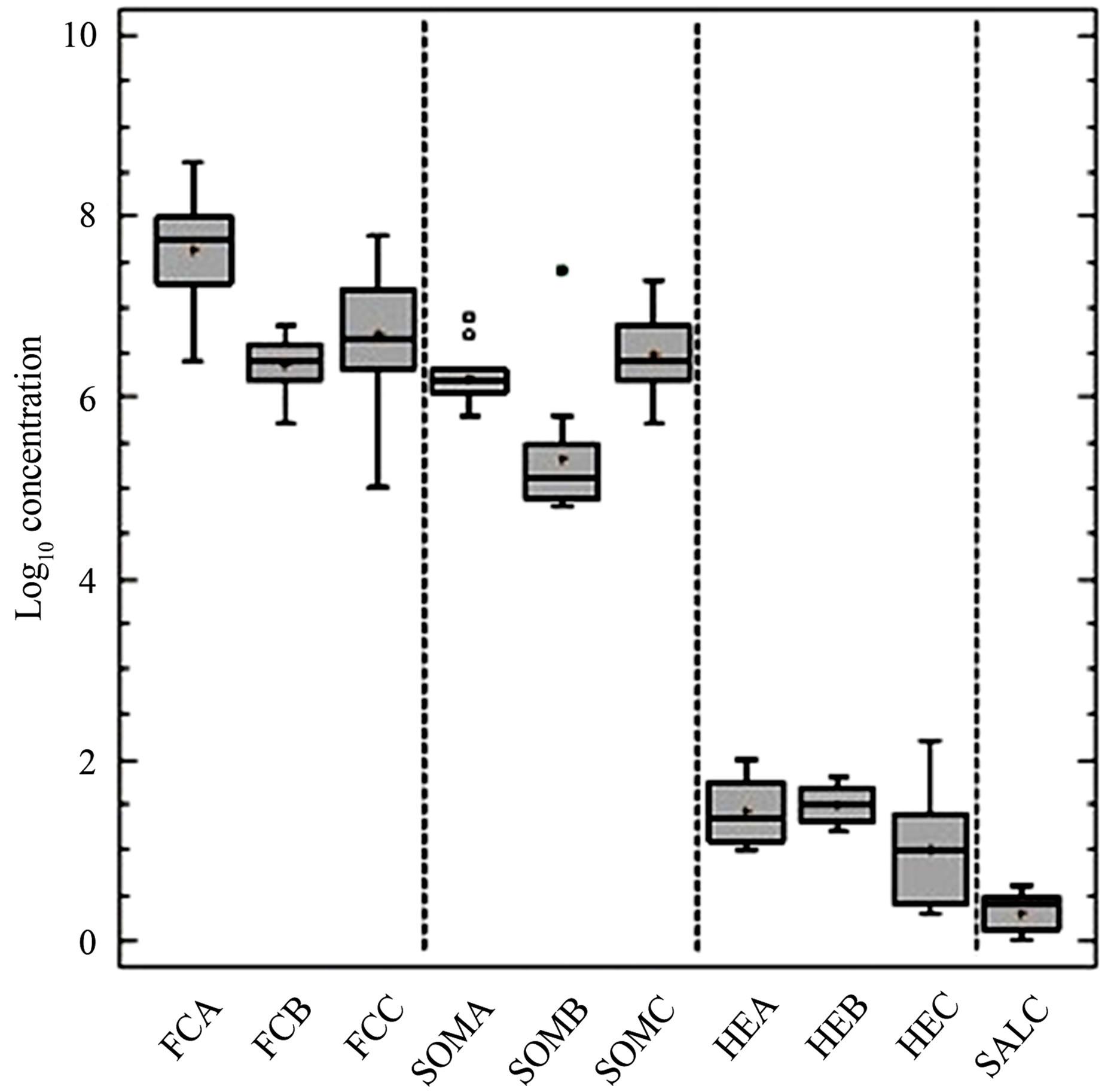
Figure 1. Box and whisker plots of counts expressed as Log10 concentrations per 10 g of dry mater (DM) of the various microorganisms analyzed in the different matrixes. Faecal coliforms (FC) in CFU, somatic coliphages (SOM) in PFU, and helminth eggs (HE) in TS (A, 20 samples), MADS (B, 13 samples) and BS (C, 52 samples); Salmonella (SAL) in MPN was only determined in BS.
The average values of helminth eggs (1.4 ± 0.4; 1.5 ± 0.2; and 1.0 ± 0.2 log10 units of viable eggs per 10 g) were not significantly different in the three matrixes. Values ranged from 10 to 97 eggs per 10 g of dry matter in TS, 16 to 62 in MADs and 2.5 to 129 in BS. In contrast with FC and SOMCPH, the differences in the numbers of eggs in the three matrixes are not significantly different (P > 0.5). These values averaged almost one order of magnitude greater than those found in industrialized countries, and are of the same order of magnitude as those of medium income countries as Brazil or Mexico [12].
Data about Salmonella sp. were only obtained for BS, where they average 0.3 ± 0.2 log10 CFU units per 10 g, with values ranging from below the detection limit (0.6 CFU per 10 g of DM) to 3.9 CFU per 10 g of dry matter. These values are of the same order of magnitude as those described elsewhere [5,27,28].
As to the possibility of using BS in agriculture, it does not fulfill the requirement for unrestricted use in agriculture according to some legislations [2,4], but can be exploited for some applications to land, as those allowed for class B biosolids according to USEPA [4].
3.2. Effects of Hygienizing Treatments
3.2.1. Thermal Treatments
Log10 reductions caused by different pasteurization treatments on the numbers of FC, SOMCPH and HE are reported in Table 1. All microbes tested were below the detection limits in all the conditions assayed, with the exception of somatic coliphages that were found in thickened raw sludge at a still relatively high concentration, averaging 4.8 log10 PFU per 10 g and ranging from 4.2 to 5.1, after 60˚C during 30 minutes. The different vulnerability of the three parameters tested agrees with information available. Thus, fecal coliforms or E. coli are quite susceptible [14,29]. Moreover, the great susceptibility to heat treatment of fecal coliforms is applicable to most bacterial pathogens. Somatic coliphages, similarly to some animal viruses, though not all [6,30], are relatively resistant [14,29], and are good surrogates for monitoring the disinfecting action of thermal treatments because of their intermediate susceptibility. The viability of HE is affected to such extent that they are not found after treatments [5,13]. However, as it can be seen in the log10 reduction figures reported in Table 1, as well as in information reported elsewhere, HE do not seem to be more susceptible than viruses to thermal treatments, since because of the low numbers in sludge prior to treatment, it is not possible to calculate log10 decreases as they were calculated for bacteriophages.
All the treatments assayed provide biosolids of sufficient microbiological quality for unrestricted use for agriculture [2,4].
3.2.2. Liming
Log10 reductions achieved after treatments and storage in quick lime (25%) of the biosolid (BS) are shown in Table 2. As in the case of thermal treatments, fecal coliforms are quite weak to lime treatment, and no fecal coliforms were detected neither at day 1 in the assays performed in the pilot treatment, nor after 7 days in the full scale treatment. This high effect of liming biowastes on fecal coliform bacteria and bacterial pathogens has been reported elsewhere [5,31]. Somatic coliphages were still found after seven days in the pilot treatment, but not in the full scale treatment where log10 decrease exceeded 4.0; in the pilot plant the log10 decreases at days 1 and 7 were two units greater than those of HE, and at day 21 were still higher than 3.5 logs. The scarce existing information on the effect of liming on viruses and bacteriophages indicates, as data reported here do, that coliphages can be useful surrogates of viruses in monitoring the disinfection in sludge treatments [31,32]. The slow abatement of helminth eggs by storage of lime treated sludges has been reported elsewhere [5,31].
The lime treatments assayed will provide biosolids sufficient microbiological quality for unrestricted use for agriculture [2,4] only after more than three weeks of storage of lime-treated biosolid, because of the slow inactivation of HE.
3.2.3. Abatement in Soil
Data on persistence of FC, SOMCPH and HE after application of mixtures of biosolids and soil into soil are shown in Figures 2-4. Figures 5 and 6 only show data on FC and SOMCPH. Since no significant differences (P > 0.5) were observed in samples after mixing different proportions of soil and biosolid (data of experimental settings 1, 2 and 5 reported in (Figures 2, 3 and 6), all the mixed samples have been treated as a single unit. Figure 4 shows the data in which sludge diluted in water was applied on the field by aspersion. In spite of the different types of soil studied and different environmental conditions regarding temperature and rainfall during our experiments the same trends can be observed in all the cases. First of all, with the exception of HE in the experiment reported in Figure 3, the addition of biosolids to soil increases significantly (P < 0.5) the concentrations of the three parameters studied. And secondly, though with some differences, all persist for long periods of time before returning to the levels found in soil before application of the sludges. In fact long persistence of bacterial indicators and pathogens [8,9] and HE [8] has been reported elsewhere, and though with less extent also viruses and bacteriophages [16,17]. All the values tended to go down after application to land, and values at a
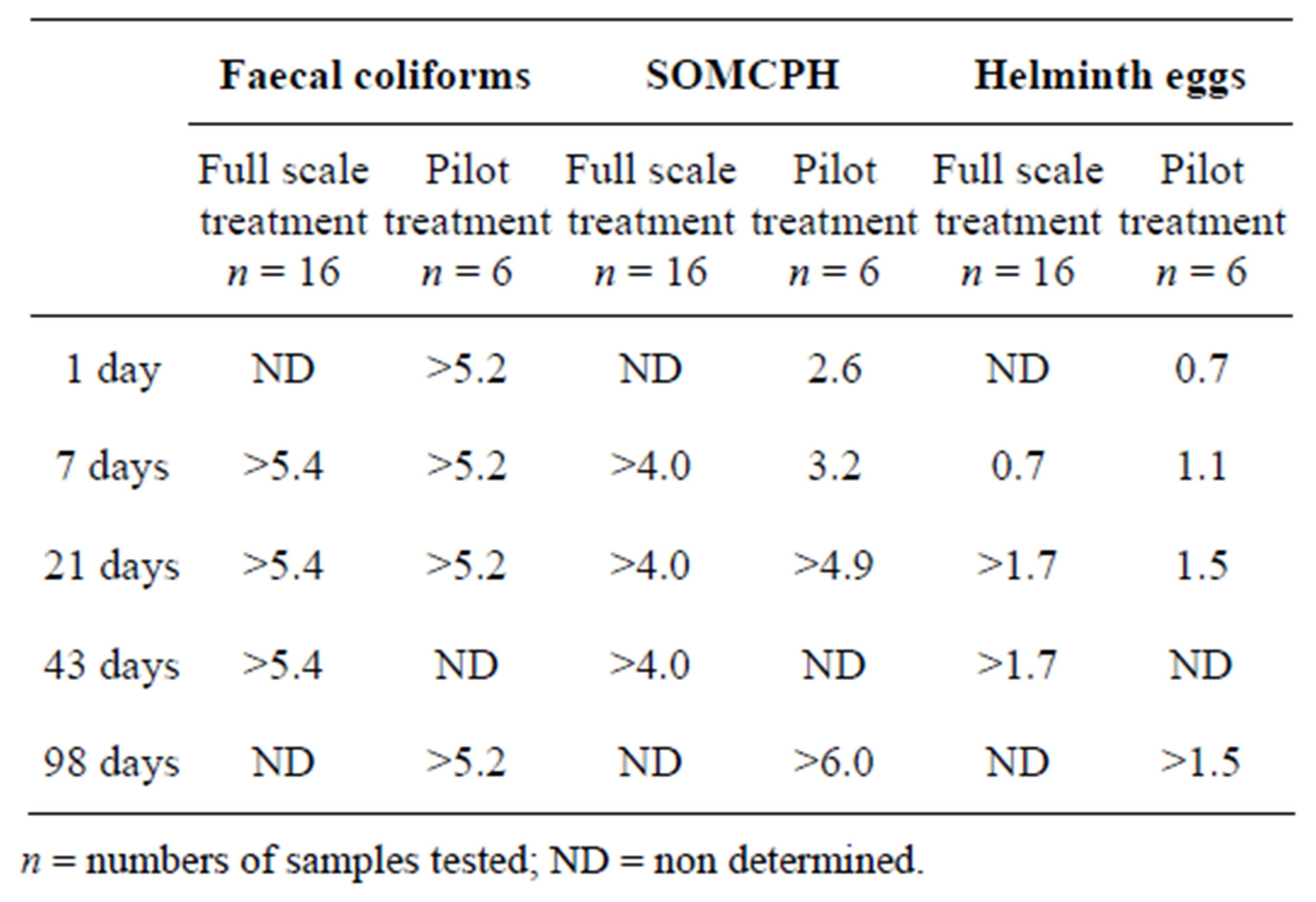
Table 2. Storage in quick lime (25%) blended biosolid. Log10 reductions after different storage times (difference between the average value before and after the treatment).
given sampling day were lower than the values of the precedent sampling day with a few exceptions. The first exception was a non-significant increase (P > 0.5) observed for SOMCPH and HE at day 15 in experimental setting 1 (Figure 2); this can be due to some disaggregation of sludge particles occurring in the first days in soil rather than replication, which is impossible for HE and very unlikely for somatic coliphages [26]. The second exception was at day 120 of experimental setting 2 (Figure 3) for HE; we have no explanation for this behavior, because human helminths do not replicate in soil and recontamination does not seem to have occurred, since neither FC nor SOMCPH show increase in numbers.
The third exception was the increase in the numbers of FC at day 60 in experimental setting 5 (Figure 6); this may be due to regrowth of fecal coliforms in soil, which has been reported to occur under some environmental conditions [33,34]. Regarding the rates of abatement, they differed among experimental settings and among parameters. HEs were always the most persistent of all, with abatements that exceeded 0.3 log10 units in any of the three experiments performed. The log10 reductions of FC and SOMCPH are reported in Table 3 and they varied depending on both the extent of the reduction and the reduction rate of the two indicators; thus in three of the five experiments, FC disappeared faster than SOMCPH, whereas in the other two the opposite occurred. However the difference on the abatement of FC and SOMCPH are not very big. Kinetics of SOMCP abatements seems more regular than that of FC and differences among experimental settings are also smaller than those of FC.
In three of the experimental settings, biosolid (BS) alone was placed on the soil surface in the same conditions than the mixture soil׃biosolid. Results of abatement of FC and SOMCPH in soil and BS reported in Table 3 do not show differences greater than those attributed to the uncertainty of the complete assay proce-
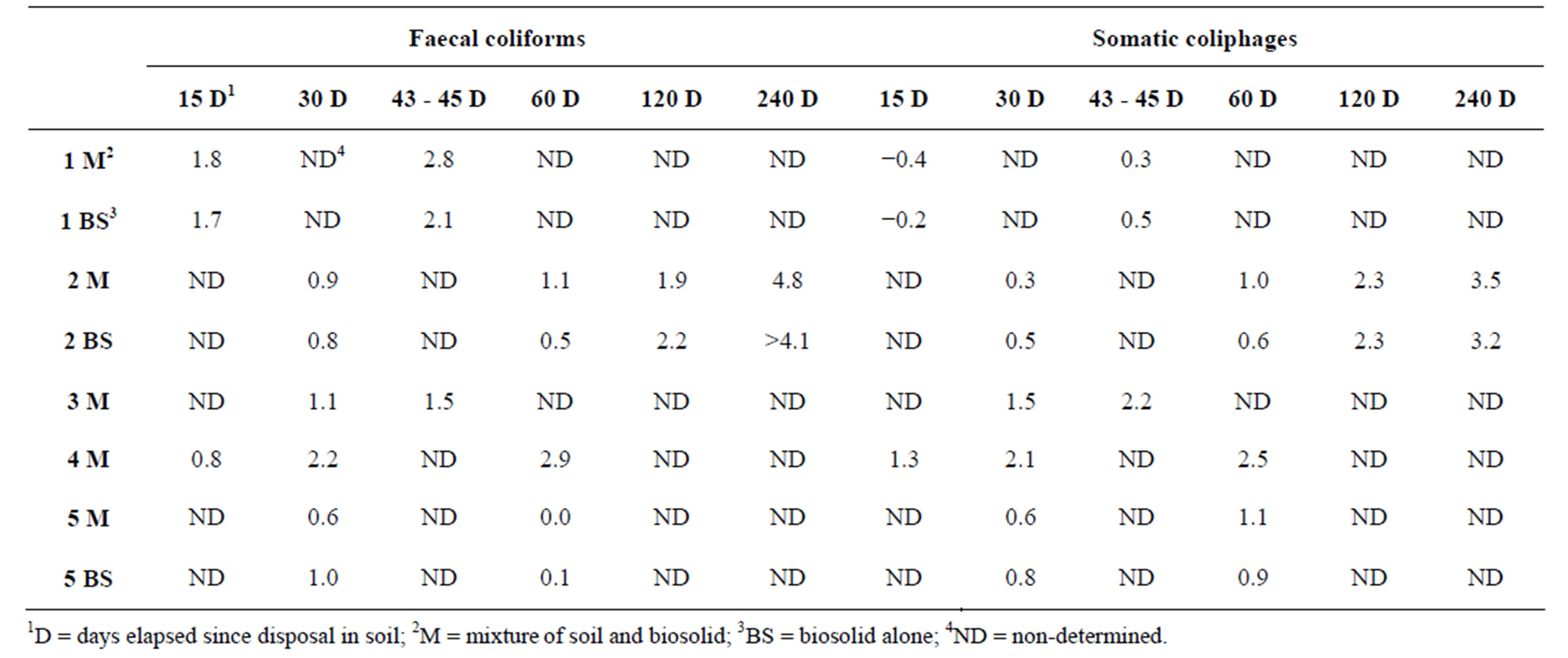
Table 3. Comparison of decay in log10 units of FC and SOMCPH in the mixture soil-biosolid and in biosolid alone.
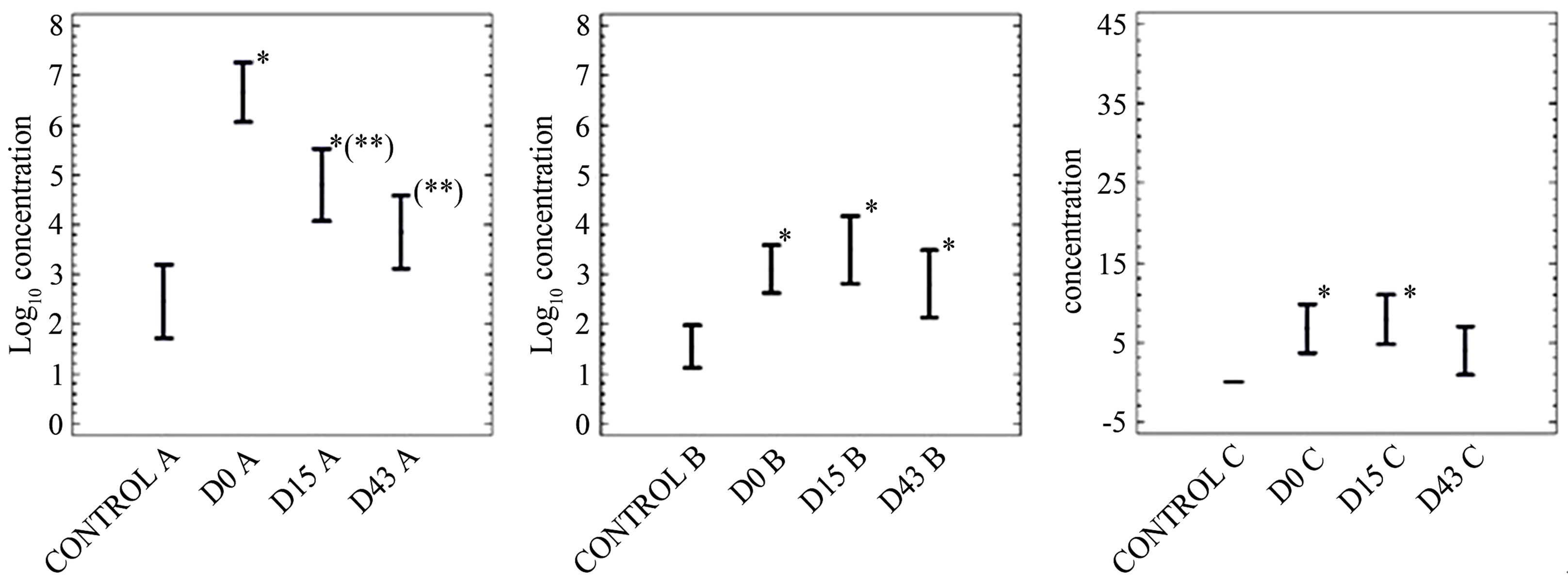
Figure 2. Log10 values of the concentrations (arithmetic means of log10 values and 95% confidence levels) of indicators (A, fecal coliforms in CFU per 10 g; B, somatic coliphages in PFU per 10 g) and helminth eggs (C) concentrations (arithmetic mean of the non-log transformed values and 95% confidence levels) in soil in experimental setting 1. In the x axis the control refers to non-inoculated soil and D stands for the days after the beginning of the experiment. *Values significantly different (P < 0.5) from the control value; **values significantly different (P < 0.5) from the D0 value.
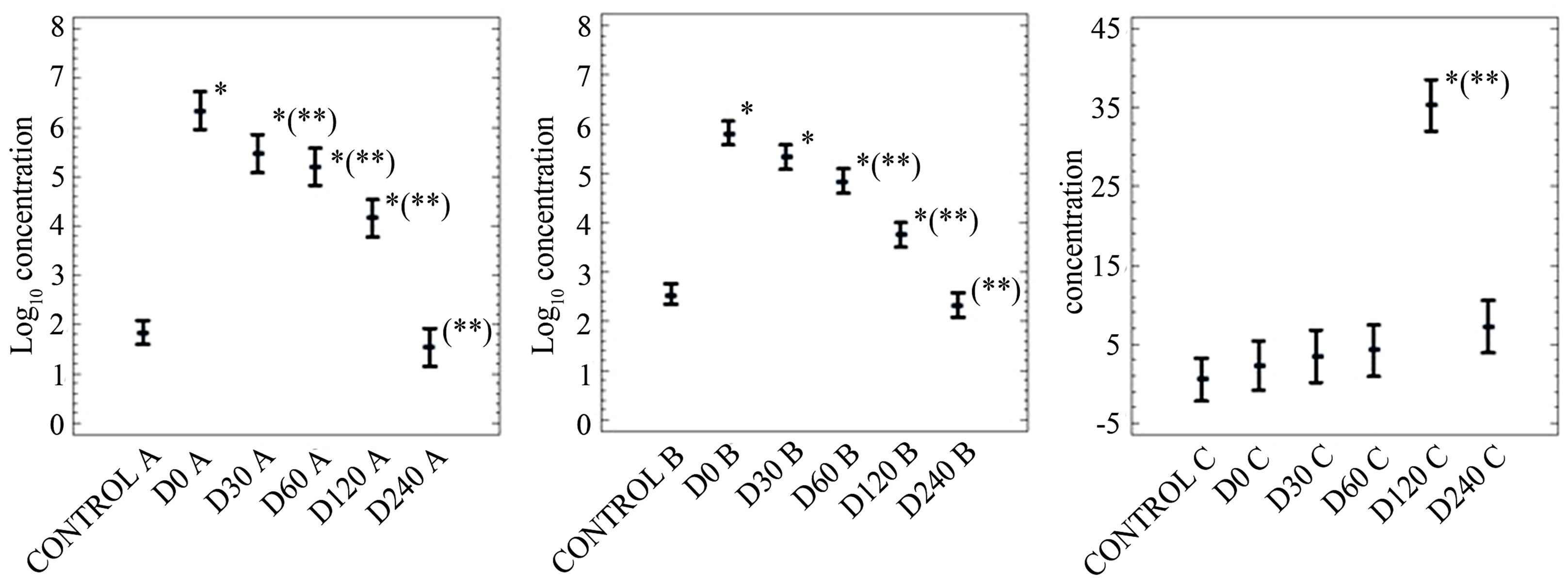
Figure 3. Log10 values of the concentrations (arithmetic means of log10 values and 95% confidence levels) of indicators (A, fecal coliforms in CFU per 10 g; B, somatic coliphages in PFU per 10 g) and helminth eggs (C) concentrations (arithmetic mean of the non-log transformed values and 95% confidence levels) in soil in experimental setting 2. In the X axis the control refers to non-inoculated soil and D stands for the days after the beginning of the experiment. *Values significantly different (P < 0.5) from the control value; **values significantly different (P < 0.5) from the D0 value.
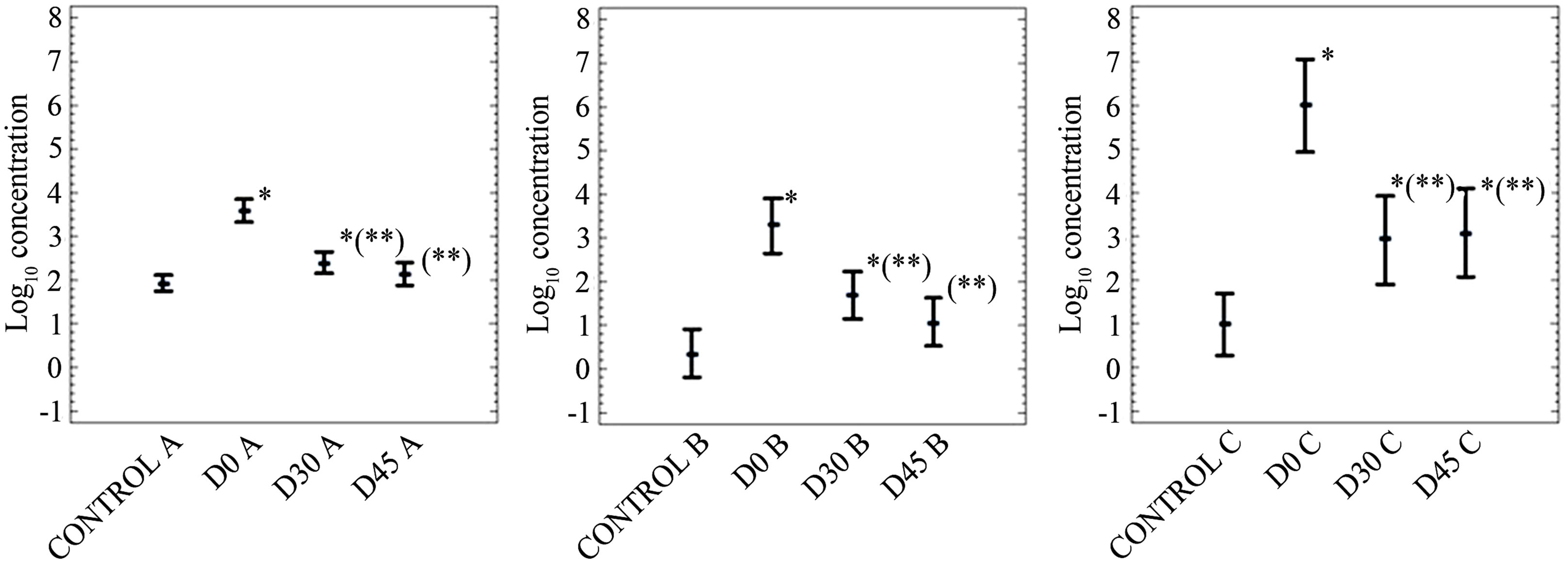
Figure 4. Log10 values of the concentrations (arithmetic means of log10 values and 95% confidence levels) of indicators (A, fecal coliforms in CFU per 10 g; B, somatic coliphages in PFU per 10 g) and helminth eggs (C) concentrations (arithmetic mean of the non-log transformed values and 95% confidence levels) in soil in experimental setting 3. In the X axis the control refers to non-inoculated soil and D stands for the days after the beginning of the experiment. *Values significantly different (P < 0.5) from the control value; **values significantly different (P < 0.5) from the D0 value.
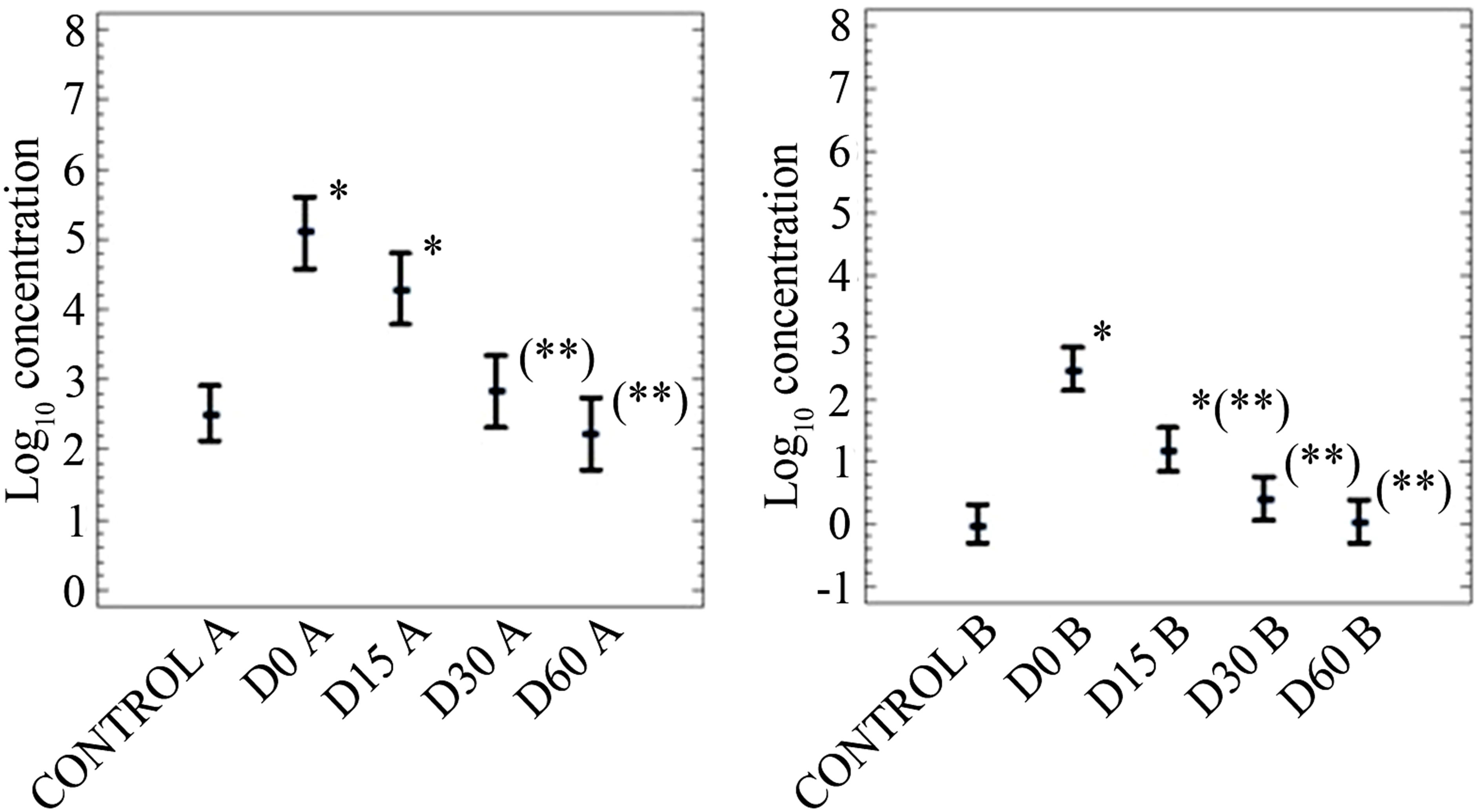
Figure 5. Log10 values of the concentrations (arithmetic means of log10 values and 95% confidence levels) of indicators (A, fecal coliforms in CFU per 10 g; B, somatic coliphages in PFU per 10 g) in soil in experimental setting 4. In the X axis the control refers to non-inoculated soil and D stands for the days after the beginning of the experiment. *Values significantly different (P < 0.5) from the control value; **values significantly different (P < 0.5) from the D0 value.
dure. Therefore, it will appear that in the three settings studied, the soil characteristics do not play a significant role in the abatement, and suggest that environmental conditions (temperature and relative humidity) played the main role in inactivating the microorganisms.
Because of the lesser variations observed in abatement kinetics from settings of somatic coliphages and because of their most unlikely replication in soil [26], we suggest somatic coliphages as a good surrogate for monitoring bacterial and viral abatement in soil. In fact some authors have reported that somatic coliphages have good concentrations in soil and can be tracked long time after application by aspersion of sewage of liquid slurries [16,17].
In spite of some differences between the persistence of the contaminant microbes studied, all of them remained for long periods in soil after application of the biosolids to land. This long persistence strongly indicates that the
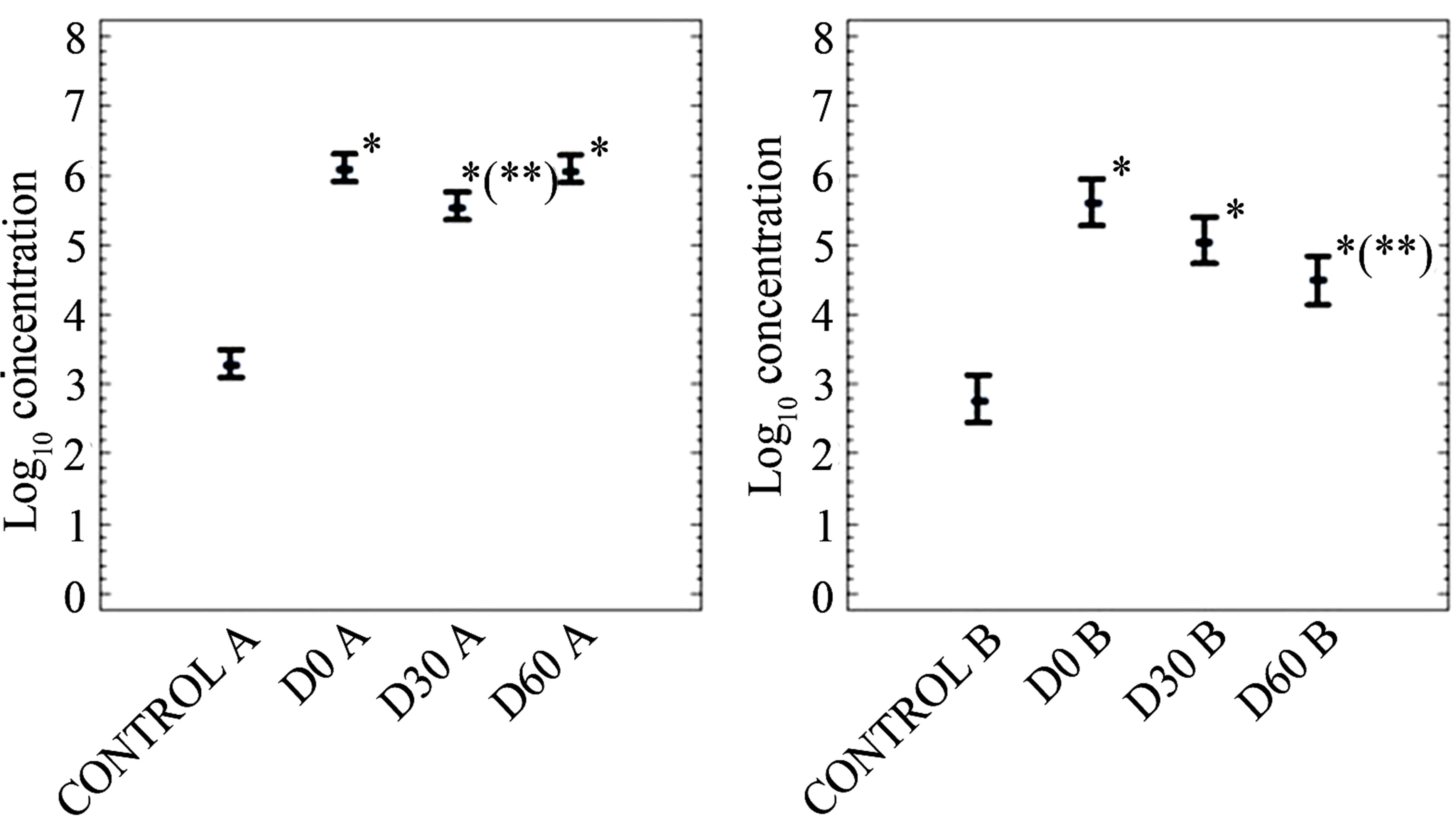
Figure 6. Log10 values of the concentrations (arithmetic means of log10 values and 95% confidence levels) of indicators (A, fecal coliforms in CFU per 10 g; B, somatic coliphages in PFU per 10 g) in soil in experimental setting 5. In the X axis the control refers to non-inoculated soil and D stands for the days after the beginning of the experiment. *Values significantly different (P < 0.5) from the control value; **values significantly different (P < 0.5) from the D0 value.
studied biosolids have the capability of sequestering the contaminant microbes, both pathogens and indicators, and consequently making improbable their release to the surrounding surface and subsurface waters in areas with rainfall regimes similar to the ones of the area during the described experiments. In summary the contribution of sludge to diffuse pollution reaching water bodies by mobilization of contaminant microbes is unlikely under the weather conditions similar to those in the study area and with amounts and type of sludges applied to soil similar to those described here.
4. Conclusions
Raw and stabilized sludges studied contain amounts of bacterial and viral indicators, helminth eggs and Salmonella comparable to those reported in developed countries.
Hygienization accomplished by thermal treatment and liming renders biosolids suitable for unrestricted use in agriculture and other land spreading applications.
Abatement in soil took several months to reach background values of contaminant microbes, with environmental conditions (temperature and relative humidity) likely playing a more important role than soil characteristics.
Under the predominant weather conditions in the area studied, the mobilization and consequent contribution to diffuse contamination, and hence to contamination of water bodies, of the contaminant microbes do not seem very important.
Because of the lesser variations observed in the abatement kinetics of somatic coliphages among different settings and because of their most unlikely replication in soil, they can serve as good surrogates for monitoring bacterial and viral abatement in soil.
5. Acknowledgements
We thank “Empresa de Acueducto y Alcantarillado de Bogotá (EAAB), Empresas Públicas de Medellín” (EPM) and “Secretaría del Medio Ambiente de Bogotá” for supporting the research reported in this paper.
REFERENCES
- E. Epstein, “Pathogenic Health Aspects of Land Application,” BioCycle, Vol. 39, No. 6, 1998, pp. 62-67.
- Journal Officiel, “Prescriptions Techniques Applicable aux Epandages de Boues sur sols Agricoles en Application du Decret nª 97-1133,” Gouvernement de France, Paris, 1998.
- European Community, “Working Document—Biological Treatment of Biowaste,” 2nd Draft, 2001. http://europa.eu.int/comm/environment/waste/factsen.htm
- USEPA, “Biosolids Technology Fact Sheet. Use of Land Filling for Biosolids Management,” United States Environmental Protection Agency, 2008, p. 103.
- C. Gantzer, L. Gillerman, M. Kuznetsov and G. Oron, “Adsorption and Survival of Faecal Coliforms, Somatic Coliphages and F-Specific RNA Phages in Soil Irrigated with Wastewater,” Water Science and Technology, Vol. 43, No. 12, 2001, pp. 117-124.
- L. Sahlström, “A Review of Survival of Pathogenic Bacteria in Organic Waste Used in Biogas Plants,” Bioresources Technology, Vol. 87, No. 2, 2003, pp. 161-166.
- C. Guzmán, J. Jofre, M. Montemayor and F. Lucena, “Occurrence and Levels of Indicators and Selected Pathogens in Different Sludges and Biosolids,” Journal of Applied Microbiology, Vol. 103, No. 6, 2007, pp. 2420- 2429.
- J. Santamaria and G. A. Torzanzos, “Enteric Pathogens and Soil: A Short Review,” International Microbiology, Vol. 6, No. 1, 2003, pp. 5-9.
- A. Pourcher, F. Picard-Bonnaud, V. Ferré, A. Gosinska, V. Stan and G. Moguedet, “Survival of Faecal Indicators and Enteroviruses in Soil after Land-Spreading of Municipal Sewage Sludge,” Applied Soil Ecology, Vol. 35, No. 3, 2007, pp. 473-479.
- IAWPRC, Study Group on Health Related Water Microbiology, “Bacteriophages as Model Viruses in Water Quality Control,” Water Research, Vol. 25, No. 5, 1991, pp. 529-545.
- P. Payment and E. Franco, “Clostridium perfringens and Somatic Coliphages as Indicators of the Efficiency of Drinking Water Treatment for Viruses and Protozoan Cysts,” Applied and Environmental Microbiology, Vol. 59, No. 8, 1993, pp. 2418-2424.
- B. Jimenez, “Helminth Ova Control in Sludge: A Review,” Water Science and Technology, Vol. 56, No. 9, 2007, pp. 147-155.
- B. Jiménez, C. Maya, E. Sánchez, A. Romero, L. Lira and J. A. Barrios, “Comparison of the Quantity and Quality of the Microbiological Content of Sludge in Countries with Low and High Content of Pathogens,” Water Science and Technology, Vol. 46, No. 10, 2002, pp. 17-24.
- L. Mocé-Llivina, M. Muniesa, H. Pimenta-Vale, F. Lucena and J. Jofre, “Survival of Bacterial Indicator Species and Bacteriophages after Thermal Treatment of Sludge and Sewage,” Applied and Environmental Microbiology, Vol. 69, No. 3, 2003, pp. 1452-1456.
- F. Lucena and J. Jofre, “Potential Use of Bacteriophages as Indicators of Water Quality and Wastewater Treatment Processes,” In: P. M. Sabour and M. W. Griffiths, Eds., Bacteriophages in the Control of Foodand Waterborne Pathogens, ASM Press, Washington DC, 2010, pp. 103- 118.
- C. Gantzer, P. Gaspard, L. Galvez, A. Huyard, N. Dumouthier and J. Schwartzbrod, “Monitoring of Bacterial and Parasitological Contamination during Various Treatment of Sludge,” Water Research, Vol. 16, No. 16, 2001, pp. 3763-3370.
- P. Gessel, N. Hansen, S. Goyal, L. Johnston and J. Webb, “Persistence of Zoonotic Pathogens in Surface Soil Treated with Different Rates of Liquid Pig Manure,” Applied Soil Ecology, Vol. 25, No. 3, 2004, pp. 237-243.
- WHO, “The Global Burden of Disease. 2004 Update,” World Health Organization, Geneva, 2008. http://www.who.int/healthinfo/global_burden_disease/en/
- A. Prüss-Üstün and C. Corvalan, “Preventing Disease through Healthy Environments. Towards an Estimate of the Estimate of the Environmental Burden of Disease,” WHO Press, World Health Organization, Genev, 2006, p. 105.
- J. Lasobras, J. Dellundé, J. Jofre and F. Lucena, “Occurrence and Levels of Phages Proposed as Surrogate Indicators of Enteric Viruses in Different Types of Sludges,” Journal of Applied Microbiology, Vol. 86, No. 4, 1999, pp. 723-729.
- ISO, “ISO 10705-2: Water Quality—Detection and Enumeration of Bacteriophages—Part 2: Enumeration of Somatic Coliphages,” International Organisation for Standardisation, Geneva, 2000.
- Norma Oficial Mexicana, “Lodos y Biosólidos. Especificaciones y Límites Máximos Permisibles de Contaminantes Para su Aprovechamiento y Disposición Final,” NOM-004-ECOL-2002, 2002, 61 p.
- S. Astals, C. Venegas, M. Peces, J. Jofre, F. Lucena and J. Mata-Alvarez, “Balancing Hygienization and Anaerobic Digestion of Raw Sewage Sludge,” Water Research, Vol. 46, No. 19, 2012, pp. 6218-6227.
- K. Cheung, M. Lang and S. Smith, “The Effects of Centrifugation Dewatering on Escherichia coli Numbers in Digested Sewage Sludge,” Proceeding of the Joint CIWEM Aqua Environmental Technology Transfer 8th European Biosolids and Organic Residuals Conference, Wakefield, 24-26 November 2003.
- M. C. Monteleone, D. Furness, B. Jefferson and E. Cartmell, “Fate of E. coli across Mechanical Dewatering Processes,” Environmental Technology, Vol. 25, No. 7, 2004, pp. 825-831.
- J. Jofre, “Is the Replication of Somatic Coliphages in Water Environments Significant?” Journal of Applied Microbiology, Vol. 106, No. 4, 2009, pp. 1059-1069.
- B. Jimenez, C. Maya and G. Salgado, “The Elimination of Helminth Ova, Faecal Coliforms, Salmonella and Protozoan Cysts by Various Physicochemical Processes in Wastewater and Sludge,” Water Science and Technology, Vol. 43, No. 12, 2001, pp. 179-182.
- P. Jatinder, J. P. S. Sdihu and S. G. Toze, “Human Pathogens and Their Indicators in Biosolids: A Literature Review,” Environmental International, Vol. 35, No. 2, 2009, pp. 187-201.
- USEPA, “Environmental Regulations and Technology. Control of Pathogens and Vector Attraction in Sewage Sludge, Appendix F—EPA/625/R-92/013,” Office of Research and Development, 1999.
- S. K. Spillmann, F. Traub, M. Schwyzer and R. Wyler, “Inactivation of Animal Viruses during Sewage Sludge Treatment,” Applied and Environmental Microbiology, Vol. 53, No. 9, 1987, pp. 2077-2081.
- C. Bean, J. Hansen, A. Margolin, H. Balkin, G. Batzer and G. Widmer, “Class B Alkaline Stabilization to Achieve Pathogen Inactivation,” International Journal of Research in Public Health, Vol. 4, No. 1, 2007, pp. 53-60.
- B. Mignotte-Cadiergues, C. Find all citations by this author (default).Orfilter your current searchGantzer, L. Find all citations by this author (default).Schwartzbrod, “Evaluation of Bacteriophages during the Treatment of Sludge,” Water Science and Technology, Vol. 46, No. 4, 2002, pp. 189-194.
- R. Gibbs, C. Hu, G. Ho and I. Unkovich, “Regrowth of Faecal Coliforms and Salmonellae in Stored Biosolids and Soil Amended with Biosolids,” Water Science and Technology, Vol. 35, No. 11, 1996, pp. 269-275.
- K. J. Zaleski, K. L. Josephson, C. Gerba and P. Pepper, “Potential Regrowth and Recolonization of Salmonellae and Indicators in Biosolids and Biosolid Amended Soil,” Applied and Environmental Microbiology, Vol. 71, No. 7, 2005, pp. 3701-3708.
NOTES
*Corresponding author.

