Journal of Water Resource and Protection
Vol.5 No.11(2013), Article ID:40076,11 pages DOI:10.4236/jwarp.2013.511109
Environmental Issues of a Marine Protected Area in a Tectonic Estuary in the Tropical Eastern Pacific: Uramba (Malaga Bay Colombia): Context, Biodiversity, Threats and Challenges
1Research Group in Estuaries and Mangroves of Colombian Pacific, Departamento de Biología, Universidad del Valle, Cali, Colombia
2Research Group in Taxonomy, Systematic and Marine Ecology, Instituto de Investigaciones Marinas y Costeras “José Benito Vives de Andreis”, Santa Marta, Colombia
Email: *jaime.cantera@correounivalle.edu.co
Copyright © 2013 Jaime Ricardo Cantera Kintz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 18, 2013; revised February 21, 2013; accepted March 18, 2013
Keywords: Protected Marine Areas; Resources Conservation; Estuaries; Tropical Eastern Pacific
ABSTRACT
The environmental protection of tropical marine and coastal areas faces different challenges due to the diversity of aspects related to these areas, which include natural, social and economical issues. Despite these challenges, efforts for the protection of these areas are urgent nowadays because of the dramatical increase of human related threats like habitat destruction and population growth. Malaga Bay (MB) is a tectonic estuarine system located in Panama Bight (central region of the Colombian Pacific coast), which due to its origin shows important environmental characteristics of few sites in the Tropical Eastern Pacific (TEP) match. For these reasons, the government of Colombia declared in 2010, the bay’s marine area as a Marine National Natural Park in order to preserve its unique estuarine marine biodiversity. Despite this measure, MB presents several conditions that make environmental protection a very difficult task. In this paper, we present the geographical context, biodiversity and natural resources, environmental threats, the complexity of economic and social context, and the institutional and legal context of MB, to exemplify the difficulty that the protection of marine areas face in the TEP.
1. Introduction
The environmental protection of highly diverse tropical marine and coastal areas (e.g. estuaries, mangroves, sand beaches, mud flats, coral reefs and rocky shores), faces three main challenges: (1) the gathering of biological knowledge about the diversity of ecosystems and species useful for conservation, (2) the inclusion of local communities in facilitating this knowledge and participating in decision-making processes, and (3) the complexity of the environmental issues and the conflicts that such complexity generates. It is necessary to be aware of all these aspects in order to guarantee the generation of better policies and actions to allow conservation and sustainable use of resources and ecosystems. These actions are even more important in vulnerable areas such as tropical estuaries where environmental conditions are very adverse.
As human population growth and habitat destruction increase dramatically, marine and coastal species may face higher extinction risks [1]. This issue has been detected by the loss of marine biodiversity and the worldwide decline of fishery catches during the last century, which has increased the extinction risk of some poorly known or unknown species. Estuarine areas are largely especially prone to the loss of biodiversity and should be studied before important processes of extinction take place. These ecosystems should be preserved for all the environmental values, including its biodiversity richness, and ecological processes that occur in them and for the goods and services they provide to human beings.
Malaga Bay (MB) is a tectonic estuarine system located in Panama Bight, in the central region of the Colombian Pacific coast within the Tropical Eastern Pacific (TEP) region (Figure 1). It has a ca. area of 136 km2 and an average depth in the range of 12 - 15 m [2]. The geographical, geological, and oceanographic conditions as well as the high species and ecosystem richness make MB a special place with high environmental importance that few other sites in the TEP present.
The bay has been recognized environmentally due to the presence of areas for breeding and larval development of invertebrates and fishes [3]. This region was acknowledged by Haffer [4] and Prance [5] as a refuge area during the late Pleistocene, enhancing the current biodiversity hot spot. In addition, MB has iconic species such as the humpback whale (Megaptera novaeangliae), breeding colonies of marine and shorebirds and seawater and freshwater turtle populations [6]. Besides presenting natural conditions of strategic importance for the conservation of biodiversity, MB also provides a variety of environmental goods and services; however, MB has been subjected to various factors of pressure and threats, mostly due to the development unsustainable production practices [7].
The government of Colombia recognized the great importance of MB and in order to hamper the environmental deterioration and the loss of species richness; declared the waters of the bay as a National Natural Park and some zones as Coastal Protected Areas: making it a valuable natural heritage for the country. Despite this measure, MB faces several conditions that make environmental protection a very difficult task. Hence, the objective of the this paper is to address the main issues that make the marine and estuarine biodiversity conservation in MB difficult, by examining five main issues: (1) geographical context, (2) biodiversity and natural resources, (3) environmental threats, (4) complexity of the economic and social context at a local scale, and (5) the legal and institutional context.
2. Geographical Context
MB is a tectonic estuarine system located in the central region of the colombian Pacific coast (3˚15'60'' - 4˚10'50'' N; 77˚11'90'' - 77˚12'10''W) within the TEP region (Figure 1). As a tectonic estuary, it has a recent geological origin and is associated with major geological processes as faulting, erosion and, subsidence. This estuarine system is special, because most of other estuaries of the Colombian Pacific coast are flooded river valleys [8].
The bay has a length of approximately 25 km and an
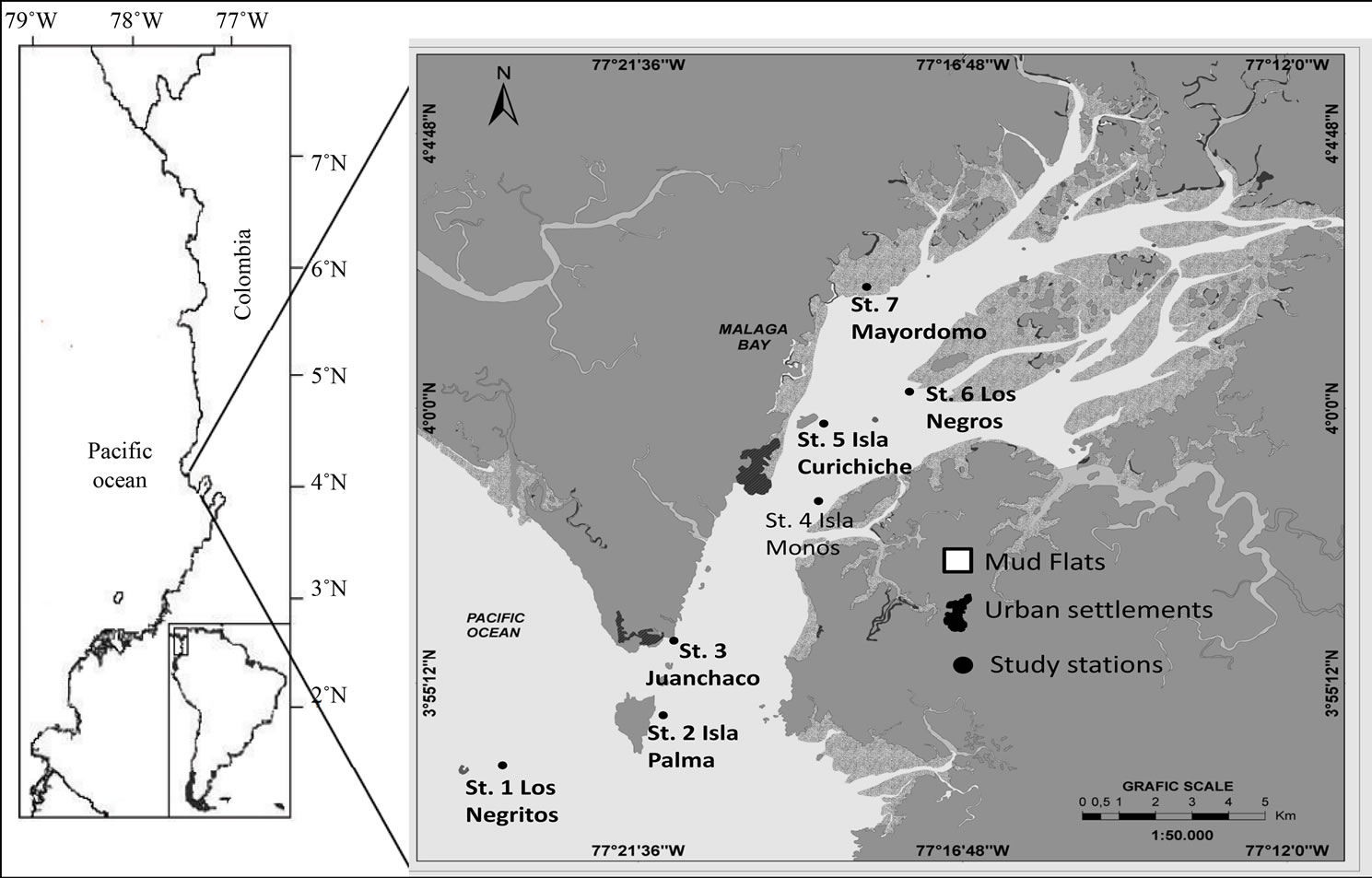
Figure 1. Sampling localities at Malaga Bay. Main populated sites are: Juanchaco and Ladrilleros (hereafter considered as Juanchaco), and the Navy Base near Curichichi (Modified of Guevara et al., 2011).
area of 136 km2, has a bearing of 351 N-NE and is surrounded by tertiary rocky cliffs (200 - 300 m high). The bay is subjected to a semidiurnal high mesomareal to macrotidal range (ca. 4 m), which affects both salinity and depth. The tidal current flux is ca. 20000 m3∙s−1 between the bay and the open ocean. The north reach is characterized by a geological fault (Malaga, also known as the Magdalena Fault) and the south reach is limited by the uplifting of the Pichido Isthmus [8,9].
The bay has two channels, one with hard substrate and average depths of around 15 m (maximum near 40 m), and the other with soft sediments and depths near 5 in low tide and 10) m in high tide. These channels converge in the narrow part of the bay, increasing the speed of the tidal current, which reaches up to 2.0 ms−1 [10,11]. In the inner part, the bay is shallow with muddy bottoms. Water temperature ranges from 24˚C to 30˚C. The area where the bay is located has one of the highest precipitation rates (6000 mm∙year−1) of the lowlands worldwide [12]. Climatic conditions vary from less humid (dry) between JuneJuly and December-January to more humid (rainy) in February-May and September-November.
Variation in salinity of the bay is low for an estuary, but it is basically explained by three aspects: 1. The tectonic formation of bay, 2. the presence of two creeks in the middle of the bay and in the inner zone of the bay, and 3. the influence of the river San Juan in periods of high rainfall in the outer zone. These characteristics make BM geographically different from others estuaries.
3. Biodiversity and Natural Resources
3.1. Marine Biodiversity Assessment
The MB geological and climatic features support a significant biological diversity. MB harbors a wide variety of habitats for aquatic and terrestrial (water-dependent) species from both, the continental shelf and the marine, estuarine and coastal waters (Table 1). Several species inhabiting the bay permanently or temporarily are considered of global importance from ecological, economic and social views (e.g., marine birds, turtles and marine mammals, as well as populations of migratory freshwater and marine fishes that use the bay for reproduction) [13]. It is an area particularly rich in fisheries with few endemic species and high biodiversity When comparing the biodiversity of MB with other areas of the Atlantic and Pacific coasts of South America, it is difficult to find another place with a larger inventory of marine taxa [14]. The majority of the other bays in the Pacific coast of Colombian barely reach half of the species number known for MB. Additionally, the number of species of MB may increase significantly when certain groups become better known e.g., zooplankton, which has been omitted from this report due to its limited knowl
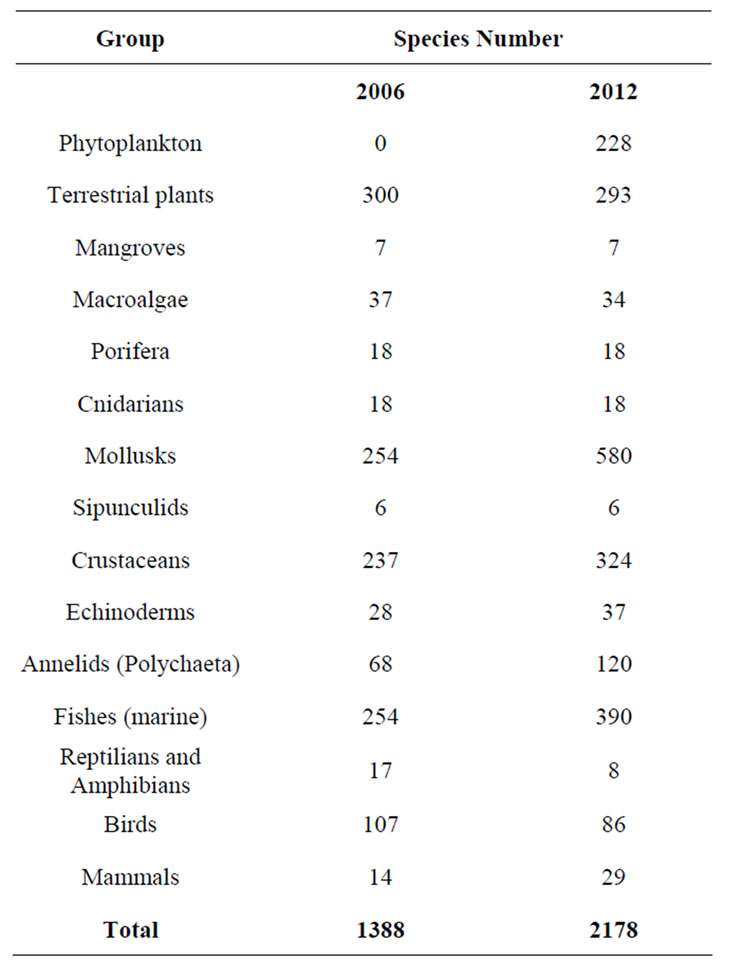
Table 1. Number of recorded species in Malaga Bay (MB) protected area by taxonomic group. Sources: [20,23].
edge (a preliminary estimate shows that there might overcome least more than one hundred species in 21 major groups, with Copepods as the most diverse with 6 or 7 genera and about 50 species) [15].
MB is one of the main resting, breeding and feeding grounds for marine and migrant shorebirds in the Colombian Pacific region. There have been reported, by the Calidris Foundation 24 species of birds that use the area for nesting, feeding and resting; 58% of which are nonbreeding residents. On the other hand, MB serves as a wintering ground for several species of which the 13% comes from the subtropical Pacific, 28% from the TEP, 42% from North America, 17% from the Arctic and only 1% from the Atlantic [16].
MB is also renowned worldwide for the seasonal migration (highest densities during September and October) of the humpback whale Megaptera novaeangliae [11] [17], where it carries out basic activities of its life cycle, such as mating, parturition, lactation and breeding as well as resting and socializing [18]. According to studies conducted during several years by the Yubarta Foundation, MB is a very important area for the reproduction of this species and the rearing of its calves. They have reported that 58% to 71% of the groups observed in MB during the season have calves [18,19]; these groups tend to spend most of their timenear Isla Palma, Los Negritos and inside of the bay, including areas with shallow waters (less than 25 meters) [17,19]. Other mammals common to the waters of MB are the Bottlenose dolphins (Tursiops truncatus) and the pan-tropical Spotted Dolphin (Stenella attenuata). These species have been reported as using the waters of MB as feeding ground [17].
3.2. Marine and Coastal Ecosystems
Sand beaches like Juanchaco and Ladrilleros are the main features of the external western reaches of MB. These beaches have been formed by the accumulation of sand carried by rivers and marine hydrodynamic processes. There are also some small islands located at the entrance of the bay, the main island is locally known as Isla Palma, others of important size are Morro Chiquito and Morro del Medio; there is also an important intertidal offshore rocky reefs (Los Negritos) and many inside of the bay similar ecosystems, of which Los Negros is the biggest and better known. Another important and very interesting feature of this bay is an archipelago located at the interior of MB; this archipelago (La Plata), is composed of more than 10 small islands and islets surrounded by mangrove and muddy flats [9].
MB harbors most of the habitats and marine life zones presented in the Colombian Pacific coast. These ecosystems are: low hills, sandy beaches of high and low energy, cliffs with bioerosion processes, intertidal and shallow subtidal rocky shores, submerged areas (with bottoms of different types: mud, gravel, rock), mud flats, creeks, and about 3000 hectares of mangroves (Rhizophora mangle, R. racemosa, Avicennia germinans, Laguncularia racemosa, Conocarpus erecta, Pelliciera rhizophorae and Mora oleifera) in good condition (there are some dwarf mangroves growing on rocky substrates), and pelagic and littoral habitats. In addition, there are few rivers that limit fresh water inputs, which is low compared to most estuaries of the Colombian Pacific coast [20]. Each of these environments has a major biological community that maintains the ecological processes necessary to sustain biodiversity and ecosystem productivity [21]. All of these ecosystems present a relatively high number of associated species (Table 2).
Table 2 Species richness by taxa in the different ecosystems found at Malaga Bay (MB). One species can inhabit more than one ecosystem. Mangrove: Mangrove forest; Bottoms: submerged bottoms; Sand: sand beaches; Mud: Mud flats; Rock: Rocky shores and cliffs; Pelagic: Pelagic environment. Sources: [20,22,23].
Of the 2178 species present in MB up-to-date, most can be found in the Pelagic environment (649 species: 29.8%), followed by rocky ecosystems (508 species: 23.3%), submerged bottoms (469 species: 21.5%), sandy beaches (408 species: 18.7%) and mud flats (428 species:
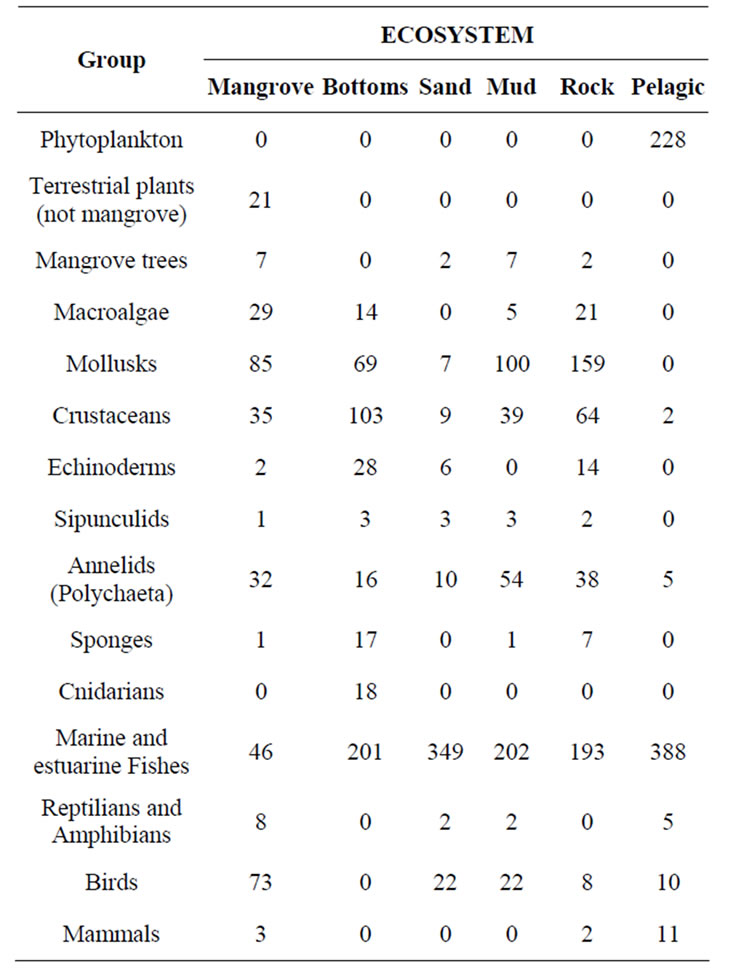
Table 2. Species richness by taxa in the different ecosystems found at Malaga Bay (MB). One species can inhabit more than one ecosystem. Mangrove: Mangrove forest; Bottoms: submerged bottoms; Sand: sand beaches; Mud: Mud flats; Rock: Rocky shores and cliffs; Pelagic: Pelagic environment. Sources: [20,22,23].
19.7%); Mangrove forests harbor the lowest species richness (336 species: 15.4%). The most common organisms found in this ecosystem are algae, vascular plants, mollusks, crustaceans and birds. Crustaceans, mollusks, echinoderms, sponges and cnidarians are richer in submerged bottoms, rocky shores and cliffs. Polychaetes and sipunculids are abundant in sandy beaches and muddy flats. Fishes are abundant in pelagic environment and submerged bottoms [8,21].
3.3. Fisheries Resources
With respect to fisheries resources of MB, Rubio [24] reports that around 100 commercially important fish species can be found in the area, most of which support the artisanal and industrial fisheries. He highlights the families Clupeidae (sardines), Engraulidae (anchovies), Carangidae (jacks), Mugilidae (mullets), Centropomidae (bass), Sciaenidae (croakers, grunts), Ariidae (catfish, goats) and Gerreidae (sunfish) as the most important fish resources for the area. In particular, MB contributes with perhaps the greatest proportion of the small pelagic (engraulids) fisheries in the Colombian Pacific coast [25].
Other fisheries include the fishing of “Jaiba” (Callinectes toxotes), Blue-crab (“Cangrejo azul” in Spanish) (Cardissoma crassum),” Mapara” (Gecarcinus lateralis), “Halacho” (Ucides occidentalis), White shrimp (Litopenaeus occidentalis, L. vannamei, L. stylirostris), Tiger shrimp (Rimapenaeus byrdi), and “Titi” shrimp (Xiphopenaeus kroyeri). There are also several species of mollusks that are used by fishermen, such as the “Piangüa” (Anadara tuberculosa, A. similis), “Sangara” (Grandiarca grandis),” Piacuil” (Littoraria zebra, L. fasciata), Beach clams (Donax assimilis), “Chorga” (Iliochione subrugosa), and “Burgao” (Melongena patula) [26].
There are more than 1500 artisanal fishermen and the annual catch per year is approximately 200 million represented almost 170 ton fishes and 200 ton shrimp
3.4. Focal Species
The species considered in this issue can be: species in risk of extinction, endemic, species supporting goods and services to the inhabitants of the bay, and bioengineering, keystone and flagship species [7]. There are important quantitative variations of focal species along MB, even at short spatial scales. Los Negritos, the external rockysandy reef complex, has around 40 - 61 focal species while Isla Palma, located ca 4.5 km from the former, has around 30 - 39 focal species [20].
Endemism in the marine coastal ecosystems of MB is poorly known and nowadays, it is represented by four species of crustaceans: Pinnotheres malguena, Hypolobocera malaguena, Cleantoides vonprahli and Synalpheus arostris. Most of the marine invertebrate fauna of MB belongs to the fauna of the Panamic Pacific Province; however, some species of the Peruvian-Chilean and the Indo-Pacific provinces have been found. In MB, several new species for science have also been reported; these include: Alpheus wickstenae, Alpheus colombiensis, Cleantioides vonprahli, Synalpheus arostris, Philocheras lepillus, Neopontonides henryvonprahli and Lepido-phthalmus bocourti [20]. They are considered as endemic at present time In terms of food supply, the most important species for the inhabitants of the area include the “piangüa”, with two species Anadara tuberculosa, and A. similis, the “sangara” (Grandiarca grandis), mangrove crabs: Cardisoma crassum and Ucides occidentalis, swimming crabs Callinectes toxotes and C. arcuatus, and shrimps, among them, Litopenaeus occidentalis, L. vanamei, and Xiphopenaeus riveti. Concerning the fish fauna present in the area of MB, there are 57 families and about 219 species of fish that serve the inhabitants as food, actual or potential. Among these species, the most representative include the following: “lisas” (Mugilidae), “gualajos” and “machetajos” (Centropomidae), “meros” (Serranidae), “corvinas” (Sciaenidae), “pargos” (Lutjanidae) and “jureles” (Carangidae) [20,27,28].
Castellanos-Galindo et al. [7], after a comprehensive review of redbooks of threatened species, scientific and gray literature, and surveys to expert scientists and local people in the bay, were able to asses changes on biodiversity over time. They listed 45 invertebrates and fish species with risk of local extinction by different threats. The most important are: Anadara tuberculosa, A. similis, Grandiarca grandis, Pinctada mazatlanica, Lobatus galeatus, Iliochione subrugosa, Litopenaeus occidentalis, Panulirus gracilis, Carcharhinus limbatus, C. porosus, Pacifigorgia spp., Cetengraulis mysticetus, Lutjanus spp., Pristis pristis, Sphyrna lewini, Epinephelus quinquefasciatus and Hippocampus ingens. The Humpback Whale (Megaptera novaeangliae), has been declared Vulnerable (VU) by IUCN [11].
4. Environmental Threats
4.1. Threats to Biodiversity
Due to the threats that have been rising during the last years, there is a need for the protection of MB’s biodiversity. The threats include: 1) degradation of marine ecosystems such as beaches, mangroves, estuaries and rocky shores marked loss of habitat, 2) disruption of biological cycles and decreasing of stocks of fished populations as a consequence of overexploitation or navigation practices and operations of new ports, 3) sediment re-suspension and settlement, 4) pollution: introduction and recycling of toxic pollutants to the bottom and the water column (waste disposal, hydrocarbons, sewage, mainly by the floating population), 5) ingestion and accumulation of contaminants by wildlife, 6) increasing water turbidity and decreasing O2 concentration, 7) modification of bathymetry causing changes in circulation, 8) alteration of species diversity and structure of benthic communities, (9) eutrophication processes, finally 10) dredging of channels causing the suspension of sediments in the water column, affecting mainly benthic populations, both adult and larval forms. Additionally, ENSO events (El Niño), the ocean hydrodynamics (waves, currents, tides), the bioerosion of cliffs and beach erosion and accretion are environmental factors with high impact on the bay. The main biological processes affected by these threats are shown in Table 3 where we can see that the marine taxonomic group that presents the major risks of deleterious changes in bioecological processes is fishes, especially those of economic importance. Moreover most affected biological processes are related to reproduction (mating, eggs, larvae, youth and recruitment given the condition nursery of the waters, ecosystems and bottoms of the bay. Similarly the probability of increased mortality of marine biodiversity affects marine biodiversity.
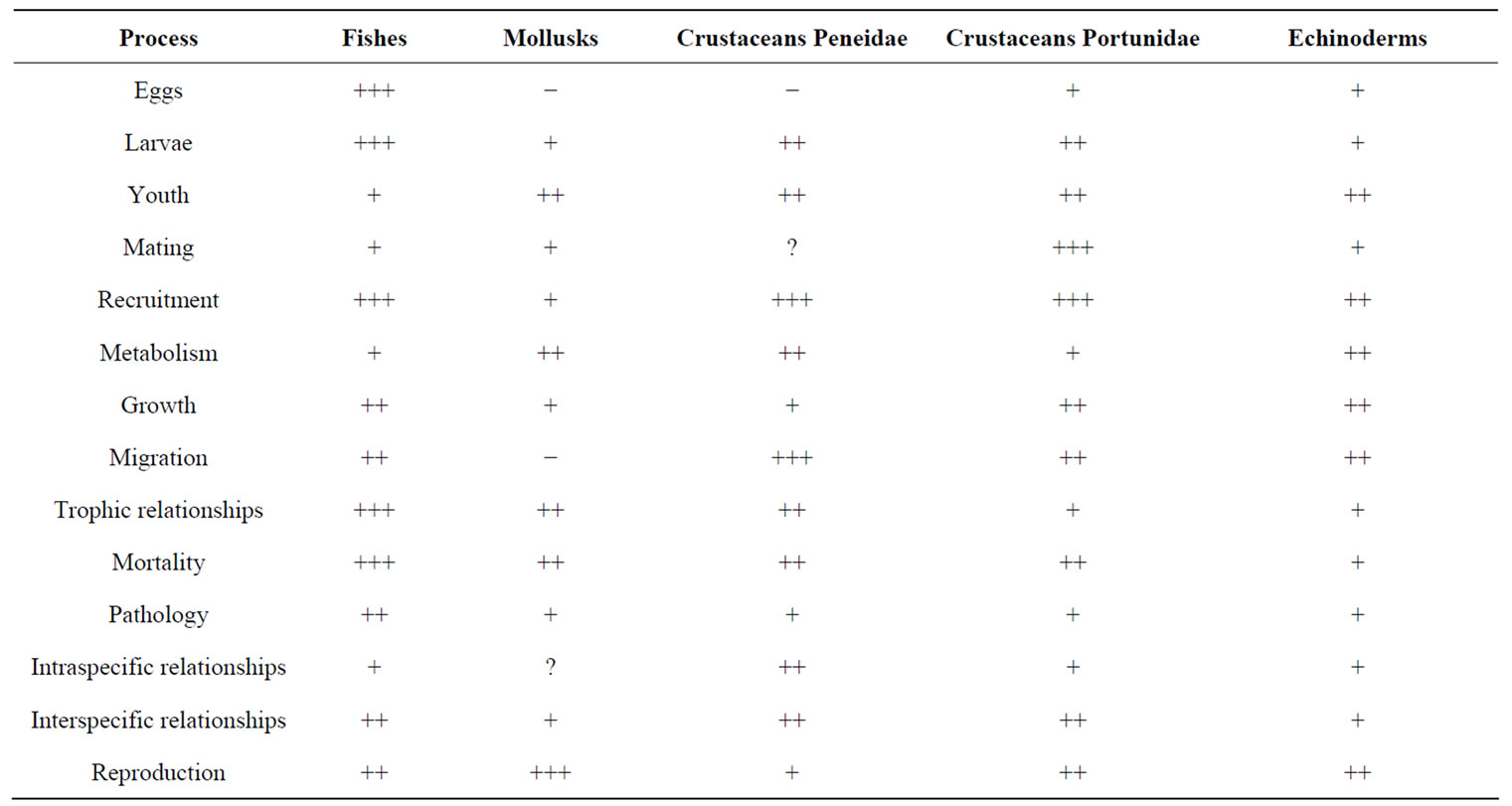
Table 3. Biological processes affected, by the main threats occurring in MB, in the major taxonomic groups. (−): Non affected; (+): mildly affected; (++): moderately affected (+++): strongly affected; (?): without information, sources: [22,23].
4.2. Environmental Vulnerability
Considering that there are, on average, 166 trees/ha (in an area of 3299.3 Ha), the mangroves of MB can be classified as moderately disturbed. For this reason, since 2006, the government has implemented policies about cutting and commercializing mangroves in order to ensure the conservation of this resource.
The outer parts of the bay are continuously exposed to strong wave action and subjected to heavy erosion. The action of the sea is reinforced by some organisms (bioeroders) that graze on algae and bore on the substrate, a process that accelerates the falling of big parts of the cliffs. As a result, the geomorphology of the coast changes rapidly and continuously, leaving islands and peninsulas [9]. For example, Isla Palma, which is exposed to strong tidal currents, due to its location in the outer bay, suffers active bioerosion processes that have determined the formation of extensive abrasion platforms of hard substrates, dominated by sedimentary rocks of sandstone and shale.
Sandy beaches of MB represent one of the most highly dependent marine environments on physical conditions, being directly affected by tidal action. According to López-Victoria et al. [29], MB shows a number of factors related to the deterioration of some beaches, such as solid waste pollution (e.g. glass, cans, and plastics); compaction of the sand (due to tourism), sand mining (for housing construction) and erosion. These factors contribute to the loss of beaches and therefore of species biodiversity, even of human settlements like Juanchaco, where people living at the shoreline has to continuously relocate their homes [30].
Regarding submerged bottoms and pelagic zone, sources of stress include the sediments that are supplied in abundance in the internal part by La Sierpe River and other creeks, and in the external part, mainly by the plume of the San Juan River. In MB, there are also direct discharges of wastewaters due to the absence of sewage systems. However, it does not affect the water quality of the bay, possibly due to the hydrodynamics. Recent data from physicochemical studies of the water in several locations in the bay reported values within a range that do not significantly affect the ecosystem [31].
5. Complexity of Economic and Social Context at a Local Scale
The region’s population reaches around 10000 inhabitants [32]; it is composed mainly by Afro-Colombians (90%), different ethnics of Colombian aborigines (3%), and white-mestizos (7%). Social differentiation is imperceptible, for this reason, local residents have multiple activities and should combine fishing with farming, hunting, timber extraction and often “unskilled labor” in the countryside or in the city [33]. The education in BM is deficient in terms of coverage, quality and internal efficiency. The Afro-Colombian population has the lowest rates of school attendance among 7 - 11 years old and the illiteracy rate of the population over 12 years in Buenaventura is 28% for indigenous, 9.5% for afrocolombians and 3.9% for non ethnics people [34]. Traditional productive activities are not oriented to accumulate wealth, but to satisfy immediate needs for food, shelter, transportation and if there is any production surplus, for trade [35].
Juanchaco, Ladrilleros, and the Navy Base are the major urban settlements. These small towns (Juanchaco and Ladrilleros) were formed by families of nomads that settled on the coastal landscapes centuries ago (ca. 300 years). Today the percentage of Afro-Colombians of Ladrilleros and Juanchaco remains above 90%. Although these localities are well recognized as important beaches for tourism in the Colombian Pacific coast, their population remains reduced because there is a balance between the continuous birth of children and the migration of young to nearby cities as Buenaventura or Cali. On the other hand, during the so-called tourist season, the population in the area can double [35].
Juanchaco and Ladrilleros communities derive their economy mainly from tourism. Residents provide accommodation services, restaurant and commercial facilities, tours to nearby natural landscapes, etc. It has been estimated that during this decade, whale sightseeing has produced US $6,000,000, representing an important source of income [11,36]. Fishing is another important economic activity, in which 10% to 20% of the population uses traditional fishing gears. The communities of La Barra and La Plata, whose residents retain a great degree of their traditional culture, derive their subsistence from several simultaneous activities including artisanal fisheries, selective timber cutting, hunting and sowing in the riversides and streams. In the Pacific Afro-Colombian communities, men and women have different types of work. The men are engaged in fishing while women are gatherers, mainly of mollusks. Another economic activity is the exploitation of forests, 6% of the total population is dedicated to timber logging used for the construction of boats and houses [35]. The aborigines are represented by Wounan, Embera-Eperara and Sapidara. They are located in the northern part of the San Juan River Basin (Embera-Eperara), Bongo, Cocalito and Cerrito-Bongo (Wounan) and a traveling community (Sapidara). They are gardeners (orchard crops, or “conuco” or “chagra”, or shifting itinerant agriculture) and hunters [35].
All BM human communities travel along the area through the sea, estuaries and rivers, using means of transportation that are amiable with the environment and have gotten a high degree of communion with nature and its biological system. The human-nature-culture interaction is presented as an environmental philosophy, which defines rhythms of life and establishes rights for human beings, the fauna and the flora, in relation with the air, the rivers, the sea and the rain. It is a flexible territorial system characterized by the respect for life and the coexistence of visible and invisible beings, spirits that haunt all aspects of the social and cultural particularities of these human communities [35].
6. Institutional and Legal Context
Recently, the MB marine and estuarine was declared as the Uramba-Bahía Málaga National Natural Park (NNP) and is under the jurisdiction of the “Ministerio de Ambiente y Desarrollo Sostenible” (Ministry of Environment) and therefore is subject to different legal and institutional instruments of environmental protection. This NNP has an extension of 47,094 Ha. There are also other government authorities that have jurisdiction over BM, including the Ministry of National Defense, which has a reserved area for a Navy Base and an information system for the regulation and control of navigation. BM is under the political jurisdiction of Valle del Cauca department, and its environmental authority, the CVC (Corporación Autó- noma Regional del Valle del Cauca), is in charge of the protection of the terrestrial zones, the river basins and the coastal zones (down to the low water mark); this corporation is in charge of generating policies, coordinate other institutions and implement regulations for the integrated management of the coastal zone.
The main legal provisions and related regulations, in chronological order, on environmental management of marine and estuarine areas of MB are presented in Table 4.
7. Conclusions
Unlike other conservation areas in the world, MB is quite pristine and has not reach a high level of development involving an immediate threat to biodiversity and to marine and coastal ecosystems. There are not large human populations and the existing settlements tend to decrease due to the lack of new jobs and opportunities for the young people. The only threat to looms is the remote possibility of building and operating of a deep water port for large vessels.
However, despite two major natural hazards: coastal erosion (accelerated by the participation of organisms) and bioerosion (substrates movements due to changes in marine hydrodynamics), there are some man-made hazards due to the lack of planning and organization between communities and environmental authorities.
These threats include the overexploitation of resources (mainly crustaceans, mollusks and some fish species by artisanal practices), the destruction of natural habitats by cutting mangroves, pollution by seasonal tourism (beach activities, whale watching) and the disorderly movement of communities.
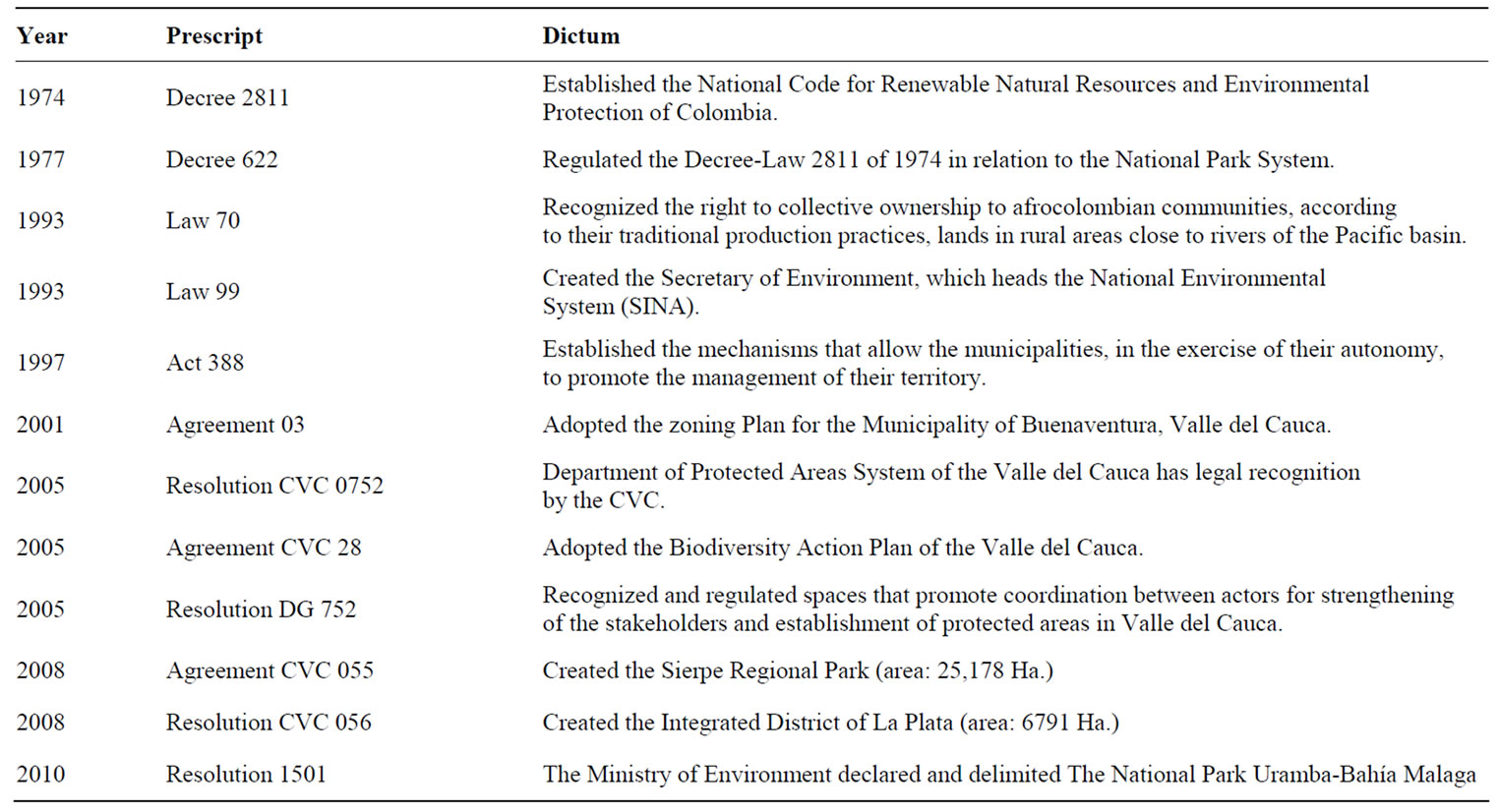
Table 4. Legal provisions on management of MB areas.
In this context the main reasons to establish an appropriate management and ensure the conservation of this protected area are:
1. MB is one of the areas with the highest marine and coastal biodiversity of the South American Pacific coast. Currently, it has 2178 recorded species related to a surface of ca. 136 km2 distributed in a great variety of taxa. This number may increase in future inventories with the inclusion of other groups (e.g. zooplankton) and when other areas and taxa get better sampled.
2. MB has a complex and rich ecosystem structure, a high number of habitats as well as important marine and coastal communities. Many of them are strategic ecosystems which give a value added to the area for its high biodiversity, high habitat heterogeneity and its significant biological and geological processes.
3. More than 250 aquatic species living in MB have actual or potential economic importance or represent one or more category of focal species that can be considered as conservation criteria.
4. More than 200 species use BM for reproduction or for other vulnerable stages of their life cycle in MB
5. Most of the bay is still in very good environmental status, without destruction of ecosystems and without high levels of pollution
6. Scientific research conducted primarily by the University of Valle, the Institute of Marine Research INVEMAR Colombia, NGOs and other universities, has created a depth knowledge in most natural key issues.
7. The existing legal basis with abundant instruments, including different levels (national departmental and local) facilitates the generation and implementation of specific conservation measures.
8. Regional human communities have sufficient knowledge about conservation values of the area and have already understood the importance of preserving this unique region. They have also acquired a degree of commitment and involvement much higher than in most other regions of Colombian Pacific coast However, some challenges still need to be overcome to achieve the conservation of MB. On the one hand, the permanent application of scientific, environmental and human knowledge and on the other hand, ensure that social and economic development is achieved based on ecosystem goods and services of the bay, without affecting its natural values and environmental conditions.
Regarding MB, decision-making institutions have build up policies based on the knowledge generated by research institutions, universities and non-governmental organizations (NGOs). The process to declare MB as a Marine Protected Area was accomplished due to the participation of community members, scientists working in academic and research institutions along with environmental authorities. This interaction allowed setting research goals and an adequate management plan.
In this process, the main role of science was to create objective knowledge at integrated ecosystem level and supply it to decision makers. This was a crucial step because it is known that the exchange of information between scientists and decision makers is difficult in most of Latin American countries resulting in limited planning of strategies especially in important economic sectors. Mutual distrust arises between scientists and decision makers, because while scientists believe that decision makers are not interested in using scientific information for the decision-making process, decision makers believe scientists create knowledge with no possible application or take too long to produce applicable information. Presently, there are a lot of documents about geology, oceanography, biodiversity, ecology, social aspects, economy as well as the legal and institutional framework related to MB. Converting all the information into an effective environmental protection and a habitat restoration plan, with governmental program and measures, is the greatest challenge these initiatives face. Once the diagnosis is completed and recommendations are made, there is a long and winding road before approval and adoption by the institutions in charge of issuing the governmental planning regulations, monitoring programs and providing education.
In MB, today the native society survives mainly due to the primary sector of the economy: fishing, gathering, logging and agriculture. The secondary economic sector consists of collection of small plants fish, shrimp and shellfish known as “piangua”.
There are also activities of harvesting of natural products such as wood in the coastal mangrove (mangroves, palms and other plants). All the area is currently under political and economic pressure due to regional, national and international interests (open policy economic, patents and genetic resources, biodiversity, natural resource exploitation, economic development plans).
In this context, the aim to ensure the conservation of natural resources is not an easy task, but the proper use of scientific information should enable natural means of production and exploitation of the landscape. This can be balanced with sustainability which requires the maintenance and improvement of the population’s living conditions. Alternative activities resulting from the eight reasons mentioned above to preserve the bay, as well as the conservation and sustainable development and application of programs in this area, should contribute to the achievement of that goal.
8. Acknowledgements
Funding for execution of the Project Vulnerabilidad de los ecosistemas marinos y costeros de Bahía Málaga (Pacífico colombiano): amenazas naturales y antrópicas has been supported by Colciencias, through grant No. 110652128786, RC 315-2011, Universidad del Valle (Cali, Colombia), and Invemar (Santa Marta, Colombia). The authors wish to express their appreciation to the communities of Bahía Málaga and to Ecomanglares research group for assistance in the field. Finally, the authors thank the valuable comments of Maria Emma Cantera Cadena which greatly improved the manuscript. Thanks to the Bank of inversions of projects BPIN INVEMAR the publication was funded according to the resolution number 0085 of February 2013.
REFERENCES
- J. E. M. Baillie, C. Hilton-Taylor and S. N. Stuart, “IUCN Red List of Threatened Species. A Global Species Assessment,” IUCN Gland, Switzerland and Cambridge, 2004. http://dx.doi.org/10.2305/IUCN.CH.2005.3.en
- Gobernación del Valle del Cauca, INCIVA, Universidad del Valle, CVC, Armada Nacional-Fuerza Naval del Pací- fico & Alcaldía de Buenaventura, “Documento Base Diagnostico Bahía Málaga,” Comité Departamental de Biodiversidad: Mesa de Trabajo Bahía Málaga, Valle del Cauca, Colombia, 2001.
- Invemar y Univalle, “El Papel de la Salinidad en las Asociaciones de Larvas de Organismos Marinos en Bahía Málaga (Pacífico colombiano): Valoración de la Importancia de esa área Como Salacuna y su Comportamiento con otros Estuarios en Hábitats Tropicales,” Cali, Colombia, 2011.
- J. Haffer, “General Aspects of the Refuge Theory,” Biological Diversification in the Tropics, Vol. 1, No. 1, 1982, pp. 6-24.
- G. T. Prance, “Forest Refuges: Evidence from Woody Angiosperms,” Biological Diversification in the Tropics, Vol. 1, No. 1, 1982, pp. 137-157.
- A. J. Capella and L. Flórez-González, “Changes in Winter Destinations and the Northern Most Record of Southeastern Pacific Humpback Whales,” Marine Mammal Science, Vol. 14, No. 1, 1999, pp. 189-196.
- G. A. Castellanos-Galindo, J. R. Cantera-Kintz, S. Espinosa-Guerrero and L. M. Mejia-Ladino. “Use of Local Ecological Knowledge, Scientist’s Observations and Grey Literature to Assess Marine Species at Risk in a Tropical Eastern Pacific Estuary,” Aquatic Conservation: Marine and Fresh Water Ecosystems, Vol. 21, No. 1, 2011, pp. 37-48. http://dx.doi.org/10.1002/aqc.1163
- C. E. Guevara-Fletcher, J. R. Cantera-Kintz, L. M. MejíaLadino and F. A. Cortés, “Benthic Macrofauna Associated with Submerged Bottoms of a Tectonic Estuary in Tropical Eastern Pacific,” Journal of Marine Biology, Vol. 1, No. 1, 2011, pp. 1-13. http://dx.doi.org/10.1155/2011/193759
- J. R. Cantera, “Etude Structurale des Mangroves et des Peuplements Littoraux des Deux Baies du Pacifique Colombien (Málaga et Buenaventura). Rapport avec les Conditions du Milieu et les Perturbations Anthropiques,” Thèse d’Etat Sciences, Université d’Aix-Marseille II, 1991.
- WWF y Cenipacifico, “Declaratoria de la Zona Marina y Estuarina de Bahía Málaga Como Área Marina Protegida,” Documento Técnico 056, 2007.
- L. Flórez-González, I. C. Ávila, J. Capella, P. Falk, F. Félix, J. Gibbons, Guzmán, B. Haase, J. C. Herrera, V. Peña, L. Santillán, I. C. Tobón and K. Van Warebeek, “Estrategia Para la Conservación de la Ballena Jorobada del Pacífico Sudeste. Lineamientos de un plan de Acción Regional e Iniciativas Nacionales,” Fundación Yubarta, Cali, Colombia, 2007.
- J. Eslava, “La Precipitación en la Región del Pacífico Colombiano (Lloró el sitio más Lluvioso del Mundo),” Revista Zenit, Vol. 3, No. 1, 1992, pp. 47-71.
- J. R. Cantera, A. Giraldo, V. Castrillon, F. Cortes, A. Guzman, L. M. Mejia-Ladino, H. Saenz, O. D. Solano and E. Montoya, “Informe del Estado de los Ambientes y Recursos Marinos y Costeros en Colombia. Año 2008,” Instituto de Investigaciones Marinas y Costeras Invemar, Santa Marta, Colombia, 2009.
- P. Miloslavich, E. Klein, J. M. Díaz, C. E. Hernández, G. Bigatti, L. Campos, F. Artigas, J Castillo, P. E. Penchaszadeh, P. E. Neill, Alvar-Carranza, M. V. Retana, J. M. Dıaz, M. Lewis, P. Yorio, M. L. Piriz, D. Rodrıguez, Y. Yoneshigue-Valentin, L. Gamboa and A. Martin, “Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps,” PLoS ONE, Vol. 6, No. 1, 2011, pp. 1-43. http://dx.doi.org/10.1371/journal.pone.0014631
- P. Espinal, A. Giraldo, M. Londoño and L. M. Mejía-Ladino, “Variabilidad en la Abundancia de Larvas de Crustáceos y Poliquetos en Bahía Málaga, Pacífico Colombiano (Enero-Junio de 2010),” Boletín de Investigaciones de Invemar, Vol. 41, No. 2, 2012, pp. 355-373.
- L. Castillo, L. Naranjo and A. Aparicio, “Importancia de la Bahía de Málaga Como Sitio de Descanso y Reproducción para aves Marinas en el Pacífico Colombiano. Documento Técnico de la Asociación para el Estudio y la Conservación de las Aves Acuáticas en Colombia,” Calidris, Cali, Colombia, 2005.
- L. Flórez-González, J. Capella, J. C. Herrera, P. Falk, I. C. Ávila, R. Londoño, A. Tobón, I. Tobón and V. Peña, “Distribución Espacial de la Ballena Jorobada en la Bahía de Málaga y Alrededores, Pacífico Colombiano,” XII Seminario Nacional del Mar, Santa Marta, Colombia, 2003, pp. 47.
- I. C. Ávila, “Patrones en la Conducta Superficial Diurna de la Ballena Jorobada (Megaptera novaeangliae) en la Bahía de Málaga y Zonas Aledañas, Pacífico Colombiano,” Tesis de maestría, Universidad del Valle, Cali, Colombia, 2006.
- R. Londoño, “Distribución Espacial de las Diferentes Agrupaciones de Ballenas Jorobadas (Megaptera novaeangliae), en Bahía Málaga y Alrededores, Pacífico Colombiano,” Tesis de Pregrado, Universidad de los Andes, Santafé de Bogotá, Colombia, 2002.
- Invemar, Univalle e Inciva, “BIOMÁLAGA: Valoración de la Biodiversidad Marina y Costera de Bahía Málaga (Valle del Cauca), Como uno de los Instrumentos Necesarios Para que sea Considerada un Área Protegida,” Informe Técnico, Cali, Colombia, 2006.
- L, Herrera, L. A. López de Mesa, L. M. Mejía-Ladino, C. Satizabal, A. Fuentes and J. R. Cantera, “La Función Ecológica de Salacuna del Estuario de Bahía Málaga (Pacífico Colombiano),” XIV Seminario Nacional de Ciencia y Tecnología del mar, Cali, Colombia, 2010, pp. 99-102.
- Univalle, “Estudio de Prefactibilidad Ambiental y Social Sobre la Construcción de un Puerto de Aguas Profundas en Bahía Málaga, Pacífico Colombiano,” Informe Técnico, Cali, Colombia, 2010.
- Univalle & Invemar, “Vulnerabilidad de los Ecosistemas Marinos y Costeros de Bahía Málaga (Pacífico Colombiano): Amenazas Naturales y Antrópicas,” Informe Técnico, Cali, Colombia, 2012.
- E. Rubio, “Estudio Taxonómico Preliminar de la Ictiofauna de Bahía Málaga, Colombia,” Cespedesia, Vol. 13, No. 47, 1982, pp. 97-111.
- L. A. Zapata, G. Rodríguez, B. Beltrán, G. Gómez, W. Angulo, A. Gómez, M. Ramírez, J. Morales, M. Hung, J. Herrera and C. Riascos, “Prospección de los Principales Bancos de Pesca en el Pacífico Colombiano, Durante Noviembre de 1998,” Boletín Científico INPA, Vol. 6, No. 1, 1999, pp. 111-175.
- H. V. Prahl, J. R. Cantera and R. Contreras, “Manglares y Hombres del Pacífico Colombiano,” Presencia, Bogotá, Colombia, 1990.
- J. R. Cantera, B. A. Tomassin and P. Arnaud, “Faunal Zonation and Assemblages in the Pacific Colombian Mangroves,” Hydrobiology, Vol. 413, No. 1, 1999, pp. 17-33. http://dx.doi.org/10.1023/A:1003890826741
- G. A. Castellanos-Galindo, J. A. Caicedo-Pantoja, L. M. Mejía-Ladino and E. Rubio, “Peces Marinos y Estuarinos de Bahía Málaga, Valle del Cauca, Pacífico Colombiano,” Biota Colombiana, Vol. 7, No. 2, 2006, pp. 263-282.
- M. López-Victoria, J. R. Cantera, J. M. Díaz, D. M. Rozo, B. O. Posada and A. Osorno, “Informe del Estado de los Ambientes y Recursos Marinos y Costeros en Colombia. Año 2003,” Instituto de Investigaciones Marinas y Costeras INVEMAR, Santa Marta, Colombia, 2004.
- B. O. Posada, W. Henao and G. Guzmán, “Diagnóstico de la Erosión y Sedimentación en la Zona Costera del Pacífico Colombiano,” Instituto de Investigaciones Marinas y Costeras, Santa Marta, Colombia, 2010.
- J. M. Betancourt-Portela, J. G. Sánchez-Diaz, L. M. MejíaLadino and J. R. Cantera-Kintz, “Calidad de las Aguas Superficiales de Bahía Málaga, Pacífico Colombiano,” Acta Biológica Colombiana, Vol. 16, No. 2, 2011, pp. 175- 192.
- DANE (Departamento Administrativo Nacional de Estadística), “Censo Nacional 2005: Proyección de Población Humana en el Municipio de Buenaventura, Valle del Cauca,” 2010. http://www.dane.gov.co
- N. S. Friedemann, “San Basilio en el Universo KilomboAfrica y Palenque-America,” Geografía Humana de Colombia. Los Afrocolombianos. Tomo VI, Vol. 1, No. 1, 1998, pp. 81-101.
- Corpoeducación, Fundación Corona, Empresarios por la Educación y Preal, “Informe de Progreso Educativo-2007- Valle del Cauca: Mejorar un reto Inaplazable,” Gobernación del Valle del Cauca, Cali, Colombia, 2007.
- G. N. Motta, “Identidad Étnica, Género y Familia en la Cultura Negra del Pacífico Colombiano,” Revista Enfoques, Vol. 10, No. 1, 1995, pp. 19-31.
- P. Falk, V. Peña, I. C. Ávila and L. Flórez-González, “Proceso Educativo Alrededor del Turismo de Observación de Ballenas Jorobadas (Megaptera novaeangliae) en el Pacífico Colombiano,” 11ª Reunión de Trabajo de Especialistas en Mamíferos Acuáticos de América del Sur y 5º Congreso de la Sociedad Latinoamericana de Especialistas en Mamíferos Acuáticos, 2004, Quito, Ecuador.
NOTES
*Corresponding author.

