Journal of Biomedical Science and Engineering
Vol.6 No.5(2013), Article ID:31886,8 pages DOI:10.4236/jbise.2013.65069
Protective effects of polar lipids and redox-active compounds from marine organisms at modeling of hyperlipidemia and diabetes
![]()
1Pacific Institute of Bioorganic Chemistry, Far Eastern Branch of Russian Academy of Science, Vladivostok, Russia
2Far Eastern State University, Vladivostok, Russia
Email: popovam@piboc.dvo.ru
Copyright © 2013 Alexander M. Popov, Olga N. Krivoshapko. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 23 February 2013; revised 28 March 2013; accepted 3 May 2013
Keywords: PUFA; Polyphenols; 1,4-Naphtoquinones; Diabetes; Hyperlipidemia
ABSTRACT
Cardiovascular diseases and diabetes mellitus are leading causes of mortality in modern society. The search for a novel effective remedy represents an important task for modern medicine. A total mixture of phosphoand glycolipids from sea macrophytes Sargassum pallidum, Ulva fenestrata, Zostera marina was separated and the fatty acid composition was determined. The biological activity of the mixtures of polar lipids and natural redox-active compounds (echinochrome A from the flat sea urchin Scaphechinus mirabilis and a polyphenolic complex from the sea grass Zostera marina) was studied under conditions of impairments of carbohydrate and lipid metabolism. Doses and compositions of mixtures of pola lipids and redox-active compounds possessing high corrective activity were optimized in mice with the experimental model of hyperlipidemia and diabetes. Based on these results possible mechanisms of the effects of polar lipids containing various polyunsaturated fatty acids and the investigated redox-active compounds (echinochrome A, rosmarinic acid, luteolin and its sulphate conjugates) have been proposed. The developed compositions may be used for creation of new biologically active additives and remedies.
1. INTRODUCTION
Cardiovascular diseases and diabetes mellitus are leading causes of mortality in modern society (both in developing and highly developed countries). This is attributed to the increased proportion of elderly people, growth and prevalence of obesity and stresses of various etiologies [1,2]. Thus, searching for novel effective, low toxic and easily available tools of prophylaxis and additional therapy of diabetes mellitus and hyperlipidemia among natural sources of biologically active substances exhibit various mechanisms of protective effects and corrective impaired biochemical status of the body represents an important task for modern medicine.
There is evidence [3] that diabetes mellitus and atherosclerosis may be referred to some extent to inflammatory diseases. Reactive oxygen species (ROS) refer to group of small reactive molecules that include hydrogen peroxide (H2O2). ROS are a consequence of aerobic metabolism and react avidly with other molecules, cellular lipids, proteins, and nucleic acids. Low to moderate levels of ROS have been shown to contribute to impotent functions, such as cell differentiation, migration, senescence, growth, and apopotosis. In contrast, a variety of diseases, such as cardiovascular pathologies, diabetes, and cancer, are associated with elevated ROS levels [4].
This suggests that redox-active compounds (antioxidants) may also exhibit corrective effects on impairments of carbohydrate and lipid metabolism. Antioxidants are already used in medicine due to their ability to inhibit lipid peroxidation (LPO) in biomembranes, stabilize structure and function of cell membranes and therefore to optimize conditions required for homeostasis of cells and tissues of the body exposed to various pathogenic factors [4,5].
Biological activity of echinochrome A, a polyhydroxy naphthoquinone isolated from the flat sea urchin Scaphechinus mirabilis, is attributed to its antioxidant properties [5]. Echinochrome A is an acting compound of the cardioprotector preparation “Histochrome”, but its corrective properties in impairments of carbohydrate and lipid metabolism have not been investigated so far.
Rosmarinic acid, luteolin and its sulphate analogies are main components polyphenol complex from the sea grass. Zostera marina is designated from Luromarin. Luteolin and rosmarinic acid is a common polyptenolic compound that exists in many types of plants. Having multiple biological effects such as antiinflammation, antiallergy, anticancer, and many others, luteolin and rosmarinic acid functions biochemically as redox-active compounds, i.e. either an antioxidant or pro-oxidant. The boilogical effects of luteolin and rosmarinic acid could be functionally related to each other. Besides antioxidant and antiinflammatory activity rosmarinic acid and luteolin also act as activators of peroxisome proliferator-activated receptors (PPAR) [6,7], which are important regulators of lipid and carbohydrate metabolism [8].
In this study we have investigated the possibility of correction of experimental impairments of carbohydrate and lipid metabolism by a mixture of polar lipids from sea macrophytes (Ulva fenestrata, Sargassum pallidum, Zostera marina) and its combination with natural redoxactive compounds: the polyhydroxy naphthoquinone echinochrome A from the flat sea urchin Scaphechinus mirabilis and a polyphenolic preparation Luromarin prepared using the sea grass Zostera marina. The latter preparation contains rosmarinic acid, sulphated luteilin in sort monoand disulphates, and bioflavonoids (luteolin, apigenin, and others) in approximate percentage (45:45: 10), correspondingly. In Figure 1 represented chemical structures of tested redox-active compounds.
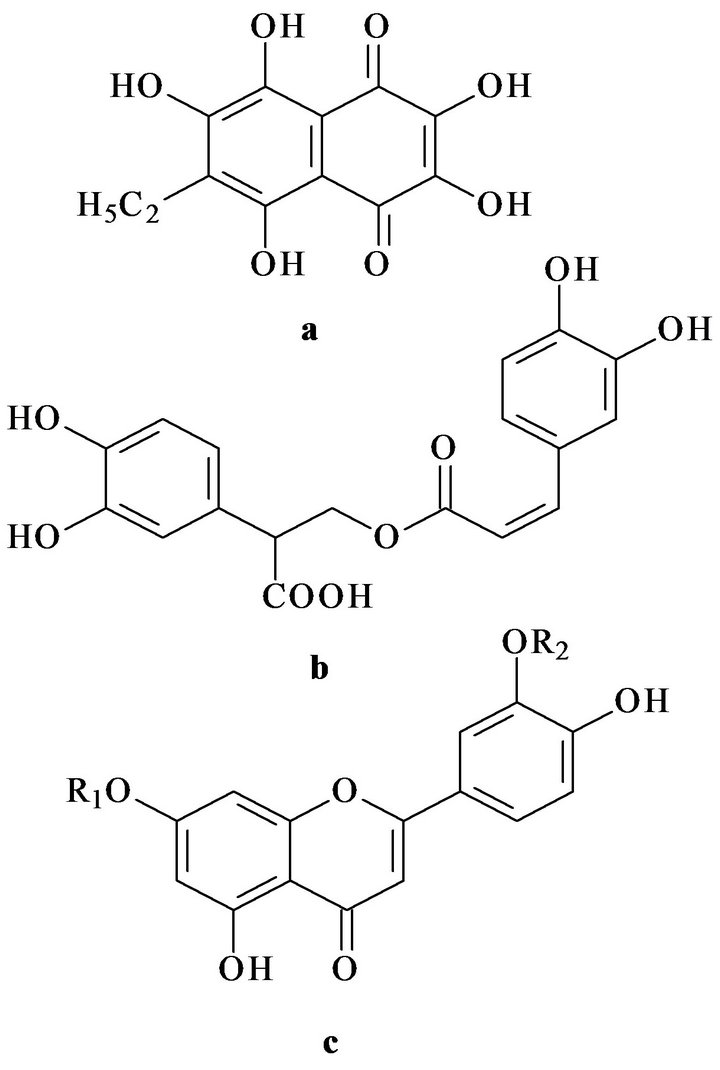
Figure 1. Chemical structures of echinochrome A (a), rosmarinic acid (b) and luteolin (c). R1 and R2—sulphate groups.
2. MATERIAL AND METHODS
2.1. Lipid and Antioxidant Isolation
A mixture of phosphorand glycolipids was obtained from the total lipid extracts of sea macrophytes Zostera marina, Ulva fenestrata, Sargassum pallidum, fished in the Sea of Japan during autumn periods of 2004-2005 at 20˚C (Kievka bay, Popov island). Total lipids were extracted by the method of Folch [9]. Crude lipid separation was performed by column chromatography. Fatty acid composition was analyzed as corresponding methyl esters using method of gas liquid chromatography [10].
The polyphenolic preparation Luromarin composed percentage from rosmarinic acid (about 45%), luteolin sulphates (about 45%) and bioflavonoids (luteolin, apigenin and ather (approximately 10%) was prepared from the sea grass Zostera marina as described in patent [11].
Echinochrome A was also isolated from the flat sea urchin Scaphechinus mirabilis by researchers of the Laboratory of Biotechnology of the Pacific Institute of Bioorganic Chemistry using the previously published method [12].
2.2. Animals
Investigations with the use of experimental animals were conducted in accordance with the rules of laboratory practice (GLP), the Order No. 267 of Ministry of Health of the Russian Federation of June 19, 2003 On Approval of the Rules of the Laboratory Practice and Instruction on Experimental (Preclinical) Study of New Pharmacological Preparations (2005). The test animals were kept in accordance with the rules accepted by the European Convention for the protection of vertebrates used for experimental and other scientific purposes (Strasbourg, 1986).
Female CBA mice (8 - 10 weeks old) is bred in vivarium of Pacific Institute of Bioorganic Chemistry. All animals were healthy, housed in five mice per cage at 20˚C. Water and a standart diet were available ad libitum.
2.3. Induction of Alloxan Diabetes and Hyperlipidemia
CBA mice (18 - 20 g) were used in experiments. Diabetes mellitus was modeled by intraperitoneal administration of alloxan (120 mg/kg in saline) to the overnightfasted animal’s according to published method with minor modifications [4,11]. Briefly, animals were subdivided into the following groups (n = 7 in each group): 1) intact mice; 2) diabetic mice (negative control); 3) diabetic mice treated during four days before and four days after (4 + 4) induction of alloxan diabetes.
Changes in carbohydrate metabolism induced by alloxan diabetes were determined on the 9th day after the beginning of the experiment in the glucose tolerance test (60 min after peroral administration of 4 g/kg glucose). Blood glucose was determined using a Satellite glucometer (Elta, Russia).
Hyperlipidemia was modeled by a single dose administration of Tyloxapol (Triton WR-1339; 40 mg/100 g of body weight) as previously described [4,11]. Animals were subdivided into the following groups (n = 7 in each group): 1) intact mice; 2) hyperlipidemic mice (negative control), 3) hyperlipidemic mice treated during three days before induction of hyperlipidemia. The mixtures of polar lipids (3 mg/kg) and bioantioxidants (5 mg/kg) were administered per os. At the end of experiments blood samples were taken for determination of the main parameters characterizing changes in lipid and carbohydrate metabolism and also aspartate amino transferase (AST) and alanine aminotransferase (ALT) activity using a biochemical analyzer (ROCHE, Switzerland).
2.4. Statistical Analysis
Statistical and graphical treatment of experimental data was performed using Microsoft Excel. Results were expressed as mean ± SD. Statistical differences were evaluated using paired Student’s t-test. Differences were considered as statistically significant at p < 0.05.
3. RESULTS AND DISCUSSION
GLC-analysis has shown (Tables 1 and 2) that in sea macrophytes Sargassum pallidum, Ulva fenestrata, and Zostera marina, the molar ratio ω-3/ω-6 PUFA is 1.3:1, 3.1:1, and 5:1, respectively.
Subsequent pharmacological studies have demonstrated an important role of these ratios for corrective activity of the investigated mixtures of polar lipids.
3.1. Pharmacological Studies on the Model of Alloxan Diabetes
Table 3 shows that induction of alloxan diabetes increased blood glucose in negative control animals by 3.4
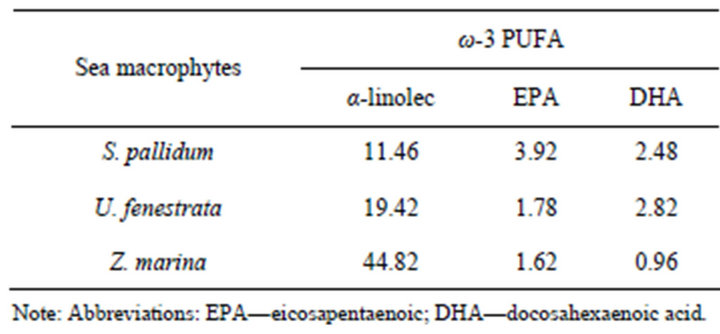
Table 1. Fatty acid composition of the total fraction of phosphoand glycolipids exhibiting anti-inflammatory (ω-3 PUFA) properties (percent of total fatty acids).
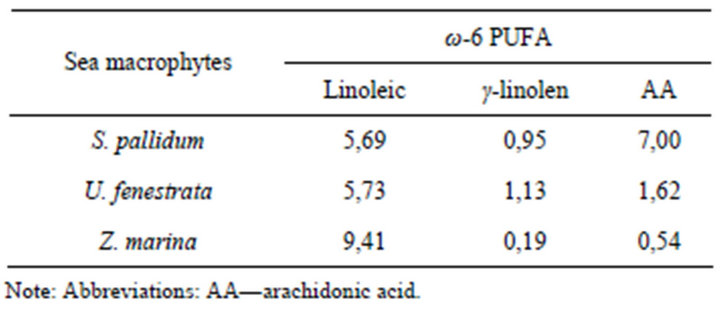
Table 2. Fatty acid composition of the total fraction of phosphorand glycolipids exhibiting proinflammatory (ω-6 PUFA) properties (percent of total fatty acids).
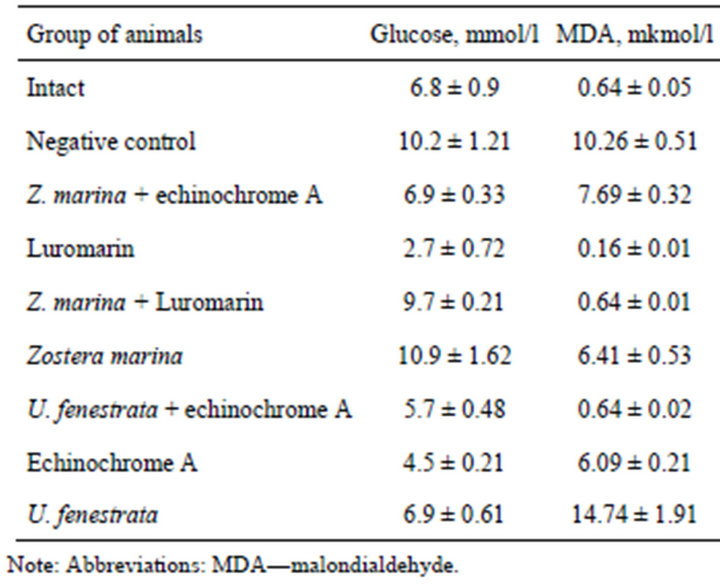
Table 3. Evaluation of protective action of the mixture of phospho-and glycolipids alone and in combination with polyphenolic complex “Lyuromarin” and echinochrome A at modeling of experimental alloxan diabetes.
mmol/l compared with intact animals. The increase in blood glucose suggests significant damage of pancreatic β-cells during induction of alloxan diabetes. The polyphenolic preparation Luromarin demonstrated the most effective normalizing effect of blood glucose: it decreased this parameter by 7.5 mol/l compared with negative control. Echinochrome A also exhibited high corrective activity and decreased blood glucose in treated diabetic mice by 5.7 mmol/l.
Combination of the lipid complex from Ulva fenestrata with echinochrome A decreased blood glucose in diabetic mice by 4.5 mmol/l. The complex of polar lipids from Zostera marina in combination with echinochrome A, and also the complex of polar lipids from Ulva fenestrata normalized blood glucose of diabetic mice up to the level of intact control. Polar lipids from Zostera marina alone or in combination with the polyphenolic preparation Luromarin exhibited insignificant influence on the level of blood glucose.
Malondialdehyde (MDA) content increased 16-fold in diabetic mice (the group of negative control) compared with intact animals. The most effective correction of the MDA level was observed after treatment of diabetic mice with the polyphenolic preparation Luromarin, polar lipids from Zostera marina combined with Luromarin, and also polar lipids from Ulva fenestrata combined with echinochrome A. These preparations normalized blood plasma MDA of the experimental groups up to the level of intact animals. Less pronounced (but normalizing effect) was observed after treatment of diabetic mice with polar lipids from Zostea marina and echinochrome A, which decreased the MDA level by 3.85 and 4.17 mmol/l, respectively, compared with negative control (untreated diabetic mice). Interestingly, treatment of diabetic mice with polar lipids from Zostera marina combined with echinochrome A decreased blood MDA only by 2.57 mmol/l, whereas polar lipids from Ulva fenestrata insignificantly influenced MDA level in blood serum.
Analysis of blood aminotransferase activities suggests that echinochrome A alone or in combination with polar lipids from Zostera marina caused the most effective normalizing action: they decreased blood ALT activeity by 0.78 and 0.35 mmol/l × h, respectively (Table 4) and AST activity by 0.57 and 1.06 mmol/l × h, respecttively, compared with untreated diabetic mice (negative control). The normalizing effect on the enzyme activity was also found after treatment with polar lipids from Ulva fenestrata combined with echinochrome A, which decreased ALT activity by 0.44 mmol/l × h. Treatment of diabetic mice with polar lipids Zostera marina combined with echinochrome A decreased serum triglycerides by 2.16 mmol/l compared with untreated diabetic mice (negative control), while the polyphenolic preparation Luromarin decreased this parameter by 1.17 mmol/l. In other experimental groups (mice treated with
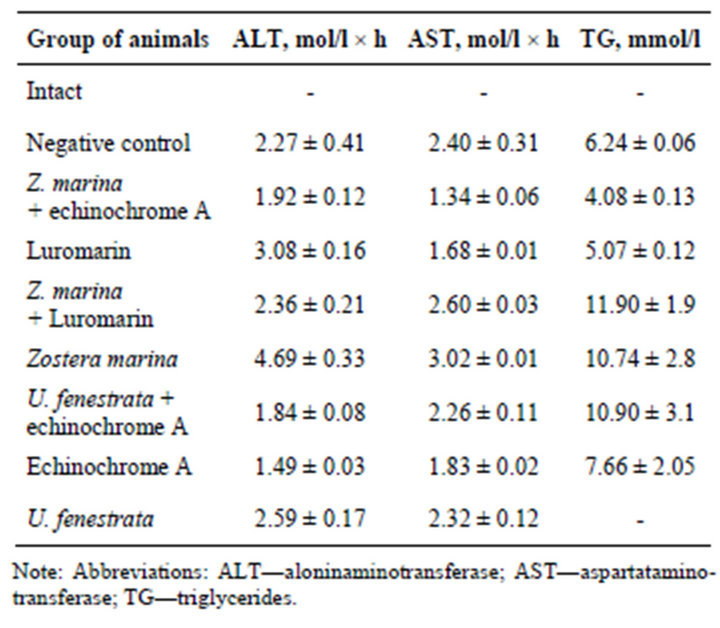
Table 4. Evaluation of protective action of the mixture of phospho-and glycolipids alone and in combination with polyphenolic complex “Lyuromarin” and echinochrome A at modeling of experimental alloxan diabetes.
polar lipids from Zostera marina combined with Luromarin, polar lipids from Zostera marina and Ulva fenestrate combined with echinochrome A, and echinochrome A alone) we did not find any significant changes in serum triglycerides compared with negative control (Table 4).
3.2. Pharmacological Studies on the Model of Experimental Hyperlipidemia
Table 5 summarizes results of serum triglyceride analysis in hyperlipidemic animals. According to these data, treatment of mice with Tylaxopol induced marked hyperlipidemia: triglyceride level increased several-fold, reaching 4.2 mmol/l (24 h after Tylaxopol administration) in the group of negative control compared with intact mice (0.03 mmol/l). In experimental groups of hyperlipidemic mice treated with echinochrome A, polar lipids from Ulva fenestrata alone and combined with echinochrome A, and polar lipids from Sargassum pallidum combined with thepolyphenolic preparation Luromarin there was a significant decrease in triglyceride level by 4.06, 4.04, 4.09, and 3.94 mmol/l, respectively, compared with untreated hyperlipidemic mice (negative control).
Induction of experimental hyperlipidemia was accompanied by a 19-fold increase in the MDA level (negative control) compared with intact animals. Treatment of hyperlipidemic mice with echinochrome A and polar lipids from Ulva fenestrata produced a significant therapeutic effect and decreased LPO products in blood
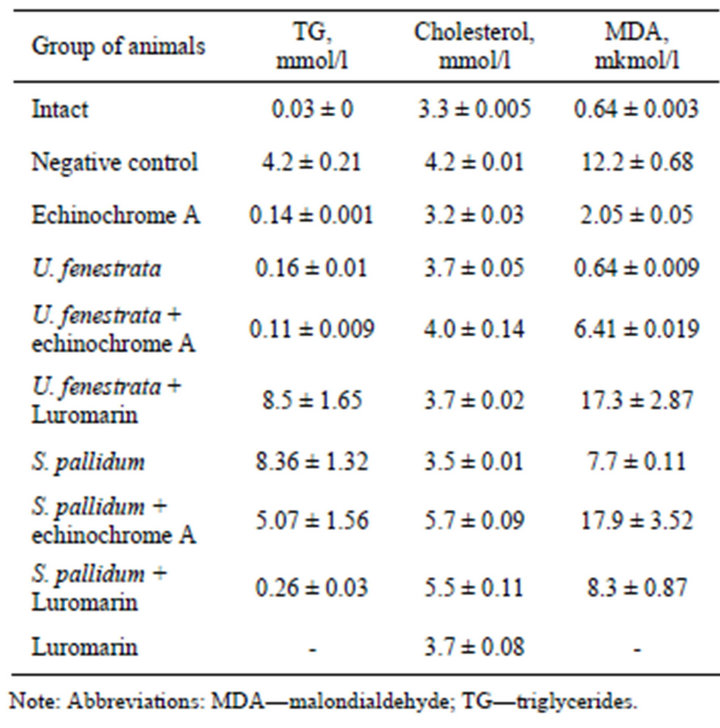
Table 5. Evaluation of protective action of the mixture of phosphoand glycolipids alone and in combination with polyphenolic complex “Lyuromarin” and echinochrome A at modeling of experimental hyperlipidemia.
plasma of experimental animals by 10.15 and 11.56 mmol/l, respectively. Clear tendency to normalization of the MDA level was also observed in the groups of hyperlipidemic animals treated with polar lipids from Ulva fenestrata combined with echinochrome A, polar lipids from Sargassum pallidum alone and in combination with Luromarin.
Analysis of aminotransferase activity revealed (Table 6) insignificant changes in ALT activity and significant (8-fold) increase in AST activity in the group of untreated hyperlipidemic mice (negative control) compared with intact mice. This is due to the fact that in cardiovascular diseases the level blood AST activity is higher than that of ALT, because myocardium contains insignificant amounts of ALT and so the serum ALT level in cardiovascular diseases usually remains within the normal range.
The highest corrective effect on blood aminotransferase activity was found in mice treated with echinochrome A, which caused a 5-fold decrease in AST activeity compared with negative control. Clear tendency to normalization of serum AST level in mice treated with echinochrome A suggests its cardioprotective action. Positive effect was also observed in hyperlipidemic mice treated with polar lipids from Ulva fenestrata and also with polar lipids from Sargassum pallidum combined with echinochrome A, which caused about 3-fold decrease in serum AST. Other preparations also decreased blood AST compared with negative control: Luromarin caused a 2-fold decrease, while polar lipids from Ulva fenestrata in combination with bioantioxidants (echinochrome A, Luromarin) decreased this parameter by 1.5- fold (Table 6).

Table 6. Evaluation of corrective action of the mixture of phosphoand glycolipids of marine macrophytes alone and in combination with polyphenolic drug “Lyuromarin” and echinochrome A model of experimental hyperlipidemia.
Summarizing all results of these experiments it should be noted that natural bioantioxidants (echinochrome A, Luromarin) and the mixture of polar lipids from Ulva fenestrane combined with echinochrome A demonstrated the highest corrective effect in both experimental models of alloxan diabetes and hyperlipidemia. The mixture of polar lipids from Ulva fenestrata was more effective in the model of experimental hyperlipidemia. Polar lipids from Sargassum pallidum alone and in combination with the polyphenolic preparation Luromarin were less effecttive in the model used.
It is known [1-4] that impairments in lipid and carbohydrate metabolism lead to the development of the metabolic syndrome. Obesity plays a key role in the development of this disease. Impairments in lipid metabolism are associated with the increase in triglyceride-rich lipoproteins. However, diacylglycerols (DAG), the main products formed during the first step of polar lipid transformation in the intestine, can effectively correct hyperlipidemia induced by increased consumption of triglycerides. It should be emphasized that DAG prevents excessive accumulation of fat by increasing its utilization after feeding and thus positively influencing triglyceride metabolism in the human body [13,14].
In our viewpoint biological activity of sea DAG is determined by a high content of polyunsaturated fatty acids (PUFA). The major role of PUFA is associated with polar lipids involved into structure-functional organization of biological membranes and biosynthesis of eicosanoids (prostaglandins, thromboxanes, leukotrienes), the mediators of metabolic reactions [1,3,15].
Eicosanoids formed from ω-3 PUFA have significant advantages over eicosanoids formed from ω-6 PUFA: they often inhibit development of inflammation. Since ω-3 PUFA suppress production of proinflammatory eicosanoids, products of ω-6 fatty acid transformation, they may be referred to potential anti-inflammatory agents [1,15].
Recently, a new family of lipid mediators, derivatives of ω-3 PUFA eicosapentaenoic and docosahexaenoic acids, widely distributed in sea macrophytes, has been described and denominated as resolves (E and D series) [15].
It is possible that these mediators may account for the antiinflammatory effects of ω-3 PUFA derivatives present in the polar lipids used in this study. In addition, a modified profile of fatty acids and lipid mediators may also significantly influence production of cytokines and transcription factors, which regulate inflammatory gene expression [1,15].
However, the viewpoint that ω-6 arachidonic acid derivatives always exhibit proinflammatory properties is extremely simplified as some eicosanoids exhibit antiinflammatory activity; for example, prostaglandin E2 inhibits production of proinflammatory cytokines, leukotrienes, and lipoxin A4 promoting inflammation [15].
The mechanism of the anti-inflammatory effect of PUFA is rather complex and is realized via different pathways. The mixture of polar lipids from Ulva fenestrata demonstrated the best therapeutic effect (among all mixtures of polar lipids studied) in the experimental hyperlipidemia. This effect may be attributed to the molar ratio of ω-3:ω-6 PUFA of 3:1 in polar lipids from Ulva fenestrata, which appears to be optimal compared with the ω-3:ω-6 PUFA ratios of 5:1 and 1.3:1 in polar lipids from Zostera marina and Sargassum pallidum, respectively.
Clear therapeutic effect of echinochrome A normalizeing blood plasma biochemical parameters of treated animals versus negative controls has been demonstrated in both experimental models (alloxan diabetes and hyperlipidemia). Echinochrome A is highly soluble in water (~1 mM) compared with other lipid-soluble antioxidants; this increases possibility of its action as a scavenger of water-soluble radical ions such as superoxide anion radical. Echinochrome A not only exhibits the peroxy radical scavenging capacity it also decreases concentration of oxidation initiator by chelating Fe2+ ions [5]. The key role in the antiradical activity and chelating properties of echinochrome A as well as redox conversions and autooxidation are determined by its hydroxyl groups at 2, 3, and 7 positions [5,17].
Echinochrome A effects are associated with its high redox potential. Oxidation of this polyhydroxynaphthoquinone by oxygen in the presence of Ca2+ ions is accompanied by hydrogen peroxide formation [16,17]. There is convincing evidence that optimal physiological concentrations of H2O2 may act as second messenger in the intracellular signaling and in signal transduction during intercellular communication [18]. One may suggest that physiological concentrations of hydrogen peroxide formed in the presence of echinochrome A may be involved into intracellular signaling and adaptation by regulating gene expression and biosynthesis of proteins and enzymes related to stress and antioxidant defense of the cell (Figure 2).
Results of the present study allow us to postulate the
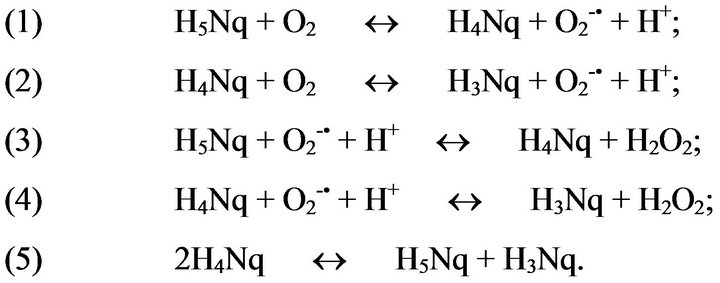
Figure 2. A scheme of autoxidation echinochrome A where Н5Nq—echinochrome A, Н4Nq—naphthosemiquinone echinochrome A, Н3Nq—tetraketon.
following mechanisms of echinochrome A effects in impairments of lipid and carbohydrate metabolism: 1) echinochrome A stimulates glucose metabolism and thus decreases its blood level; 2) echinochrome A interacts with endothelial cell DT-diaphorase production and this results in H2O2 production; 3) H2O2 exhibits vasodilatation effect; 4) under ischemic conditions and hypoxia H2O2 is an additional source for catalase-dependent oxygen production; 5) H2O2 acts as a signal molecule, which cases a sharp increase of various PPAR, the main regulators of carbohydrate and lipid metabolism [3,19].
H2O2 predominantly oxidizes thiol groups rather than leading to non-targeted crosslinking of cellular macromolecules. As a result of reversible redox reactions or by undergoing posttranslational modifications, such as glutathionylation, acetylation, nitrosylation, sulfhydration or metal binding, Cys residues are not only involved in controlling catalytic sites, but also function as sensors or modulators of signal transduction processes, in particular in response to oxidative stress, thereby modulation enzymes associated with energy metabolism, anti-oxidative stress and cellular redox control [20].
The polyphenolic preparation Luromarin as well as echinochrome A exhibits marked antidiabetic and antihyperlipidemic effects by promoting essential normalizetion of blood biochemical parameters compared with untreated animals.
One may suggest that the positive effect of Luromarin, which substantially consists of luteolin sulphates and rosmarinic acid, is associated with the known modulating effect of these polyphenolic compounds on different isoforms of PPAR. The PPAR-α, δ and γ isoforms are cell receptors that play a key role in defense against various pathologies, which appear during the development of the metabolic syndrome. After activation various isoforms of PPAR form heterodimers with retinoid X-receptor, which may bind to the PPAR responsive elements in the promoter regions of corresponding genes and modulate (increase or decrease) their transcription. PPAR-γ are preferentially expressed in adipose tissue and are used for therapy of diabetes mellitus type 2. PPAR-γ antagonists prevent obesity and exhibit antidiabetic activity [6-8].
PPAR-α are mainly expressed in the liver, brown adipose tissues, kidneys, heart and skeletal muscles. PPAR- α activation stimulates expression of lipoprotein lipase and a sharp increase in the level of this enzyme results in the decrease of triglycerides in chylomicrons and very low density lipoproteins. In addition, PPAR-α activation increases high density lipoprotein (HDL) cholesterol by increasing expression of hepatic apolipoproteins A-I and A-II and also promotes cholesterol transfer from cells to HDL [6-8].
PPAR-δ are expressed abundantly. At the cell level activation of this receptor increases fatty acid oxidation and energy consumption in muscles, stimulates lipogenesis in adipocytes and decreases glucose production. This is accompanied by improved lipoprotein profile and decreased levels of triglycerides and insulin resistance of the body. This explains why PPAR-δ represents a target for potential drugs for treatment of obesity. All three subtypes of PPAR may play a role in improvement of the hyperlipidemic status by inhibiting inflammation [6-8].
In addition, it was earlier shown [21] that rosmarinic acid and luteolin provide trapping, stabilization and detoxification ROS; they protect protein, enzyme, and DNA against ROS and also demonstrate the antiinflammatory effects realized via different biochemical pathways: 1) they inhibit formation of enzymes (phospholipase A2, cyclooxygenase, and lipoxygenase) involved into eicosanoid formation; this decreases content of proinflammatory molecules (prostaglandins and leukotrienes); 2) they inhibit activation of transcription factors modulating expression of proinflammatory genes (cyclooxygenase-2, inducible NO synthase, tumor necrosis factor-α, and also interleukin-1 and interleukin-6).
Sulfation—an important way of the metabolism flavonoids in plants, including of flavone luteolin. In sea grasses family Zosteraceae (Zostera marina and Z. asiatica) us is discovered large quantity of 7,3’-disulphate luteolin (DSL) and is designed available way of its isolation [11]. It was shown [4,11] that the pharmacological activities of DSL in many events much above than beside luteolin. Possible expect that DSL—natural water soluble form of luteolin, after oral injection capable to absorption and bioavailability into animal and human plasma through intestine, avoiding stage of the modification of intestine and liver cells and increase efficiency its of physiological action. In this connection development medical-preventive remedies on base DSL introduces more perspective than on base luteolin, which is already presently broadly used in medical practice [21].
Results of the present study suggest that manipulating the ω-3:ω-6 PUFA ratio is possible to decrease signs of inflammation, inhibit pathways inducing inflammatory processes in the organism, neutralize reactive oxygen species, and block free radical chain reactions by adding redox-active compounds (bioantioxidants). However, in order to achieve the best therapeutic effect it is important to find an optimal combination of polar lipids and different redox-active compounds.
Thus, polar lipids containing ω-3 and ω-6 PUFA and different redox-compounds such as echinochrome A, rosmarinic acid and luteolin sulphates can counteract the modeling pro-inflammatory status, which is believed to primary cause in many formidable diseases. Therefore determination of optimal ratios, and also elucidation of mechanisms of their action during combined application represent an important basis for the most rational their application as remedy for prophylaxis and therapy. In addition, complete randomized clinical trials in humans are also needed to confirm the potential use of polar lipids containing ω-3 and ω-6 PUFA in the prevention of diseases with inflammation constituents, e.g. diabetes, cardiovascular diseases, cardiometabolic syndrome, and ageing.
4. ACKNOWLEDGEMENTS
The authors are grateful to V.P. Glasunov (PIBOC FEB RAS) for spectral testing of peptide samples. This work was supported by a grant from the Russian Foundation for Basic Research (No. 04-04-08083).
REFERENCES
- Calder, P.C. (2012) Long-chain fatty acids and inflammation. Proceedings of the Nutrition Society, 71, 284-289. doi:10.1017/S0029665112000067
- Bays, H.E., Goldberg, R.B., Truitt, K.E. and Jones, M.R. (2008) Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: Glucose and lipid effects. Archives of Internal Medicine, 168, 1975-1983. doi:10.1001/archinte.168.18.1975
- Krivoshapko, O.N., Popov, A.M. and Artyukov, A.A. (2011) Peculiarities of the corrective effects of polar lipids and bioantioxidants from sea hydrobionts in impairments of lipid and carbohydrate metabolism. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 5, 152-157. doi:10.1134/S1990750811020053
- Popov, A.M., Krivoshapko, O.N. and Artyukov, A.A. (2012) Mechanisms of the protective pharmacological activity of flavonoids. Journal of Biopharmaceuticals, 4, 27-41.
- Lebedev, A.V., Ivanova, M.V. and Levitsky, D.O. (2008) Iron chelators and free radical scavengers in naturally occurring polyhydroxylated 1,4-naphthoquinones. Hemoglobin, 32, 79-165. doi:10.1080/03630260701700017
- Mueller, M., Lukas, B., Novak, J., Simoncini, T., Genazzani, A.R. and Jungbauer, A. (2008) Oregano: A source for peroxisome proliferator-activated receptor gamma antagonists. Journal of Agricultural and Food Chemistry, 56, 11621-11630. doi:10.1021/jf802298w
- Ding, L., Jin, D. and Chen, X. (2009) Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. Journal of Nutritional Biochemistry, 21, 941-947. doi:10.1016/j.jnutbio.2009.07.009
- Barbier, O., Torra, I.P., Duguay, Y., Blanquart, C., Fruchart, J.C., Glineur, C. and Staels, B. (2002) Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 717-722. doi:10.1161/01.ATV.0000015598.86369.04
- Folch, J., Lees, M. and Sloane Stanley, G.H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226, 497-509.
- Sanina, N.M., Goncharova, S.N. and Kostetsky, E.Y. (2004) Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry, 65, 721-730. doi:10.1016/j.phytochem.2004.01.013
- Popov A.M., Artyukov A.A., Krivoshapko O.N., Krilova N.V. and Kozlovskaya E.P. (2011) Remedy possessing antioxidant, cardioprotective, antiinflametory, hepatoprotective, antidiabetes, antitumor and antiviral effects. Russian Federation Patent No. 2432959.
- Nishibori, K. (1961) Isolation of echinochrome A from the spines of the sea urchin, Stomopneustes variolaris (Lamarck). Nature, 192, 1293-1294. doi:10.1038/1921293a0
- Yanai, H., Tomono, Y., Ito, K., Furutani, N., Yoshida, H., and Tada, N. (2007) Diacylglycerol oil for the metabolic syndrome. Nutrition Journal, 6, 43-57. doi:10.1186/1475-2891-6-43
- Baylin, A., Kim, M.K., Donovan-Palmer, A., Siles, X., Dougherty, L., Tocco, P. and Campos, H. (2005) Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: Comparison with adipose tissue and plasma. American Journal of Epidemiology, 162, 373-381. doi:10.1093/aje/kwi213
- Calder, P.C. (2009) Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie, 91, 791-795. doi:10.1016/j.biochi.2009.01.008
- Perry, G. and Epel, D. (1981) Ca2+-stimulated production of H2O2 from naphtoquinone oxidation in Arbacia eggs. Experimental Cell Research, 134, 65-72. doi:10.1016/0014-4827(81)90463-8
- Veal, E.A., Day, A.M. and Morgan, B.A. (2007) Hydrogen peroxide sensing and signaling. Molecular Cell, 26, 1-14. doi:10.1016/j.molcel.2007.03.016
- Lebedev A.V., Ivanova M.V. and Levitsky D.O. (2005) Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sciences, 76, 863-875. doi:10.1016/j.lfs.2004.10.007
- Artyukov А.А., Popov А.М., Tsybulsky A.V., Krivoshapko O.N. and Polyakova N.V. (2012) Pharmacological activity echinochrome а singly and consisting of BAA “Timarin”. Biomeditsinskaia Khimiia, 59, 281-290.
- Jones, D.P. (2010) Redox sensing: Orthogonal control in cell cycle and apoptosis signalling. Journal of Internal Medicine, 268, 432-448. doi:10.1111/j.1365-2796.2010.02268.x
- Kim, H.P., Son, K.H., Chang, H.W. and Kang, S.S. (2004) Anti-inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Sciences, 96, 229-245. doi:10.1254/jphs.CRJ04003X

