Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18051 , 10 pages DOI:10.4236/cm.2012.31005
Comparative Antiproliferative Action of Two Extracts from Tilia x viridis on Normal and Tumoral Lymphocytes: Relationship with Antioxidant Activity
Institute of Chemical and Drug Metabolism (IQUIMEFA-CONICET), Pharmacognosy Unit, Faculty of Pharmacy and Biochemistry, University of Buenos Aires, Buenos Aires, Argentina
Email: canesini@yahoo.com.ar
Received December 6, 2011; revised December 27, 2011; accepted January 16, 2012
Keywords: Antioxidant Activity; Antiproliferative Activity; Tilia x viridis
ABSTRACT
Tilia species have been used in Asia, Europe and in America to treat anxiety and also for the treatment of colds and inflammation. The oxygen reactive species (ROS) (hydrogen peroxide (H2O2) and the superoxide anion (O2) are involve in the balance cell proliferation/death in lymphocytes. It was reported the presence of flavonoids in Tilia species which possess antioxidant properties. The aim of this study was to determine comparatively the effect of an aqueous (AE) and ethanol (E) extract from Tilia x viridis, on the proliferation of tumoral and normal concanavalin A stimulated murine lymphocytes in relation to antioxidant activities such as peroxidase (Px), catalase (CAT) and superoxide dismutase (SOD) activities involves in H2O2 modulation. Also a phytochemical pattern of the two extracts in relation to flavonoids content was determined. Both extracts presented antiproliferative action on both type of lymphocytes but E was more selective on the tumoral lymphocytes inhibition (EC50 (µg/ml, Mean ±SEM) (tumoral): 50 ± 4; EC50; (normal lymphocytes): 323 ± 20); this action was related to a high polyphenols content (150 ± 10 mg/g extract) and high “per se” SOD and low Px activities. In conclusion, the extracts could be a source of antioxidant compounds which contribute to a selective antiproliferative action on tumoral cells, acting through the modulation of H2O2 levels.
1. Introduction
Natural antioxidants from plants such as flavonoids and other polyphenols are known to play an important role in the protection of cells from oxidative damage. Consequently, the antioxidant activity is related to anticancer activities including pro-apoptotic, DNA damaging, antiangiogenic, and immunostimulatory effects [1].
Among polyphenols, flavonoids are the principal secondary metabolites that characterize the genus Tilia [2]. Tilia specie widely distributed and used in Argentine folk medicine is Tilia x viridis. This plant is an hybrids between Tilia tomentosa (native to Europe and western Asia) and Tilia Americana. Previously, an ethanol extract from the flowers presented a selective antiproliferative action on a lymphoma cell line named BW 5147 in relation to free radical scavenging activity; in addition, one of its main compounds, the flavonoid rutin, showed antioxidant peroxidase activity related to the inhibition of normal cells proliferation by hydrogen peroxide modulation [3]. In addition, an aqueous extract from other specie of Tilia, T. cordata presented antiproliferative action on BW 5147 cells [4].
Furthermore, hydrogen peroxide plays an important role in the modulation of cell proliferation, stimulating cell proliferation but also leading cells to apoptosis [5,6]. Previously it was shown that BW 5147 cells possess low level of hydrogen peroxide and high level of superoxide anion in comparison with normal lymphocytes in response to a low level of superoxide dismutase activity [7].
The aim of this study was to determine the effect of an aqueous extract (AE) from T. x viridis, and compare it with the effects exerted by the ethanol extract (E), on tumoral and normal lymphocytes proliferation in relation to their antioxidant activities such as: linoleic acid peroxidation inhibition, DPPH scavenger activity, catalase (CAT), peroxidase (Px) and superoxide dismutase (SOD) “per se” activities. To determine the participation of Polyphenols in the effect of the extracts, polyphenols were quantified; too the major flavonoids compounds were identified and quantified by HPLC analysis.
2. Materials and Methods
2.1. Plant Material Preparation
Tilia x viridis (Bayer) Simonk nothosubspecie moltkei (Dippel) Xifreda flowers were collected in the province of Buenos Aires in January and authenticated by Dr Gustavo Giberti (IQUIMEFA-CONICET). A voucher specimen is deposited at Museum of Pharmacobotanic, Faculty of Pharmacy and Biochemistry, UBA (BAF 17414). To prepare the ethanol extract (E), the dried and ground flowers (120.3 g) were extracted twice by maceration with ethanol overnight and filtered. The extracts were pooled, evaporated to dry. A yield of 24.36 g (20.25%) was obtained.
The aqueous extract (AE) was prepared by extraction of 1.5 g of plant material with 20 ml of distilled water at 52˚C during 45 min, followed by maceration at 5˚C for 72 h. The resulting extract was filtered, homogenized and lyophilized, yielding 266 mg of residue. For the experiments, AE was re-dissolved in water and E in absolute ethanol, so that the concentration of ethanol in the wells for cell culture was 0.5%.
2.2. Total Phenols Determination
The total phenol content was determined by the FolinCiocalteu colorimetric method described by Singleton [8]. The absorbance was measured at 765 nm and compared with a gallic acid calibration curve. The result was expressed as mg gallic acid equivalents per gram of dry plant (GAE/g).
2.3. HPLC: Flavonoids Quantification
The HPLC method used was that of Filip et al. [9]. Briefly, a C18 column was used (Gemini 5 μm, 150 × 4.6 mm) with a Rheodyne injector fitted with a 20 μl loop. Solvent A: H2O/AcOH (98:2), solvent B: MeOH/AcOH (98:2), gradient: 15% B to 40% B, 30 min; 40% B to 75% B, 10 min; 75% B to 85% B, 5 min. Flow rate: 1.2 ml/min. Detection: diode array detector at 263 nm. Rutin and kaempferol were identified by comparison of their retention times to those of authentic standards, and quantification was carried out by use of an external standard as described previously [9].
2.4. Cell Culture, Antiproliferative Activity and Viability Studies
The tumour cell line BW 5147 (Institute fur Virologie und Immunobiologie der Universitat Wurzburg, Germany) is a T cell lymphoma cell line that expresses the H-2 k haplotype, is CD3+ and has a TCRab as determined by flow cytometric analysis. Cells were cultured at optimal concentrations of 3 × 105 cells/ml in RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). Lymphoid cell suspensions from C3H (H-2d) inbred male mice (2 - 4 months old) were prepared from lymph nodes obtained aseptically from nylon wool purification of T cells as described previously [10]. Normal cells, at a concentration of 1 × 106 cells/ml, were cultured in the same medium as the tumor cells, but in the presence of a polyclonal mitogen, concanavalin A (Con A) 2 µg/ml [10]. Cells were cultured at a final volume of 0.2 ml in 96-well flat-bottom microtiter plates (Nunc) during 24 h and then pulsed with [3H]TdR (20 Ci/mmol) for the last 6 h as previously described [11]. Results were expressed as cell proliferation (% of inhibition): [dpm control – dpm treated/dpm control × 100]. Data represent the mean ± SEM of three experiments performed in triplicate. The effective concentration 50 (EC50) values were calculated from data obtained graphically, using a mathematical method based on the principle of the right-angled triangle: CE50 = D – [(A – 50% max response)X]/Y [12]. Animals were used according to the Guide for the Care and Use of Experimental Animals (DHEW, NIH 80-23). Cell viability was determined by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma). Briefly, the cells (1 × 106/ml), treated with AE and E during 24 h were incubated in 100 ml of RPMI 1640 containing 10 ml of 5 mg/ml MTT (Sigma, St. Louis, MO, USA). The untreated cells were used as controls for viability (100%) and the results were expressed as % of viability relative to control [7].
2.5. Apoptosis Assay
The percentage of cells with apoptotic morphology was determined by staining the nuclei with Hoechst dye. For this, tumoral and normal lymphocytes were incubated in 24-well plates with different concentrations of the extracts for 24 h. A control test without extracts was carried out. Aliquots of these cultures were fixed with methanol on a glass, stained with Hoechst dye during 15 minutes and then washed with PBS 1×. Nuclear morphology was observed under a fluorescence microscope (Olympus) at 400× [13].
To determine whether the ethanol extract induced nuclear changes compatible with apoptosis, 105 tumoral lymphocytes were incubated in 24 well plates, with and without different concentrations of the extract. Cells were then washed twice with PBS, resuspended in 1 × binding buffer at a concentration of 1 × 106 cells, aliquots of 1 × 105 cell were incubated with annexin V-FITC (SIGMA) and Propidium iodide (SIGMA), for 15 min at room temperature in the dark. Samples were shaken gently, diluted with binding buffer and analyzed by flow cytometry within 1 hour of processing. A control of apoptosis was done, incubating the cells in the same medium without FCS during 48 h.
2.6. Determination of the Antioxidant Activity by the Ferric Thiocyanate Method and DPPH Scavenger Activity
The general antioxidant capacity was determined by the ferric thiocyanate method (FTC) and DPPH scavenger activities. For FTC a volume of 0.8 ml of each concentration of extracts was mixed with 0.05 M phosphate buffer pH 7 and 2.5% linoleic acid in ethanol to obtain 4 ml of solution. The resulting solutions were incubated at 38.5˚C in a glass flask. Aliquots were taken at regular intervals and a FeCl2/ammonium thiocyanate solution was added in order to allow any peroxides resulting from the oxidation of linoleic acid to react, forming a complex that can be detected spectrophotometrically at 500 nm (Shimatzu UV 2101). This step was repeated every 24 h until the control (phosphate buffer plus linoleic acid) reached its maximum absorbance value. Therefore, high absorbance values indicated high levels of lino1eic acid oxidation. Phosphate buffer was used as reaction blank. The total antioxidant activity was expressed as the average of three independent determinations carried out in duplicate. The percentage of inhibition of lipid peroxidation of linoleic acid was ca1culated applying the following equation: Inhibition of lipid peroxidation (%) = 100 – [(As/Ao) × 100], where Ao is the absorbance of the control reaction (linoleic acid alone, 100% peroxidation) and As is the absorbance obtained in the presence of the sample extract or positive control of antioxidant activity (1 mg/ml ascorbic acid) [14].
Scavenging activities of the extracts on the stable free radical DPPH were assayed using the modified Blois´ method in which the bleaching rate of DPPH is monitored at a characteristic wavelength in presence of the sample [15]. A volume of 0.1 ml of an aqueous dilution of the extracts were mixed with 0.5 ml of a 500 μM DPPH solution in absolute ethanol and 0.4 ml of a 0.1 M Tris-ClH buffer pH 7.4. The mixture was kept for 20 min in the darkness and then the absorbance was read at 517 nm. The percentage of decrease of DPPH bleaching was calculated by measuring the absorbance of the sample and applying the following equation: % of inhibition = [1 – (As/A0)] × 100, where As is absorbance of sample (i.e., extracts) and Ao is the absorbance of the DPPH solution. Ascorbic acid solutions of different concentrations were used as positive controls for antioxidant activity.
The effective concentration 50 (EC50) values were calculated using the mathematical method explained before.
2.7. Catalase (CAT), Peroxidase (Px) and Superoxide Dismutase (SOD) “Per Se” Activities
To determine CAT activity, the sample, 50 µl of AE and E was added to a sodium phosphate buffer (50 mM), pH: 7 and 100 mM (v/v) H2O2. The absorbance was monitored for 5 min at 240 nm. The change in absorbance is proportional to the breakdown of H2O2, one unit of the enzyme activity was defined as the amount of the enzyme required for the breakdown of 1 µM H2O2 [16].
To determine SOD activity the effect of the extracts was evaluated on epinephrine autoxidation inhibition. The sample, 50 µl of AE and E was treated with 910 µl of sodium phosphate buffer (0.05 M) pH: 10.7 and epinephrine 1 mM. Under these conditions the epinephrine rapidly undergoes to auto-oxidation to produce the adrenochrome, which is a pink colored product that can be measured at 480 nm using a UV/visible spectrophotometer in kinetic mode. The antioxidant activity of sample was evaluated as the % of epinephrine auto-oxidation inhibition. The rate of absorbance was monitored at 480 nm, and ∆ absorbance/min was determined to calculate the % of inhibition as: [(Abs/min Control – Abs/min sample)/Abs/min Control] × 100. To calculate SOD activity, it was taken account that 50% of inhibition of epinephrine auto-oxidation represents 1 Unit/ml [16].
To determine Px activity, extracts (from 1 to 1000 µg/ml) were diluted with Krebs-Henseleit buffer (pH: 7.4) containing: 125 mM NaCl; 4.0 mM KCl; 0.5 mM NaH2PO4; 0.1 mM MgCl2; 1.1 mM Ca Cl2 and 5.0 mM glucose. The peroxidase activity was determined by the method described by Herzog and Fahimi [17]. Briefly, 50 µl of each sample were incubated with 925 µl of 5 × 10–4 M 3’, 5’. diaminobencidine tetrahidrochloride (DAB) (Sigma, St Louis, Mo, USA) and 25 µl of H2O2 (a solution of 30% H2O2, diluted 1/86 in distilled water) in a final volume of 1 ml. A DAB solution without H2O2 was used as reaction blank. In all cases the reaction was initiated by the addition of H2O2 and the change in OD readings was recorded at 30 sec intervals for 5 min using a Shimadzu recording spectrophotometer UV-240 (graphic printer PR-1) set at 465 nm. The ∆ absorbance/min was calculated. A calibration curve of peroxidase concentration vs ∆ absorbance/min was plotted using horseradish peroxidase obtaining a linear relationship in the range of 1.95 × 10–3 to 2.5 × 10–5 U/ml. The activity of samples was calculated by interpolation in the standard curve.
2.8. Statistical Analysis
Data was analyzed by the Student’s t test and one way ANOVA and Dunnett’s test. Differences were considered significant when p £ 0.05.
3. Results
Firstly, the effect of both extracts AE and E was analyzed on tumoral and normal cell proliferation and viability. Both extracts presented antiproliferative action on a lymphoma cell line BW 5147 and on normal lymphocytes previously stimulated with concanavalin A (Figures 1(a) and (b)). The antiproliferative action was directly related to extract’s concentrations. The EC50 for the antiproliferative action on tumoral cells was higher with AE than E, also on normal Con A stimulated lymphocytes, the EC50 value for antiproliferative action was higher with AE than E (Table 1). With respect to cell viability, on tumoral cells E decreased it significantly over 500 µg/ml meanwhile AE decreased it significantly at higher concentrations; on normal cells E decreased cell viability over 1000 µg/ml but AE decreased it at higher concentrations (Figures 1(c) and (d)).
To relate the effect on cell viability with apoptosis, Hoechst analysis was done. As well, E extract did not produce muclear morphology compatible with apoptosis on normal lymphocytes exerted this phenomenon on tumoral ones in relation to concentrations. By other way, AE exerted principally signals compatible with necrosis over 5000 µg/ml in both normal and tumoral lymphocytes (Table 2).
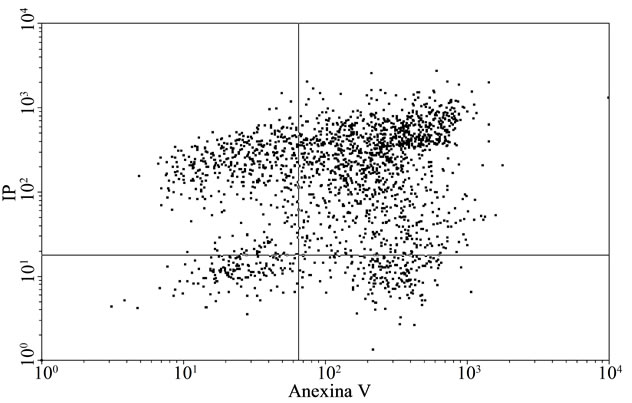
To confirm the apoptosis signal observed on tumoral cells with E, a flow citometry study was performed. Figure 2(a) and Table 3 inserted show that the apoptotic control, obtained by incubation of cells without FCS during 48 h, presented the cells principally in late apoptosis; in Figures 2(b) and Table 3 inserted, it can be seen BW 5147 cells without any treatment as control, and in Figures 2(c) and Table 3 inserted, it can be seen cells treated with E (500 µg/ml), which were in late and early apoptosis.
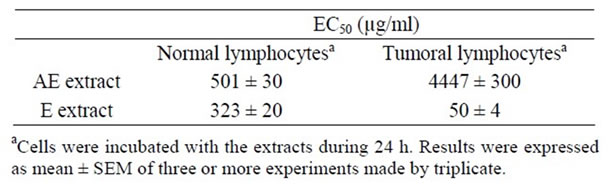
Table 1. EC50 of Tilia extracts on tumoral and normal cells proliferation.
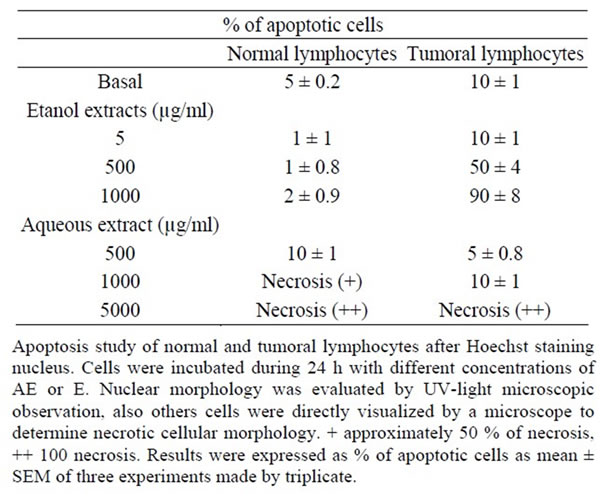
Table 2. Percentage of apoptotic cells in normal and tumoral lymphocytes induced by extracts.
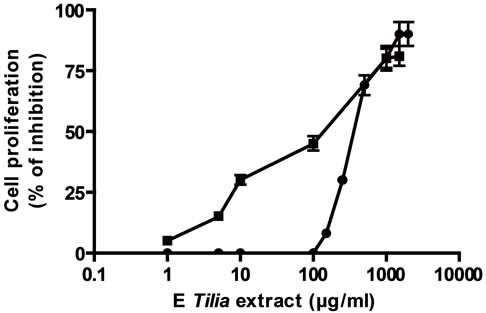 (a)
(a)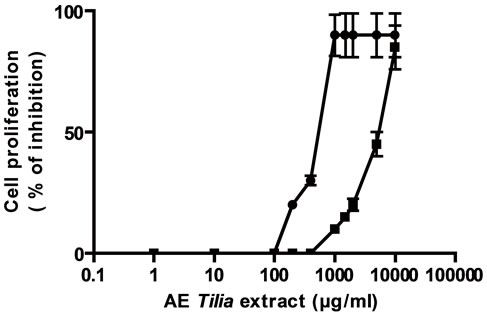 (b)
(b)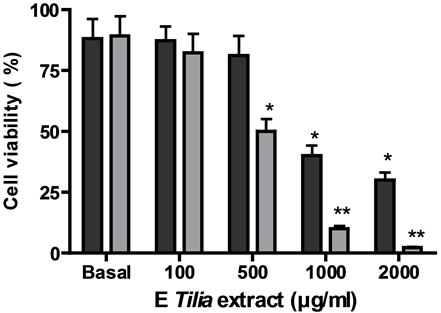 (c)
(c)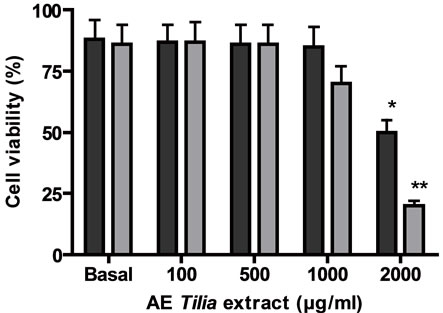 (d)
(d)
Cells ere incubated with different concentrations of E or AE from 1 to 10000 µg/ml during 24 h. In (a) and (b): ■ tumoral cells, ● normal lymphocytes. (c) and (d): black column: normal lymphocytes, grey column: tumoral lymphocytes. *p < 0.05, **p < 0.01 significantly differences between balas values and treatments, accordingly to ANOVA + Dunnett’s test.
Figure 1. Effect of extracts, E (a) and AE (b) on normal and tumoral lymphocytes proliferation and on cell viability ((c) and (d)).
 (a)
(a)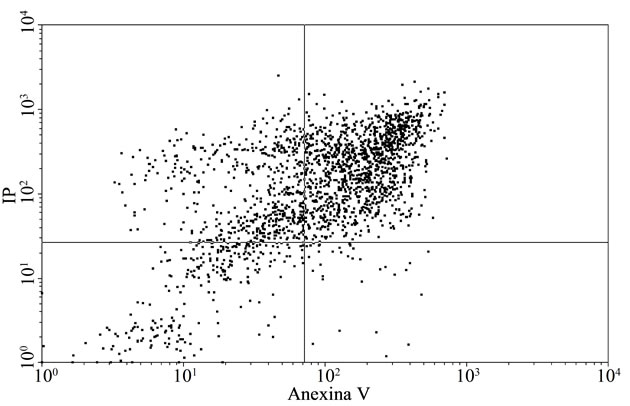 (b)
(b)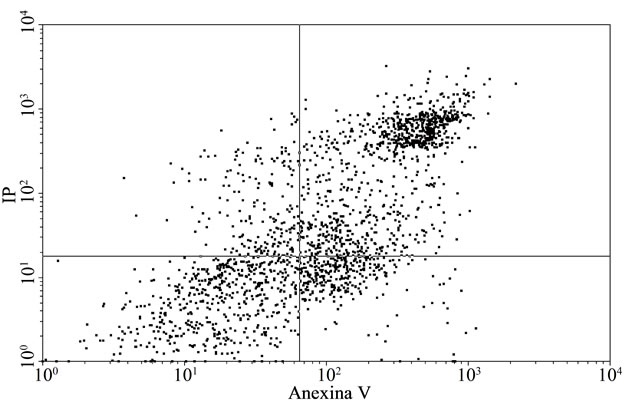 (c)
(c)
Representative dot plot analysis of BW 5147 cells stained with annexin-V FITC and iodide propidium from tree determinations. (a) Apoptosis control: cells incubated in absence of CFS; (b) BW 5147 alone and (c) BW 5147 treated with 500 µg/ml of E Upper left panel (necrosis), upper right panel (late apoptosis or necrosis), low left panel (viable cells), low right panel (early apoptosis).
Figure 2. Effect of extracts on apoptosis.
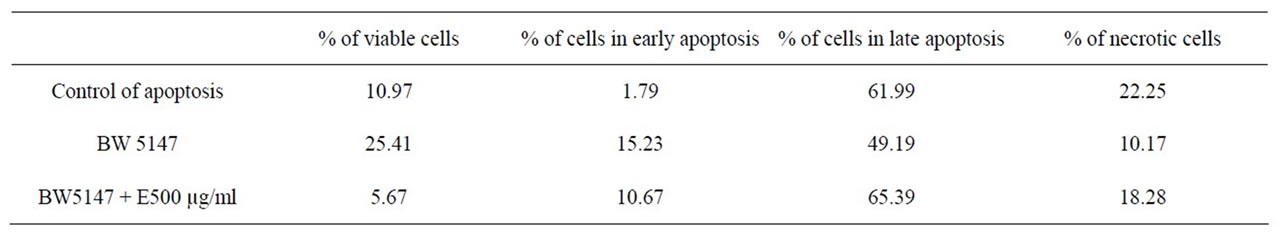
Table 3. % of cells in different stages, obtained from dot plot analysis.
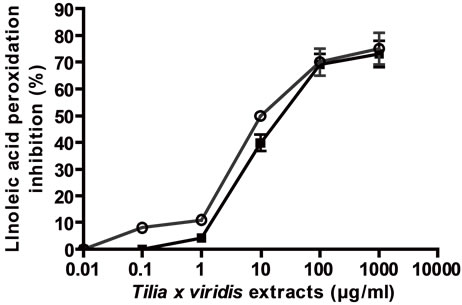
To relate the effect on cell proliferation with the antioxidant activity, the antioxidant activity in relation to linoleic acid peroxidation inhibition and DPPH scavenger activities were determined. As it can be seen in Figure 3, both extracts presented peroxidation inhibitory activity and DPPH scavenger activity in direct relation to concentrations and in comparison with vitamin C (EC50 (µg/ml) for FTC: E: 12.5 ± 1 and AE: 6.4 ± 0.5; for DPPH scavenger activity: E: 21.84 ± 2; AE: 7.24 ± 0.5.
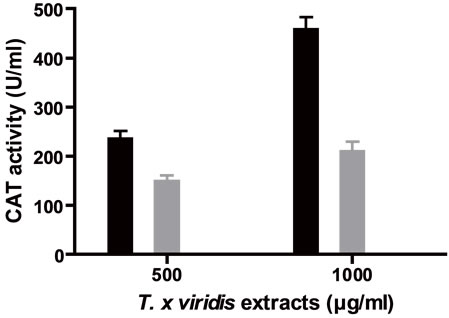
In addition, CAT, SOD and Px like activities were determined for both extracts. Both extracts presented CAT activity in relation to concentrations, AE presented a maximum CAT activity higher than E (Figure 4(a)); also, both extracts presented SOD activity in relation to concentrations (Figure 4(b)); the extracts presented too Px activity, with AE a maximum Px activity was found at 50 µg/ml meanwhile with E the activity was related to the concentrations.
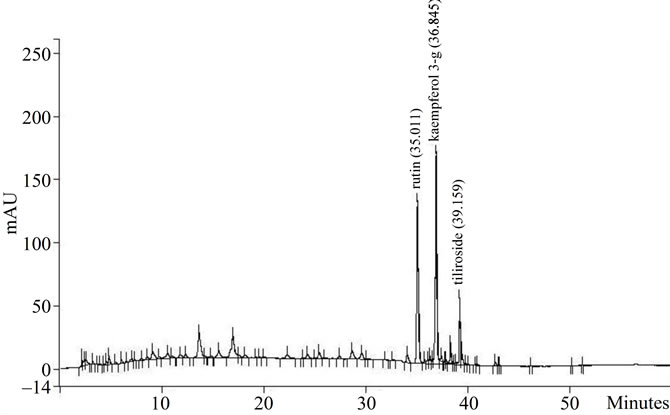
Also, the polyphenol content as well as the majority flavonoids were determined. In Figure 5(a) it can be seen the HPLC chromatogram of AE and in Figure 5(b) the chromatogram of E. The majority flavonoids were identified as rutin, kaempferol-3 glicoside and tiliroside. In addition, E presented more flavonoids and polyphenol
 (a)
(a)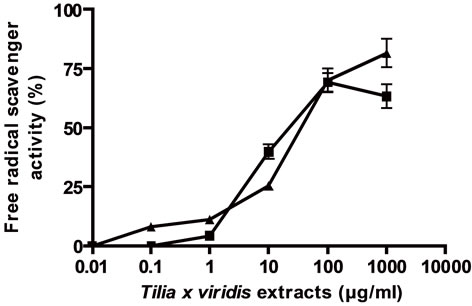 (b)
(b)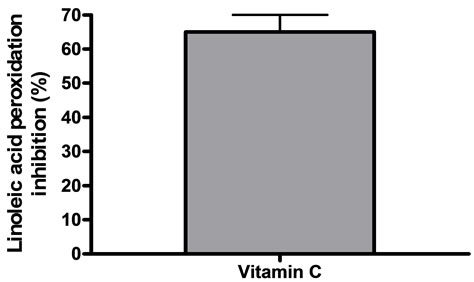 (c)
(c)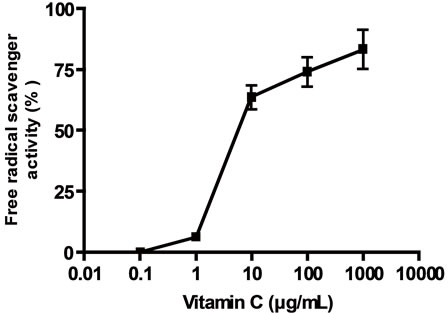 (d)
(d)
(a) linoleic acid peroxidation inhibition, (b) DPPH scavenger activity, (c) and (d) effect of vitamin C on linoleic acid peroxidation inhibition and DPPH scavenger activity respectively. Results were expressed as mean ± SEM of three experiments made by triplicate.
Figure 3. General antioxidant activity of the extracts.
 (a)
(a)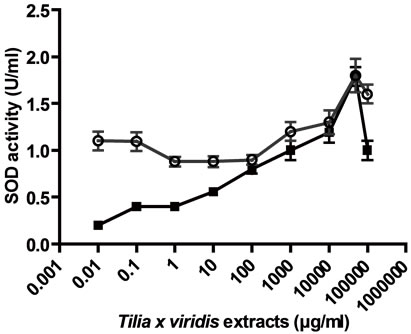 (b)
(b)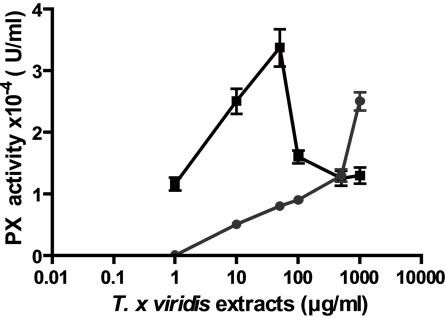 (c)
(c)
The antioxidant like activity of AE and E in different concentrations from 0.01 to 10000 µg/ml, was performed on CAT, Px and SOD activities. (a) CAT like activity; (b) SOD like activity; (c) Px like activity. Results were expressed as Mean ± SEM of three experiments performed by triplicate.
Figure 4. Enzyme “per se” activity of the extracts.
 (a)
(a) 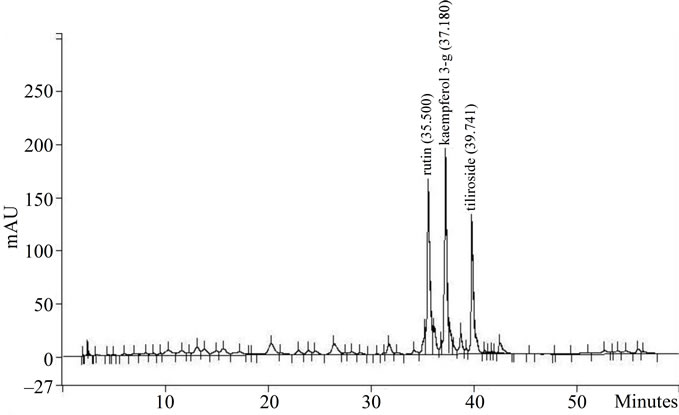 (b)
(b)
(a) AE extract and (b) E extract. The principal compounds were indicated in the graphs as: rutin, kaempferol 3-glycoside and tiliroside.
Figure 5. HPLC profile of AE and E Tilia extracts.
content in comparison with AE (Table 4).
4. Discussion
In this manuscript the effect of AE on tumoral and normal lymphocytes proliferation was demonstrated and compared to the effect exerted by E; both extracts were obtained from Tilia x viridis flowers. The extracts presented different selectively effect on both type of lymphocytes depending on H2O2 modulation, through CAT, Px and SOD like activity, and antioxidant general activity. AE was more selective in the inhibition of normal ConA stimulated lymphocytes meanwhile E was more selective to inhibit tumoral cells. Polyphenol content contribute to the antiproliferative action on tumoral cells.
AE extract exerted inhibitory action on tumoral and normal pre-ConA stimulated lymphocytes as well as E. But E was more selective on the inhibition of tumoral cells, in accord to its EC50 value, which was 6.5 times lower for tumoral cells inhibition than normal cells inhibition. By other way, AE was more selective to inhibit Con A stimulated lymphocytes as its EC50 for this activeity was 9 times lower than that of tumoral cells. The selective antiproliferative action of E was before described, but no studies on cell viability and apoptosis were done [3]. Therefore, cell viability was studied in order to determine cytostatic or cytotoxic action. Both extracts presented cytostatic and cytotoxic effects depending on the concentrations analyzed. On tumoral cells, at low concentrations, AE and E exerted cytostatic action as a decrease in cell proliferation was observed without a change in cell viability, the same could be observed on normal cells but at high concentrations a decrease in cell proliferation was accompanied by a decrease in cell viability which revealed a cyotoxic effect. Moreover, E exerted
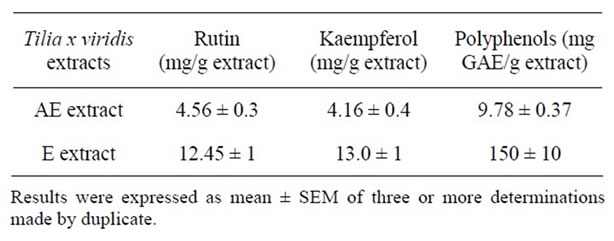
Table 4. Polyphenol and rutin content of AE and E extracts from T. x viridis.
mainly apoptosis on tumoral cells meanwhile AE exerted necrosis. The apoptosis induced by E in tumoral cells was confirmed by flow cytometry; E principally induced cells to late apoptosis and necrosis, which could explain the decrease in cell viability observed with concentrations over 500 µg/ml. These results are in accordance with results obtains with an aqueous extract from another specie of Tilia, Tilia cordata Mill [4].
In order to relate the antiproliferative action with the antioxidant activity, firstly the inhibition of linoleic acid peroxidation and DPPH scavenger activities were performed as general methods to evaluate antioxidant activity. As well as, both extracts presented general antioxidant activities, AE was more potent for both activities, as it presented low EC50 values than E. While a good correlation between general antioxidant activities and antiproliferative activity on normal Con A stimulated lymphocytes was observed with AE, it presented high EC50 values for the inhibition of tumoral cells, indicating a more selective action on normal Con A stimulated lymphocytes. Probably these effects were related to the high antioxidant activity exerted by this extract as it is well known that, Con A induced oxidative stress in mice. Shirin [18] observed that, reactive oxygen species play a role in immune-mediated Con A-induced hepatitis probably secondary to immune-mediated liver damage; also it was observed that, scavenging of reactive oxygen species by antioxidants prevents hepatitis. Others, have demonstrated that, using in vivo and in vitro models of liver cell injury, antioxidant like ellagic acid are capable of protecting hepatocytes from VK3- or ConA-induced liver damage by a mechanism consistent with its putative antioxidant activity, also these results indicate that ROS are critical mediators of hepatocyte death and liver damage in the ConA model of fulminant liver failure [19]. Furthermore, Con A is related to the production of homocysteine in lymphocytes, effect reverses by resveratrol, an important antioxidant [20]. Homocysteine in turn increases oxidative stress and is closely related to accumulation of asymmetric dimethylarginine [21].
Despite the major antioxidant activity exerted by AE extract, it presented less polyphenol and flavonoids content than E. Although, total polyphenols shows a good correlation with antioxidant activity, some authors have observed that, not always this occurs. Padilla [22] demonstrated that the pericarp of Melicoccus bijugatus presented the lowest polyphenol content (1.40 g GAE/100 g) but a high reducing power and DPPH scavenger activity. The authors noted that the difference in the chemical structure of each of the polyphenols present in the different samples can be reacted as electron donors or not, a feature that will influence the power reducer. Also authors, explained that reducing power is related to the presence of reductones, which are strong reducing agents, fairly strong acids and commonly derived from saccharides by oxidation at the carbon atom alpha to the carbonyl function, in this sense, flavonols/procianidins could act similarly to reductones.
By other way, the major potency exerted by E on tumoral cells inhibition could be related to the high content in polyphenols and flavonoids but also with it antioxidant activity related to H2O2 scavenger activity. It is known that polyphenols are related to antioxidant activity as well as antiproliferative action [23-26]. By other way, the role of H2O2 level on the modulation of cell proliferation and death was before observed. In hamster and rat fibroblast and in tumor cells such as human histiocytic leukaemia cells low concentrations of H2O2 (10 µM - 1 µM) exerts a stimulatory effect on cell proliferation [5]. In contrast, H2O2 treatment of cells with concentrations of about 10–4 M causes activation of NFkB, phenomenon related to a decrease in cell proliferation associated with DNA damage, mutations and genetic instability [6].
In order to find a correlation between the effects observed on proliferation with the antioxidant activity, the antioxidant activity of the extracts related to H2O2 modulation was analyzed. It can be said that, AE presented more CAT and Px “per se” activities, related to H2O2 scavenging activity and E exerted more SOD activity, which is related to H2O2 synthesis. Previously it was demonstrated that, BW5147 cells presented low quantity of H2O2 in comparison with normal lymphocytes, because of low SOD activity [7]; in addition it was reported that, the increase of H2O2 is related to the antiproliferative action of an aqueous extract form L. divaricata in these same cells [27]. For example, it has been demonstrated that some tumor cells, can produce variable levels of H2O2 according to the activity of antioxidant enzymes such as the Mn+2-dependent superoxide dismutase (SOD). As well as, SOD, catalase and peroxidase are the major antioxidant intracellular enzymes in mammalian cells in protecting cells from the damage by ROS, low expression of antioxidant enzymes has been observed in numerous solid tumors and the increase in Mn+2-SOD expression induced the inhibition of breath cancer cells [28,29]. Moreover, the anticancer effects of Mn+2-SOD can be reverted by the overexpression of catalase and peroxidase supporting that the anticancer effect exerted by Mn+2-SOD is mediated by increase of H2O2 [30,31].
In fact, the antiproliferative action of E and AE exerted on tumoral cells could be related to their SOD like activity. But the major SOD activity exerted by E could explain it selective action on tumoral cells inhibition as well as the presence of polyphenols.
Both extracts presented a similar chemical Pattern about flavonoids content, but E also presented quercetin and AE did not. Quercetin possess both antioxidant action but also can increase SOD activity in numerous cells, also in tumoral cells such as human hepatoma cells, increasing H2O2 level and conducing cells to apoptosis [32], so this compound could contribute to the antiproliferative action of E exerted on tumoral cells. Also it was demonstrated that quercetin presented SOD like activity [33], also this compound could contribute to the SOD like activity presented by E.
By other way, E presented more rutin content (2.7 times more than AE). Previously it was demonstrated that, rutin exerted Px “per se” activity related to H2O2 elimination [3], but also it was demonstrated that an aqueous extract from T. x viridis presents stimulating effect on normal lymphocytes without Con A pre-stimulation, not only “in vitro” but also “in vivo”, in relation to rutin presence [34]. So, taking into account the Px “per se” activity of rutin, this compound probably could not be related to the antiproliferative action of the extracts on tumoral cells. Nevertheless, more studies related to the activity of rutin on tumoral and normal lymphocytes proliferation as well as its antioxidant activity are being performed.
In conclusion, depending on the methodology of extraction a plant could exert a selective antiproliferative activity on tumoral cells or not. In this lymphoma cell line, not only the presence of polyphenols was important to exert antiproliferative activity but also, the presence of compounds capable to exert an induction of H2O2 level.
5. Acknowledgements
The study was supported by a grant PIP 0007 from CONICET.
REFERENCES
- A. C. N. Leong, Y. Kinjo, M. Tako, H. Iwasaki, H. Oku and H. Tamaki, “Flavonoid Glycosides in the Shoot System of Okinawa Taumu (Colocasia esculenta S.),” Food Chemistry, Vol. 119, No. 2, 2001, pp. 630-635. doi:10.1016/j.foodchem.2009.07.004
- G. Toker, M. Aslan, E. Yesilada, M. Memisoglu and S. Ito, “Comparative Evaluation of the Flavonoid Content in Officinal Tiliae flos and Turkish Lime Species for Quality Assessment,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 26, No. 1, 2001, pp. 111-121. doi:10.1016/S0731-7085(01)00351-X
- C. Marrassini, C. Anesini and G. Ferraro, “HPLC Fingerprint of a Flowers’ Extract of Tilia x viridis and Relationship Correlation of Antiproliferative and Antioxidant Activity,” Phytotherapy Research, Vol. 25, No. 10, 2011, pp. 1466-1471. doi:10.1002/ptr.3444
- M. L. Barreiro Arcos, G. Cremaschi, G. S. Werner, J. Coussio, G. Ferraro and C. Anesini, “Tilia cordata Mill Extracts and Scopoletin (Isolated Compound): Differential Cell Growth Effects on Lymphocytes,” Phytotherapy Research, Vol. 20, No. 1, 2006, pp. 34-40. doi:10.1002/ptr.1798
- M. Shibanuma, T. Kuroki and M. Nose, “Induction of DNA Replication and Expression of Protooncogenes cmyc and c-fos in Quiescent Balb/3T3 Cells by XanthineXanthine Oxidase,” Oncogene, Vol. 4, 1988, pp. 17-21.
- X. Park, X. You and J. A. Imalay, “Substantial DNA Damage from Submicromolar Intracellular Hydrogen Peroxide Detected in Hpx– Mutants of Escherichia Coli,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 102, No. 26, 2005, pp. 9317-9322. doi:10.1073/pnas.0502051102
- R. Davicino, M.-G. Manuele, G. Ferraro, B. Micalizzi and C. Anesini, “Modulatory Effect of Hydrogen Peroxide on Tumoral Lymphocytes Proliferation,” Immunopharmacology and Immunotoxicology, Vol. 31, 2009, pp. 130- 139.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, “Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of the Folin-Ciocalteu Reagent,” Methods in Enzymology, Vol. 299, 1999, pp. 152-178
- R. Filip, P. López, G. Giberti, J. Coussio and G. Ferraro, “Phenolic Compounds in Seven South American Ilex Species,” Fitoterapia, Vol. 72, No. 7, 2001, pp. 774-778. doi:10.1016/S0367-326X(01)00331-8
- C. Anesini, A. Genaro, G. Cremaschi, L. Sterin Borda, C. Cazaux and E. Borda, “Immunomodulatory Action of Larrea divaricata Cav,” Fitoterapia, Vol. 67, 1996, pp. 329-333.
- G. Cremaschi, P. Fisher and F. Boege, “β-Adrenoceptor Distribution in Murine Lymphoid Cell Line,” Immunopharmacology, Vol. 22, No. 3, 1991, pp. 195-206. doi:10.1016/0162-3109(91)90044-Y
- B. Alexander and D. J. Browse, S. J. Reading and I. S. Benjamin, “A Simple and Accurate Mathematical Methods for Calculation of the EC50,” Journal of Pharmacology and Toxicology, Vol. 41, No. 2-3, 1999, pp. 55-58. doi:10.1016/S1056-8719(98)00038-0
- T. Araki, A. Tamamoto and M. Tamada, “Accurate Determination of DNA Content in Single Cell Nuclei Stained with Hoechst 33258 Florochrome at High Salt Concentration,” Histochemistry, Vol. 87, No. 4, 1987, pp. 331-338. doi:10.1007/BF00492587
- T. Osawa and M. Namiki, “A Novel Type of Antioxidant Isolated from Leaf Wax of Eucalyptus Leaves,” Agricultural and Biological Chemistry, Vol. 45, No. 3, 1981, pp. 735-740. doi:10.1271/bbb1961.45.735
- M. S. Blois, “Antioxidant Determinations by the Use of a Stable Free Radical,” Nature, Vol. 26, 1958, pp. 1199- 1200.
- M. C. Carrillo, S. Kanai, M. Nokubo and K. Kitani, “Derprenyl Induces Activities of Both Superoxide Dismutase and Catalase But Not Glutathione Peroxidase in the Striatum of Young Male Rats,” Life Science, Vol. 48, No. 6, 1991, pp. 517-521. doi:10.1016/0024-3205(91)90466-O
- V. Herzog and H. D. Fahimi, “A New Sensitive Colorimetric Assay for Peroxidase Using 3,3’Diaminobenzidine as Hydrogen Donors,” Analytical Biochemistry, Vol. 55, No. 2, 1973, pp. 554-562. doi:10.1016/0003-2697(73)90144-9
- H. Shirin, H. Aeed, A. A. Zipora, M. M. Kirchner, E. Brazowski, I. Goldiner and R. Bruck, “Inhibition of Immune-Mediated Concanavalin A-Induced Liver Damage by Free-Radical Scavengers,” Digestive Diseases and Sciences, Vol. 55, No. 2, 2010, pp. 268-275. doi:10.1007/s10620-009-0732-5
- J. M. Hwang, J. Sook Cho, T. H. Kim and Y. I. Lee, “Ellagic Acid Protects Hepatocytes from Damage by Inhibiting Mitochondrial Production of Reactive Oxygen Species,” Biomedicine & Pharmacotherapy, Vol. 64, No. 4, 2010, pp. 264-270. doi:10.1016/j.biopha.2009.06.013
- K. Schroecksnadel, C. Winkler, B. Wirleitner, H. Schennach, G. Weiss and D. Fuchs, “Anti-Inflammatory Compound Resveratrol Suppresses Homocysteine Formation in Stimulated Human Peripheral Blood Mononuclear Cells in Vitro,” Clinical Chemistry and Laboratory Medicine, Vol. 43, No. 10, 2005, pp. 1084-1088. doi:10.1515/CCLM.2005.189
- N. Tyagi, K.C. Sedoris, M. Steed, A. V. Ovechkin, K. S. Moshal and S. C. Tyagi, “Mechanisms of HomocysteineInduced Oxidative Stress,” American Journal Physiology Heart and Circulatory Physiology, Vol. 289, No. 6, 2005, pp. H2649-H2656. doi:10.1152/ajpheart.00548.2005
- F. C. Padilla, A. M. Rincón and L. Bou-Rached, “Contenido de Polifenoles y Actividad Antioxidante de Varias Semillas y Nueces,” Archivos Latinoamericanos de Nutrición, Vol. 3, 2008, pp. 303-308.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, “Antioxidant Properties of Phenolic Compounds,” Trends in Plant Science, Vol. 2, No. 4, 1997, pp. 152-159. doi:10.1016/S1360-1385(97)01018-2
- L. Bravo, “Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance,” Nutrition Reviews, Vol. 56, No. 11, 1998, pp. 317-333. doi:10.1111/j.1753-4887.1998.tb01670.x
- B. Halliwell, “Establishing the Significance and Optimal Intake of Dietary Antioxidants: The Biomarker Concept,” Nutrition Reviews, Vol. 57, No. 4, 1999, pp. 104-113. doi:10.1111/j.1753-4887.1999.tb06933.x
- D. Lamoral-Theys, L. Pottier, F. Dufrasne, J. Neve, J. Dubois, A. Kornienko, R. Kiss and L. Ingrassia, “Natural Polyphenols That Display Anticancer Properties through Inhibition of Kinase Activity,” Current Medicinal Chemistry, Vol. 17, No. 9, 2010, pp. 812-825. doi:10.2174/092986710790712183
- R. Davicino, M. G. Manuele, S. Turner, G. Ferraro and C. Anesini, “Antiproliferative Activity of Larrea Divaricata Cav. on a Lymphoma Cell Line: Participation of Hydrogen Peroxide in Its Action,” Cancer Investigation, Vol. 28, 2010, pp. 13-22. doi:10.3109/07357900902849665
- C. J. Weydert, T. A. Waugh, J. M. Ritchie, K. S. Iyer, J. L. Smith, L. Li, D. R. Spitz and L. W. Oberley, “Overexpression of Manganese or Copper Zinc Superoxide Dismutase Inhibits Breast Cancer Growth,” Free Radical Biology and Medicine, Vol. 41, No. 2, 2006, pp. 226-227. doi:10.1016/j.freeradbiomed.2006.03.015
- L. W. Oberley, “Mechanism of the Tumor Suppressive Effect of MnSOD Overexpression,” Biomedicine and Pharmacotherapy, Vol. 59, No. 4, 2005, pp. 143-148. doi:10.1016/j.biopha.2005.03.006
- A. M. Rodriguez, P. M. Carrico, J. E. Mazurkiewicz and J. A. Melendez, “Mitochondrial or Cytosolic Catalase Reverses the MnSOD-Dependent Inhibition of Proliferation by Enhancing Respiratory Chain Activity Net ATP Production and Decreasing the Steady State Levels of H2O2,” Free Radical Biology and Medicine, Vol. 29, No. 9, 2000, pp. 801-813. doi:10.1016/S0891-5849(00)00362-2
- S. Li, T. Yan, J. Q. Yan, T. D. Oberley and L. W. Oberley, “The Role of Cellular Glutathione Peroxidase Redox Regulation in the Suppression of Tumor Cell Growth by Manganese Superoxide Dismutase,” Cancer Research, Vol. 60, 2000, pp. 3927-3939.
- Y.-F. Chang, Y.-C. Hsu, H.-F. Hung, H.-J. Lee, W.-Y. Lui, C.-W. Chi and J.-J. Wang, “Quercetin Induces Oxidative Stress and Potentiates the Apoptotic Action of 2-Methoxyestradiol in Human Hepatoma Cells,” Nutrition and Cancer, Vol. 61, No. 5, 2009, pp. 735-745. doi:10.1080/01635580902825571
- J. V. Formica and W. Regelson, “Review of the Biology of Quercetin and Related Bioflavonoids,” Food and Chemical Toxicology, Vol. 33, 1995, pp. 1061-1080. doi:10.1016/0278-6915(95)00077-1
- R. Davicino, G. Zettler, M. Rodriguez Brizi, C. Marrassini, G. Ferraro, R. Filip and C. Anesini, “In Vivo Immunomodulatory Effect of Tilia x viridis Extracts on Normal Lymphocyte Proliferation: A Direct and an Indirect Action,” Phytotherapy Research, Vol. 25, No. 9, 2011, pp. 1342-1347. doi:10.1002/ptr.3432

