Natural Science
Vol.5 No.10(2013), Article ID:37736,7 pages DOI:10.4236/ns.2013.510134
Effect of natural and synthetic growth stimulators on in vitro rooting and acclimatization of common ash (Fraxinus excelsior L.) microplants
![]()
Forest Biotechnology Laboratory, Branch of Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry RAS, Pushchino, Russia; *Corresponding Author: vglebedev@mail.ru
Copyright © 2013 Vadim Lebedev, Konstantin Schestibratov. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 25 August 2013; revised 25 September 2013; accepted 2 October 2013
Keywords: Common Ash; In Vitro; Plant Growth Stimulators; Rooting; Acclimatization
ABSTRACT
Application of growth stimulators can be especially effective on plantlets in vitro of tree species which are usually worse rooted and adapted in comparison with annual plants. In our work we evaluate effects of natural (dihydroquercetin, Zircon) and synthetic growth stimulators (Melafen, Fumar, Epin-Extra) on rooting and acclimatization of common ash (Fraxinus excelsior L.) microplants. The 0.05% - 0.2% Zircon and 10−5% Melafen enhanced in vitro rooting by 29% - 37% and 31%, respectively. Melafen also stimulated root formation faster compared to control plants. The dihydroquercetin concentration of 0.01% increased rooting by 24% and root number per shoot by 1.8 times. In vitro plants rooted on media supplemented with Melafen, Fumar and Zircon demonstrated enhanced ability to adapt to non-sterile conditions and accelerated growth. Two months after planting to the greenhouse, plants rooted on 0.01% dihydroquercetin were 45% taller than the control. Weekly spraying of plantlets with 0.02% Epin-Extra containing 24-epibrassinolid stimulated growth of uniform plants with large leaves. The obtained results support the use of growth stimulators for application in clonal micropropagation of common ash both for large-scale production of planting stock and for conservation of rare and valuable genotypes.
1. INTRODUCTION
The majority of forest trees are propagated by seeds and their progeny is genetically variable. Conventional methods of vegetative propagation like grafting, cutting, layering etc. for many trees are often too slow or impossible. Clonal micropropagation using tissue culture offers an attractive alternative to traditional methods of propagation of forest trees. This technology provides the possibility for high multiplication of selected superior trees and to produce genetically uniform plant material irespective of the season and weather. Micropropagation activities occur in at least 64 countries in all regions of the world and include more than 80 genera of forest trees [1]. Clonal planting material is currently the main way to increase productivity of forest plantations. In addition, clonal micropropagation is widely used for conservation of hardwood tree species, which are rare or threatened by exotic insects and diseases [2]. Current studies in this field are aimed at reducing time and cost of growing in vitro plants by reducing the steps of developmental pathways and decreasing the loss of plants during acclimatization [3]. One of the solutions to this problem is to use plant growth stimulators from diverse origins. These chemicals act on the most plant physiological and biochemical processes at very low concentrations. Their use can be particularly effective during rooting and acclimatization, which are the most critical stages of clonal micropropagation. In particular, to improve in vitro rooting of recalcitrant species, in addition to auxins, substances with phytohormonal-like activity are used [4]. A very important stage of clonal micropropagation is the acclimatization of plantlets in vitro after transfer to non-sterile environment. In vitro and ex vitro conditions are very different in parameters such as humidity, illumination, nutrient concentrations in medium or substrate, and gaseous composition of air. These changes cause stress in plants, which often leads to death or growth retardation [5]. To increase plant survival after transfer to soil, different strategies were utilized: photoautotrophic culture [6], plant growth retardants [7], arbuscular mycorrhiza inoculation [8], and various growth stimulators. In particular, there are reports on the use of substances such as triacontanol [9], chitosan [10] and humic acids [11]. Application of growth stimulators can be especially effective on plantlets in vitro of tree species which are usually worse rooted and adapted in comparison with annual plants. The aim of this work was to evaluate effects of natural (dihydroquercetin, Zircon) and synthetic growth stimulators (Melafen, Fumar, Epin-Extra) on rooting and acclimatization of common ash microplants.
2. MATERIALS AND METHODS
2.1. Plant Material
Sterile common ash (Fraxinus excelsior L.) plants were kindly provided by Prof. V. Padutov from Forest Institute at Gomel, Belarus. Stock shoot cultures were established in vitro from shoots of 60-year-old plus trees. Proliferating culture was maintained in vitro in 300-ml glass jars containing 50 ml of MS medium [12], 30 g/l sucrose, and 7 g/l agar (american type QP, Panreac), supplemented with 2 mg/l BA and 0.1 mg/l IAA. Uniform twoto three-node shoots (20 - 30 mm in length) oriented horizontally were used as explants. Cultures were transferred to fresh medium at four week intervals.
2.2. Rooting Conditions
Shoots (15 - 20 mm long, 2 - 3 pairs of leaves) were isolated and used for rooting experiments. Basal medium for rooting consisted of Woody Plant Medium (WPM) salts and vitamins [13] (half strength of macrosalts), 10 g/1 sucrose, 7 g/l agar and 0.5 mg/l NAA. The following experiments were carried out to determine the effects of growth stimulators on rooting: 1) Melafen at 10−8%, 10−7%, 10−6%, 10−5%, 10−3%; 2) Fumar at 10−4%, 10−3%, 10−2%; 3) Zircon at 0.05%, 0.1%, 0.2%; 4) dihydroquercetin (DHQ) at 0.01%, 0.03%, 0.05%, 0.1%. Since the stock solution of DHQ is a 10% ethanol solution the medium containing 0.3%, 0.5%, 1% ethanol was used as control. Rooting was carried out in 250-ml polypropylene containers containing 50 ml of medium. 4 - 6 containers with 24 shoots each constituted one experimental treatment. The percentage of rooted shoots, the number of roots per shoot (more than 2 mm) and the root length were recorded after 2, 3, 4 and 8 weeks (for DHQ only) on the rooting medium.
The pH of all media was adjusted to 5.6 - 5.8 with 1 N KOH and autoclaved at 121˚C at 1 kg/cm2 for 20 min. Growth regulators and vitamins were filter-sterilized (Millipore, 0.22 µm) and added to the media after autoclaving. All cultures were grown in a 16-h photoperiod provided by cool-white fluorescent lamps giving a photon flux density of 40 μmol m−2.s−1 at the culture level and a constant temperature of 23˚C ± 1˚C.
2.3. Acclimatization Conditions
The rooted plants were selected for acclimatization in the greenhouse. Microplants were washed with tap water, transferred to peat: perlite (3:1) and kept under relative humidity of 80% - 90%, temperature of 23˚C - 28˚C for 4 weeks. Shoots grown on control medium were treated with growth stimulator solutions weekly during 2 - 8 weeks after planting into the substrate in 2.5 ml per plant. Experiment includes the following treatments: 1) control (without treatment); 2) water; 3) Epin-Extra (0.2 ml/l); 4) Zircon (0.1 ml/l); 5) substrate treatment with Trichoderma harzianum (109 CFU/g; 250 mg/300 ml of substrate). After 1, 2 and 3 months, the number of surviving plantlets and the mean shoot height (only 2 months) were recorded. The description of the growth stimulators is presented in Table 1.
2.4. Statistical Analysis
All experiments were performed in a completely randomized block design and were repeated twice. A general analysis of variance (ANOVA) was conducted using the Statistica 7.0 program. Percentage data were transformed to arcsine values prior to analysis. Means were evaluated according to Duncan’s multiple range test at p = 0.05.
3. RESULTS
The experiment showed a positive effect of growth
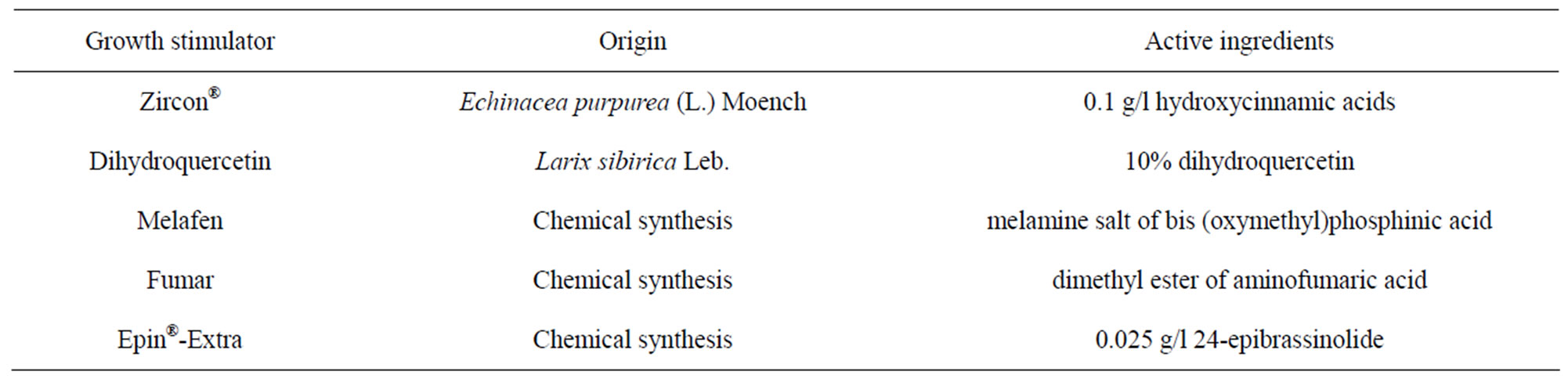
Table 1. Growth stimulators used in this study.
stimulators on rooting of common ash plants (Table 2). Addition of 10−5% Melafen and all concentrations of Zircon to the medium significantly enhanced rooting compared with control. After 4 weeks the best rooting percentage (100%) was obtained with the media containing 0.1% Zircon. The fastest root formation was observed on media with 10−5% and 10−3% Melafen. The number of roots per rooted shoot was similar in the most treatments. Only shoots on medium containing 10−6% Melafen produced a significantly greater mean number of roots compared to control, 3.4 and 2.8, respectively. The elongation of roots on media with Zircon was strongly inhibited. The lengths of the longest roots at the optimal concentrations for rooting were 7.7 mm (0.05% Zircon), 18.0 mm (10−2% Fumar) and 18.6 mm (10−8% Melafen). Moreover, the roots on medium with Zircon were thinner and had dark tips.
The addition of DHQ to the rooting medium resulted in positive effects on the percentage of rooted shoots and number of roots per rooted shoot but these effects were concentration-dependent (Table 3). The highest rooting (100%) after 4 weeks was obtained with 0.01% DHQ. Root formation on other concentrations of DHQ was slower and therefore the experiment was extended to 8 weeks. After 8 weeks the rooting percentage on 0.03% DHQ was significant higher than the control. The root number with DHQ was 4.7 - 5.8, in compare to control with only 3.2 per shoots. There was no significant dif-
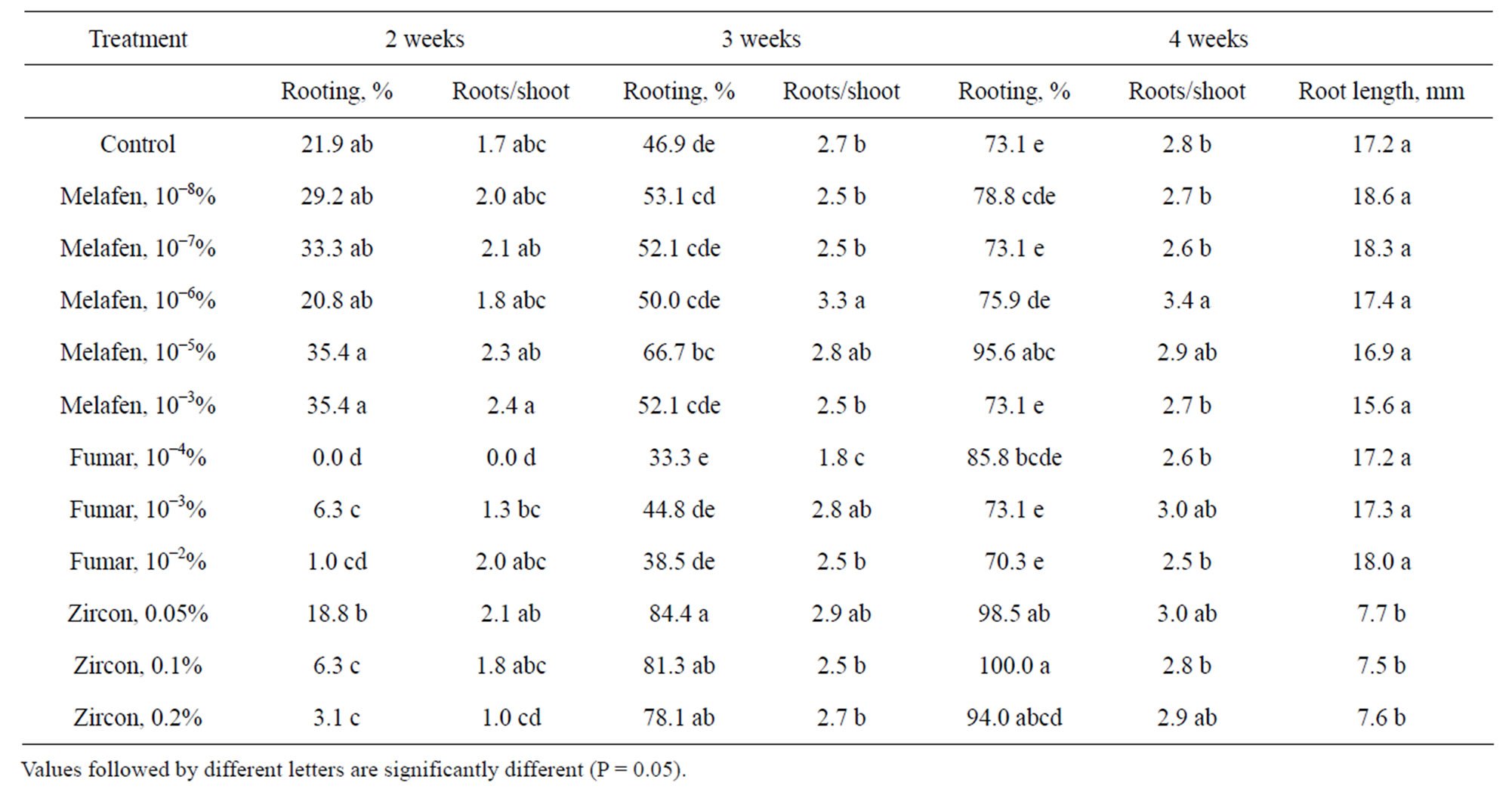
Table 2. The effect of growth stimulators on rooting of common ash in vitro.
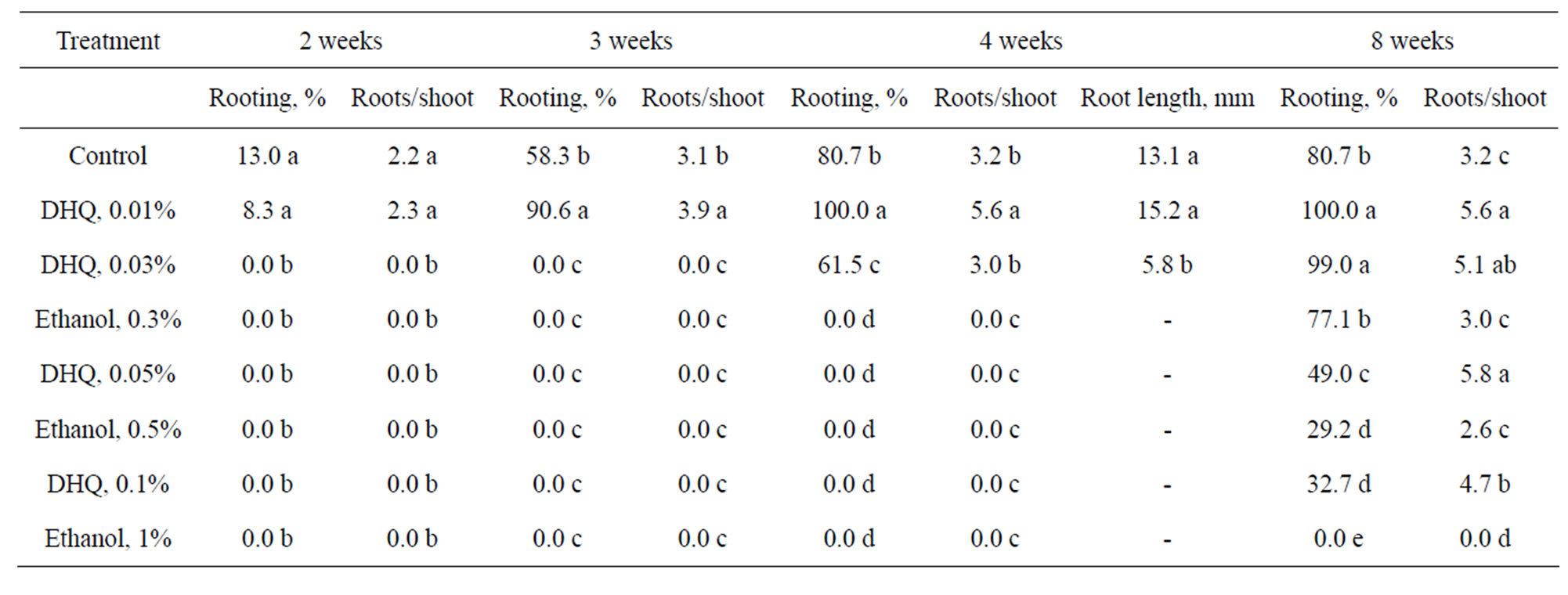
Table 3. The effect of DHQ and ethanol on rooting of common ash in vitro.
ference in the number of roots per shoot between 0.01% DHQ and control but DHQ was stimulated growth of lateral roots. Chlorotic leaves were observed on shoots rooted on 0.1% DHQ, 0.5% and 1% ethanol, probably, due to the toxic effect of ethanol. Besides shoots on medium with 0.05% and 0.1% DHQ were thicker and rougher than in all other treatments. Browning of medium around some shoots was observed at 0.1% DHQ. Unlike control DHQ was stimulated growth of shoots especially on medium with 0.01% DHQ.
Common ash plants rooted on medium with growth stimulators were acclimatized in the greenhouse (Table 4). The survival rate was high (96% - 100%) except for the variant with 0.05% Zircon. Growth stimulators had positive effect on growth of plants during ex vitro acclimatization. After 1 month the percentage of actively growing plants were significantly higher in all treatments with growth stimulators in comparison with control, 75% - 92% and 57%, respectively. Shoots rooted on 10−7%, 10−6%, 10−5% Melafen and 0.05%, 0.1% Zircon grew significantly taller (17% - 32%) than those on control medium.
Plants rooted on medium with DHQ and ethanol also were acclimatized in the greenhouse. The DHQ treatment did not produce statistically significant changes in survival percentage or growing shoot part (Table 5).
Ethanol had negative effect on subsequent acclimatization of rooted plants and this effect was correlated with concentration of ethanol. Aftereffect of DHQ significantly increases growth of plants in the greenhouse—up to 45% compared with control. Treatment of plants by growth stimulator solutions of and treatment of soil by biofungicide had no effect on mortality of plantlets during acclimatization. The differences between the treatments were not significant due to the high survival rate (96% - 99%) (data not shown). However the appearance of the shoots was different: plants treated with Epin-
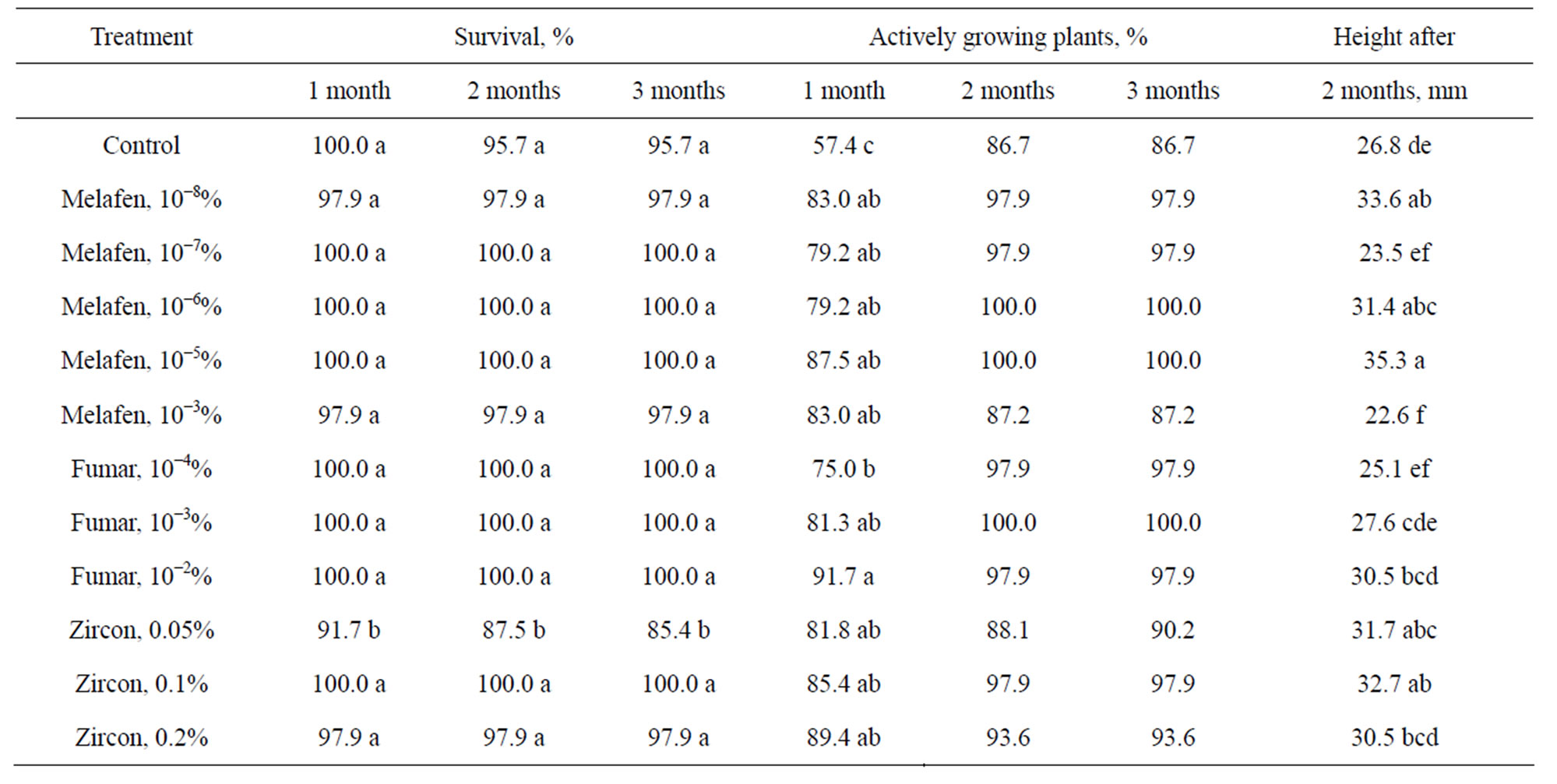
Table 4. Acclimatization of common ash plants rooted on medium with growth stimulators.
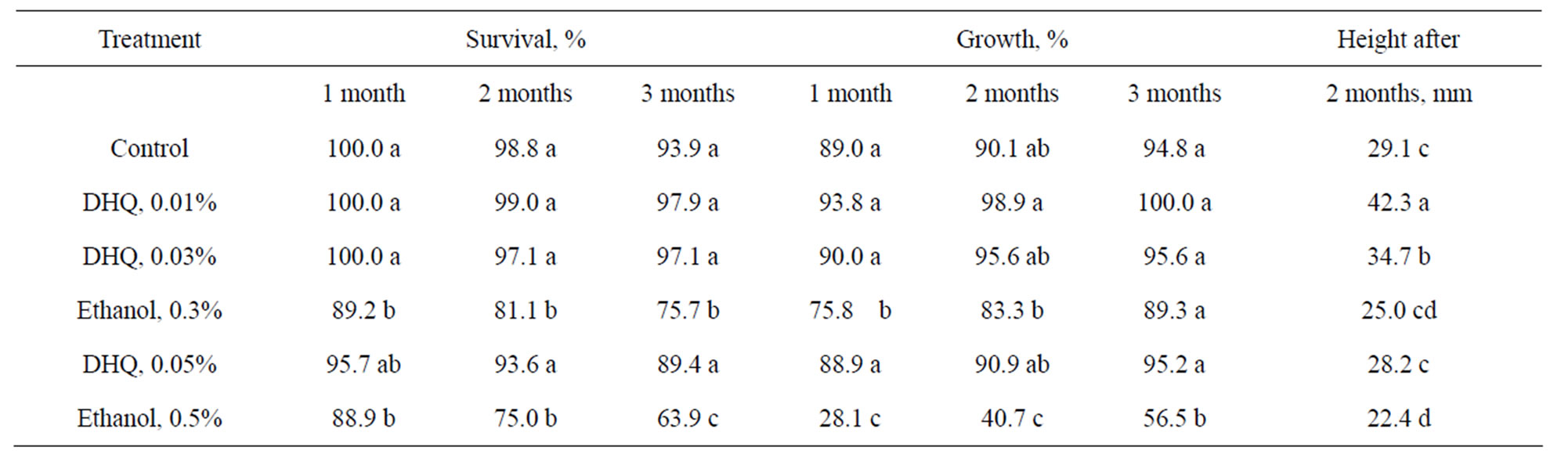
Table 5. Acclimatization of common ash plants rooted on medium with DHQ and ethanol.
Extra and less Zircon were significantly larger and more vigorous than plants in any other treatments (Figure 1).
4. DISCUSSION
The type and concentration of growth stimulators strongly influenced the rooting of common ash shoots in vitro. The highest rooting percentage (94% - 100%) was obtained on medium supplemented with Zircon at various concentrations. The natural plant growth stimulator Zircon derived from Echinacea purpurea and contained 0.1 g/l hydroxycinnamic acids. It is known that some hydroxycinnamic acids can inhibit the oxidation of IAA [14], thus improving rooting of plants. On the other hand, shoots on the medium containing Zircon produced shortest roots compared with all other treatments. Recent research has shown that exogenously applied cinnamic acid, precursor of hydroxycinnamic acids, inhibited soybean root growth [15]. Addition of Melafen to medium also had positive effect on root formation but only one of five concentrations used, 10−5%. With barley (Hordeum vulgare L.), Osipenkova et al. [16] showed that the height of seedlings treated with Melafen at concentrations of 0.5 × 10−10 and 0.5 × 10−8 M increased by approximately 10% and 20%, respectively, but at high concentrations (10−5 and 10−3 M), Melafen had no effect on the growth of seedlings. No significant differences in rooting percentage, root length and root number per shoot were observed on media supplemented with 10−4%, 10−3%, 10−2% Fumar compared with control. Thus the Fumar does not stimulate the rhizogenesis of common ash microshoots.
Dihydroquercetin (DHQ, taxifolin), a member of the flavonoids family, is one of the most prominent dietary antioxidants and has great therapeutic potential [17]. It was shown, that DHQ had strong stabilizing effect on the membranes of isolated vacuoles of common beet [18]. In vitro bioassays showed that DHQ is a strong inhibitor of Fusarium growth and macrospore formation [19]. In our experiments a DHQ isolated from Larix sibirica Ledeb significantly increased the rooting and the root number per shoot at concentration 0.01% and stimulated branching roots and growth of shoots. Slower root formation at higher DHQ concentrations could be related to the inhibitory action of ethanol since after its gradual evaporation the ability to root recovered. Higher concentrations of DHQ (0.05% and 0.1%) changed the appearance of shoots and significantly decreased rooting frequency but increased mean root number per shoot compared with control. These data suggest a powerful effect of DHQ on in vitro plants.

Figure 1. Common ash plants in the greenhouse: left—control, right—treated with Epin-Extra.
During acclimatization no significant difference occurred in the survival percentages, which were very high: 96%, 98% - 100%, 100% and 85% - 100% for shoots rooted in the control, Melafen, Fumar and Zircon media, respectively. However all growth stimulators have contributed to a more rapid acclimatization of in vitro plants, which resulted in the early beginning of growth compared with the control. Apparently, used growth stimulators have a common mechanism of action—stimulation of natural processes in the plants in vitro, which leads to better tolerance to stresses during acclimatization. Although after 2 months there was no significant difference in the percentage of growing plants, the medium containing Zircon, Melafen and DHQ produced shoots which were taller than the control. All concentrations of Zircon significantly increased the height of common ash plants. Tallest shoots were obtained after rooting on the medium supplemented with 10−5% Melafen. Other Melafen concentrations increased or decreased shoot height compared with controls plants. Our data are generally in agreement with those reported by Ladyzhenskaya et al. [20] demonstrating that Melafen could both stimulate and inhibit the growth of potato tubers depending on its concentration. Rooting on low concentrations of DHQ (0.01% and 0.03%) also stimulated subsequent growth of plants in the greenhouse. In plant cultivation a number of other growth stimulators isolated from conifers are used, but not all of them are effective. Testing several coniferous needle products on strawberries showed some positive influence on plant development and the yield but no significant effects on spreading of diseases and fruit quality were observed [21].
Very high survival (96% - 99%) of common ash plants during acclimatization doesn’t allow to determine the effect of the growth stimulator treatments on plant mortality after transfer to non-sterile conditions. However weekly spraying of plantlets with Epin-Extra and Zircon solutions stimulated growth of uniform plants with large leaves. Plant growth stimulator Epin-Extra contains 24- epibrassinolide that plays an important role in many physiological processes and adaptation to various environmental stresses [22]. It was shown that exogenous application of 24-epibrassinolide on rice seeds improved tolerance to salt stress [23]. Our data support the role of 24- epibrassinolide in plant protection from stress, in our case, the stress during acclimatization of in vitro plants. Hydroxycinnamic acids in Zircon also had promotable effect on plant growth in the greenhouse. Galis et al. suggest that some phenylpropanoids (e.g. hydroxycinnamic acids) may participate in controlling the endogenous cytokinin/auxin balance, and thus may be of a great importance in plant growth and development [24].
5. CONCLUSIONS
We show here that the plant growth stimulators, both of natural and synthetic origin have a positive impact on common ash microplants in the most critical stages of clonal micropropagation—rooting and acclimatization. The 0.05% - 0.2% Zircon and 0.01% DHQ increased in vitro rooting by 29% - 37% and 24%, respectively. Rooting on media supplemented with Melafen and Zircon enhanced ability of in vitro plants to adapt to non-sterile conditions and accelerated its growth. Plants sprayed during acclimatization by Epin-Extra solution were uniform and had large leaves. Thus, the use of above-mentioned growth stimulators can be recommended for application in clonal micropropagation of common ash both for largescale production of planting stock and for conservation of rare and valuable genotypes.
This work was financially supported by the Ministry of Education and Science of the Russian Federation under the State contract № 14.512.11.0015 from 14.03.2013.
REFERENCES
- FAO (2004) Preliminary review of biotechnology in forestry, including genetic modification. Rome.
- Pijut, P.M., Lawson, S.S. and Michler, C.H. (2011) Biotechnological efforts for preserving and enhancing temperate hardwood tree biodiversity, health, and productivity. In Vitro Cellular and Developmental Biology—Plant, 47, 123-147. http://dx.doi.org/10.1007/s11627-010-9332-5
- Durkovic, J. and Misalova, A. (2008) Micropropagation of temperate noble hardwoods: An overview. Functional Plant Science and Biotechnology, 2, 1-19.
- Gaspar, T., Kevers, C., Penel, C., Greppin, H., Reid, D.M. and Thorpe, T.A. (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cellular and Developmental Biology—Plant, 32, 272-289. http://dx.doi.org/10.1007/BF02822700
- Chandra, S., Bandopadhyay, R., Kumar, V. and Chandra, R. (2010) Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnology Letters, 32, 1199- 1205. http://dx.doi.org/10.1007/s10529-010-0290-0
- Kozai, T. (1991) Photoautotrophic micropropagation. In Vitro Cellular and Developmental Biology—Plant, 27, 47-51. http://dx.doi.org/10.1007/BF02632127
- Hazarika, B.N. (2003) Acclimatization of tissue-cultured plants. Current Science, 85, 1704-1712.
- Azcon-Aguilar, C., Cantos, M., Troncoso, A. and Barea, J.M. (1997) Beneficial effect of arbuscular mycorrhizas on acclimatization of micropropagated plantlets. Scientia Horticulturae, 72, 63-71. http://dx.doi.org/10.1016/S0304-4238(97)00120-9
- Reddy, B.O., Giridhar, P. and Ravishankar, G.A. (2002) The effect of triacontanol on micropropagation of Capsicum frutescens and Decalepis hamiltonii W and A. Plant Cell Tissue and Organ Culture, 71, 253-258. http://dx.doi.org/10.1023/A:1020342127386
- Nge, K.L., New, N., Chandrkrachange, S. and Stevens, W. F. (2006) Chitosan as a growth stimulator in orchid tissue culture. Plant Science, 170, 1185-1190. http://dx.doi.org/10.1016/j.plantsci.2006.02.006
- Baldotto, L.E.B., Baldotto, M.A., Canellas, L.P., BressanSmith, R. and Olivares, F.L. (2010) Growth promotion of pineapple “Vitoria” by humic acids and Burkholderia spp. during acclimatization. Revista Brasileira de Ciência do Solo, 34, 1593-1600. http://dx.doi.org/10.1590/S0100-06832010000500012
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, 15, 473-497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Lloyd, G. and McCown, B.H. (1980) Commercially feasible micropropagation of mountain laurel, (Kalmia latifolia) by use of shoot tip culture. Combined Proceedings of International Plant Propagators’ Society, 30, 421-427.
- Gazaryan, I.G. and Lagrimini, L.M. (1996) Tobacco anionic peroxidase overexpressed in transgenic plants: Aerobic oxidation of indole-3-acetic acid. Phytochemistry, 42, 1271-1278. http://dx.doi.org/10.1016/0031-9422(96)00096-9
- Salvador, V.H., Lima, R.B., dos Santos, W.D., Soares, A.R., Böhm, P.A.F., Marchiosi, R., Ferrarese, M.L.L. and Ferrarese-Filho, O. (2013) Cinnamic acid increases lignin production and inhibits soybean root growth. PLoS ONE, 8, e69105. http://dx.doi.org/10.1371/journal.pone.0069105
- Osipenkova, O.V., Ermokhina, O.V., Belkina, G.G., Oleskina, Yu.P., Fattakhov, S.G. and Yurina, N.P. (2008) Effect of melaphene on mxpression of Elip1 and Elip2 genes encoding chloroplast light-induced stress proteins in barley. Applied Biochemistry and Microbiology, 44, 635-641. http://dx.doi.org/10.1134/S0003683808060136
- Weidmann, A.E. (2012) Dihydroquercetin: More than just an impurity? European Journal of Pharmacology, 684, 19-26. http://dx.doi.org/10.1016/j.ejphar.2012.03.035
- Nurminsky, V.N., Ozolina, N.V., Sapega, J.G., Zheleznykh, A.O., Pradedova, E.V., Korzun, A.M. and Salyaev, R.K. (2009) The effect of dihydroquercetin on active and passive ion transport systems in plant vacuolar membrane. Biology Bulletin, 36, 1-5. http://dx.doi.org/10.1134/S1062359009010014
- Skadhauge, B., Thomsen, K.K. and von Wettstein, D. (1997) The role of the barley testa layer and its flavonoid content in resistance to Fusarium infections. Hereditas, 126, 147-160. http://dx.doi.org/10.1111/j.1601-5223.1997.00147.x
- Ladyzhenskaya, E.P., Platonova, T.A., Evsyunina, A.S., Fattakhov, S.G., Korableva, N.P. and Reznik, V.S. (2007) The effect of melamine salt of bis(oxymethyl)phosphinic acid (melafen) on the growth processes and plasma membrane function in potato tuber cells. Applied Biochemistry and Microbiology, 43, 222-226. http://dx.doi.org/10.1134/S0003683807020172
- Laugale, V. and Daugavietis, M. (2009) Effect of coniferous needle products on strawberry plant development, productivity and spreading of pests and diseases. Acta Horticulturae, 842, 239-242.
- Khripach, V.A., Zhabinskii, V.N. and de-Groot, A.E. (2000) Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Annals of Botany, 86, 441-447. http://dx.doi.org/10.1006/anbo.2000.1227
- Sharma, I., Ching, E., Saini, S., Bhardwaj, R. and Pati, P.K. (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiology and Biochemistry, 69, 17-26. http://dx.doi.org/10.1016/j.plaphy.2013.04.013
- Gális, I., Simek, P., Van Onckelen, H.A., Kakiuchi, Y. and Wabiko, H. (2002) Resistance of transgenic tobacco seedlings expressing the Agrobacterium tumefaciens C58-6b gene, to growth-inhibitory levels of cytokinin is associated with elevated IAA levels and activation of phenylpropanoid metabolism. Plant and Cell Physiology, 43, 939-950. http://dx.doi.org/10.1093/pcp/pcf112

