Materials Sciences and Applications
Vol.3 No.11(2012), Article ID:24742,5 pages DOI:10.4236/msa.2012.311113
New Nanocrystalline Matrix Materials for Sintered Diamond Tools
![]()
AGH-University of Science & Technology, Krakow, Poland.
Email: konstant@agh.edu.pl, aromansk@agh.edu.pl
Received July 9th, 2012; revised August 11th, 2012; accepted September 12th, 2012
Keywords: PM Diamond Tools; Metal Matrix; Nonocrystalline Powder
ABSTRACT
The work presents the possibility of substitution of expensive, wear resistant Co-WC powders, that have been traditionally used in the production of sintered diamond tools, with cheap iron-base counterparts manufactured by ball milling. It has been shown that ball-milled Fe-Ni-Cu-Sn-C powders can be consolidated to a virtually pore-free condition by hot pressing at 900˚C. The as-consolidated material has nanocrystalline structure and is characterised by a combination of high hardness, mechanical strength and excellent resistance to abrasion. Its properties can be widely modified by changing the milling conditions.
1. Introduction
Until relatively recently, cobalt has been the most valued matrix metal in professional diamond-impregnated tools, such as circular and frame saw blades, wire saws, core drills and grinding wheels used for cutting, drilling and shaping natural stone and construction materials. Like any other matrix material, cobalt combines excellent diamond retention properties and resistance to wear which can be widely modified by alloying with WC, WC/W2C, Fe, Cu, Sn, Ni, etc. [1].
Until the early 1990’s, the economical viability of the toolmaking process had been mostly affected by a high cost of diamond grits whereas the contribution of cobalt powders had remained at a relatively low level. The forthcoming price cuts on the diamond supply side and the price spikes of cobalt [2] raised the spectre of substitution.
The intensive search for cheaper matrix powders began in the mid-1990’s and the first cobalt substitutes were marketed in years 1997 and 1998 [3,4]. To date three families of fine copper-base and iron-base powders dedicated to fabrication of diamond-impregnated tools have been developed and launched commercially under the brand names: Cobalite (Umicore, Belgium), Next and Keen (Eurotungstene Poudres, France).
Although cobalt and its substitutes are still used for professional tools, the latest trend is towards a broader application of premixed and/or mechanically alloyed iron-base powders. Good candidates are medium-carbon steels containing metastable austenite that readily transforms to hard martensite under tribological straining [5]. The strain-induced martensitic reaction imparts wear resistance to the matrix [6] and, by generating compressive stress under the working face of the tool, favours retention of the most heavily loaded diamond crystals.
2. Experimental Procedure and Results
Basing on earlier studies [6,7], Fe-Ni-Cu-Sn-C alloys manufactured from inexpensive ball-milled powders by the hot press route were chosen as potential cobalt substitutes in the manufacture of diamond impregnated segments for abrasive applications.
Spongy (carbon reduced) iron, prealloyed water atomised tin-bronze, carbonyl nickel and synthetic graphite powders were provided by Höganäs AB, ECKA, Vale and Timcal, respectively, and used as the starting powders (Figure 1).
Prior to milling the powders were mixed in a Turbula type mixer for 10 minutes. The mixture containing 12% nickel, 8% bronze 80/20, 0.63% graphite, balance iron, was ball-milled in air for 8, 30 and 120 hours at 70% critical speed. About 50% of the milling container was filled with 12 mm hardened 100 Cr6 steel balls and ~10:1 ball-to-powder weight ratio was used.
After ball milling the powders were tested for particle size and shape, and examined metallographically. The results are shown in Table 1 and Figure 2.
The ball-milled powders were then poured into rec-

Figure 1. Starting powders: (a) NC 100.24 iron, (b) 25GR80/20-325 bronze, (c) T210 nickel, (d) Graphite TIMREX F10.
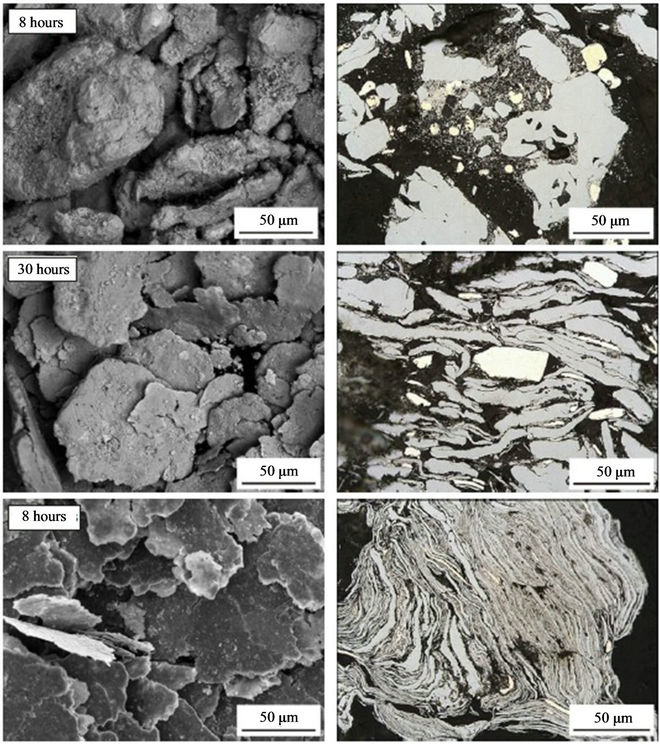
Figure 2. Particle shape (left) and microstructure (right) of powders ball-milled for 8 (top), 30 (middle) and 120 hours (bottom).
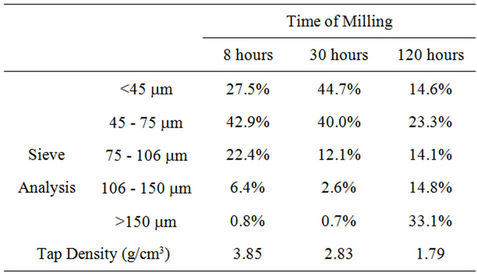
Table 1. Particle size distributions and tap densities of ballmilled powders.
tangular cavities of a graphite mould and consolidated to near-full density by passing an electric current through the mould under uniaxial compressive load. The powders were held for 3 minutes at the peak temperature of 900˚C, under 25 MPa, and subsequently cooled down at a mean rate of 6.4 K/s within the critical temperature range from the hot pressing temperature down to 550˚C.
The as-consolidated specimens were first tested for density and hardness using the water displacement method and Knoop test, respectively, and then examined metallographically by means of light microscopy (LM), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction.
The metallographic sections were wet ground on #220 SiC paper and successively polished on cloths impregnated with 9, 3 and 1 μm diamond compound. As it has been previously documented on annealed polycrystalline 70:30 brass [8], both grinding and polishing produce a plastically deformed layer. Its depth depends on the sharpness and size of the abrasive, hence the deformed layer extends to 77 μm and 0.7 μm after plain grinding on #220 SiC paper and after fine polishing on 1 μm diamond respectively. More importantly, a significant plastic deformation occurs only after grinding and extends down to ~8 μm beneath a 2 μm thick, scratched skin. Therefore by using X-ray diffraction with the CuKα radiation and Bragg-Brentano geometry it was possible to determine the proportion of (γFe) to (αFe) in the subsurface layer of the metallographic specimen both after grinding and after polishing in order to predict the amount of martensite which can be generated beneath the working face of the tool by abrasion In addition to the metallographic specimens, a thin foil was prepared from the finest material ball-milled for 120 hours to characterise its microstructure by TEM. Ion etching was used to perform the finishing polish in order to avoid deformation of the specimen.
The results of measurements are given in Table 2 whereas the as-sintered microstructures are presented in Figures 3 and 4.
3. Discussion and Conclusions
The results given in Table 2 indicate that ball-milled FeNi-Cu-Sn-C powders can be hot pressed to near-full density by a 3-minute hold at 900˚C under a pressure of 25 MPa.
During milling powder particles are repeatedly flattened, work-hardened and fractured, and welded together. After 120 hours of milling they show flaky shape and a characteristic layered microstructure with very fine lamellar spacing (Figure 2). The flaky shape increases the average particle size of the powder, as measured by sieving, and halves its tap density.
After densification the tested alloys display high hardness which increases with the milling time and amount of martensite. From Table 2 it is evident that, except for the alloy made of premixed powder, the hardness numbers and volume densities of (γFe) are inversely correlated. The contribution of abrasion-induced martensite to hardness is relatively small although 32% - 72% of the retained austenite is being transformed to martensite during abrasion. This may potentially increase the alloy’s resistance to abrasion as it has been found in similar materials produced from premixed powders [6].
From Figure 3 it is evident that all experimental alloys display fine-grained microstructure. The microscopic examination of the metallographic sections has revealed, however, marked differences in microstructural inhomogeneity of the tested specimens. Those manufactured from premixed powders show a highly inhomogeneous distribution of structural components. For prolonged time of milling strong grain refinement has been observed.
As the ball-milling operation has been performed in air, the powder picks up oxygen which increases from 0.29%
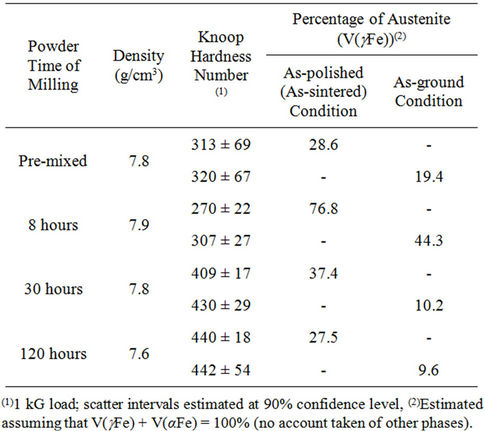
Table 2. As-consolidated densities, hardness numbers and phase compositions.
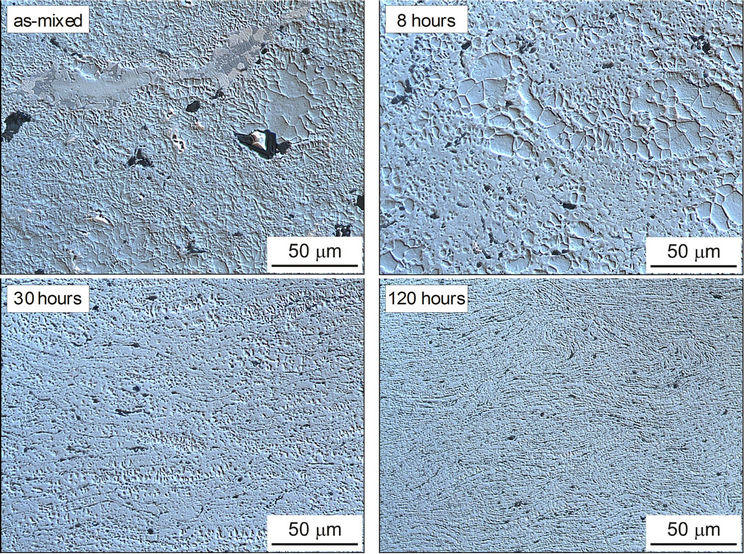
Figure 3. LM micrographs of the experimental alloys. Nomarski contrast.
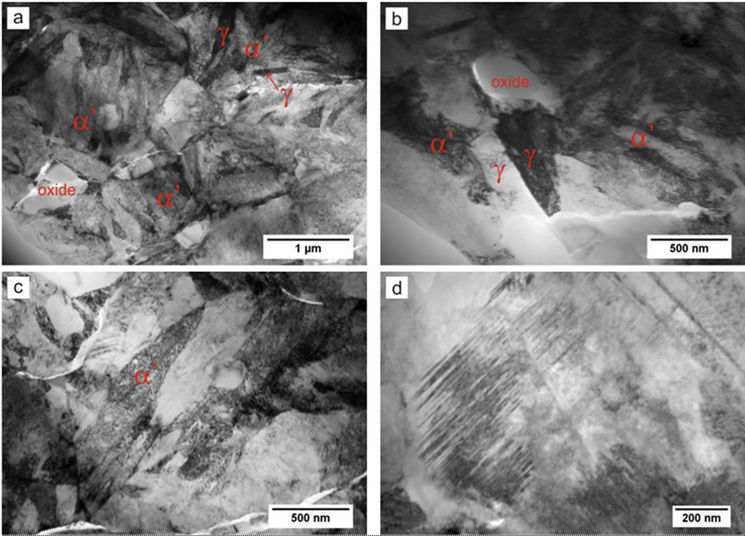
Figure 4. As-sintered microstructure of alloy made from powder ball-milled for 120 hours: (a), (b) Martensite α’, austenite γ and oxides; (c) High-carbon plate martensite; (d) Micro-twins in plate martensite.
to 1.77% after milling for 8 and 120 hours, respectively. Finely dispersed, submicron-sized oxides pin the grain boundaries and impede grain growth. As illustrated in Figure 4, the as-sintered microstructure of material made of the powder milled for 120 hours is generally submitcron-sized (nano-sized). It still remains unclear why prolonged milling decreases the volume density of retained austenite, promoting its transformation to plate martensite, characterised by high density of dislocations and microtwins (Figures 4(c) and 4(d)).
In conclusion it can be stated that:
1) Ball-milling proves to be an economical means whereby flaky powders having layered microstructure with very fine lamellar spacing can be produced.
2) The investigated powders can be readily consolidated to virtually pore-free condition by a 3-minute hold at 900˚C and under a moderate pressure of 25 MPa.
3) The amount of austenite in the investigated alloy strongly depend on powder milling conditions. The highest volume density of austenite (~77%) is retained in material produced from powder ball-milled for 8 hours. Milling prolonged to 30 and 120 hours leads to marked decrease in austenite to 37% and 27%, respectively.
4) The retained austenite is unstable and can be partly transformed to high-carbon martensite under tribological straining.
5) The strain-induced martensitic reaction generates compressive stress under the working face of the tool, which hypothetically should improve retention of working diamonds.
It should be noted that laboratory wear tests and tool field performance trials are underway to determine whether the submicron-grained iron-base matrix is superior to other materials used as a matrix in diamond-impregnated tool components.
4. Acknowledgements
The authors gratefully acknowledge Professor W. Ratuszek and Professor A. Zielinska-Lipiec for the able assistance with the XRD and TEM. The work was supported by the Polish Ministry of Science & Higher Education through contract N N507 592838 (18.18.110.994).
REFERENCES
- J. Konstanty, “Cobalt as a Matrix in Diamond Impregnated Tools for Stone Sawing Applications,” 2nd Edition, Agh-Uwnd, Krakow, 2003.
- Anon, “2011 Production Statistics,” Cobalt News, 2012, p. 3.
- Anon, “A New Generation of Powders for the Diamond Tool Industry,” Marmomacchine International, Vol. 18, 1997, p. 156.
- I. E. Clark and B.-J. Kamphuis, “Cobalite HRR—A New Prealloyed Matrix Powder for Diamond Construction Tools,” Industrial Diamond Review, Vol. 62, 2002, p. 177.
- J. Konstanty, T. F. Stephenson and D. Tyrala, “Novel Fe-Ni-Cu-Sn Matrix Materials for the Manufacture of Diamond-Impregnated Tools,” Diamond Tooling Journal, Vol. 3, 2011, p. 26.
- D. Tyrala, “Engineering Structure and Properties of Sintered Fe-Ni-Cu-Sn Materials Used as a Matrix in Diamond-Impregnated Tools (in Polish),” Ph.D. Thesis, AGHUniversity of Science & Technology, Krakow, 2010.
- J. Konstanty, D. Tyrala, A. Radziszewska and W. Ratuszek, “Properties of Iron-Base Materials Manufactured from Premixed Powders by the Hot Press Process,” Proceedings of the 5th International Powder Metallurgy Conference, Ankara, 8-10 October 2008.
- K. Geels, “The True Microstructure of Materials,” Structure, Vol. 35, 2000, p. 5.

