Materials Sciences and Applications
Vol. 3 No. 1 (2012) , Article ID: 16969 , 7 pages DOI:10.4236/msa.2012.31005
In Situ Synthesis of Titanium Matrix Composite (Ti-TiB-TiC) through Sintering of TiH2-B4C
![]()
1School of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Johannesburg, Republic of South Africa; 2Institut Keramische Technologien und Systeme, Dresden, Germany.
Email: *iakovos.sigalas@wits.ac.za
Received September 19th, 2011; revised November 1st, 2011; accepted December 5th, 2011
Keywords: Titanium; Titanium Hydride; Titanium Matrix Composite (TMC); Pressureless Sintering; In Situ; Hardness; Matrix; Composite; Inclusion
ABSTRACT
Fully densified in-situ reinforced (TiB + TiC)-Ti matrix composites have been produced from TiH2-B4C mixtures using pressure less sintering or hot pressing technique. With increasing content of reinforcing components the sintering is retarded. The materials with more than 20 - 30 vol% were only completely densified by hot pressing technique. Hardness values of the Ti matrix composites produced are up to 5 times higher than that of the sintered pure Ti produced from TiH2. This is caused beside the higher hardness of the inclusions also by hardening the matrix due to solubility of B and C in the titanium.
1. Introduction
Titanium matrix composites (TMCs) exhibit several excellent properties such as corrosion resistance, high specific strength, high specific modulus, heat resistance etc. [1]. There are two families of titanium matrix composites, continuously reinforced and discontinuously reinforced TMCs [2]. The early work on titanium matrix composites considered continuous fiber reinforced composites and the industrial use of the composites produced was restricted to highly specialized applications [3]. The reasons for this limited application of these materials were prohibitive cost of continuous fibers, complex fabrication routes, limited formability and highly anisotropic properties of composites produced [3].
Discontinuously reinforced (particulate) TMCs have attracted more attention compared to continuous fiber reinforced composites. This is because of their properties of isotropy nature, easy secondary operation, improved mechanical properties, and significant technical and economic benefits [2]. Typical applications for discontinuous or particulate titanium matrix composites include, 1) Creep resistant engine components; 2) Wear parts such as gears, bearings and shafts; and 3) erosion-corrosion resistant tubing [2]. However, particulate titanium matrix composites (TMCs) also face challenges that include selection of an ideal reinforcement, as well as high performance processing methods [3,4].
For an ideal reinforcing compound, special demands must be met: high hardness, heat resistance and rigidity, thermodynamic stability in the titanium alloys at the sintering temperature, insolubility of the reinforcing phases in the titanium matrix and crystallographically stable matrix/particle boundaries [5]. Titanium monoboride (TiB) has been judged the best compared to the existing reinforcing compounds like TiC, SiC, TiB2, B4C, Al2O3, TiN, and Si3N4 because of its superior thermodynamic properties and outstanding physical and mechanical properties [5].
In situ techniques for producing reinforcements during sintering or casting are considered promising methods [6]. Novel processing techniques based on in situ approaches has been reported to provide new opportunities for the development of titanium matrix composites (TMCs) with small grain size, uniformly distributed reinforcing particles, and improved mechanical properties [6,7]. Almost all reported studies on TMCs used commercial titanium powder as the matrix for their investigations. The commercial titanium powder, apart from being expensive also contains some impurities (e.g. oxygen, manganese, chlorine, nitrogen, iron, silicon, magnesium etc.), thereby raising questions about the total quality (purity) of the final composites.
A possible alternative source for Ti matrix could be titanium hydride (TiH2), which can be prepared at high purity and low grain sizes [5]. B4C powder is also a promising reinforcement ceramic material of Ti alloys for a variety of applications that require elevated hardness, good wear and corrosion resistance [8] and above all, it is a good source for TiB, TiB2 and TiC particles for reinforcing titanium [3,9,10].
Recent investigations have shown that the TiH2 powder has a much higher sinterability in comparison to Ti powder. TiH2 powder compacts could be sintered to full density at 1200˚C whereas Ti powder compacts were only densified to 93% of theoretical density at this temperature [11].
Therefore, this paper is aimed at investigating the feasibility of using titanium hydride powder as the source of titanium matrix in TMC with B4C to generate TiB and TiC reinforced Ti.
TiH2 reacts with B4C according to the reaction:
 (1)
(1)
If TiH2 is in excess, excess Ti will be formed. This indicates that with complete chemical reaction, TiB and TiC would be the final reinforcements in the TMC [7]. Based on this understanding and the feasibility of the thermodynamic stability of the above reaction, in situ TMC with 0 to 80 vol% (TiB + TiC) reinforcements were produced with TiH2-B4C powder mixture as starting materials and their properties were investigated.
2. Experimental
High purity titanium hydride and boron carbide powders obtained from M/S Alfa Aesar (Germany) were used for the present investigation. Detailed chemical analysis of the powders as per the manufacturer’s specification has shown that titanium hydride (99% purity) contains 3.7 wt% hydrogen and maximum 0.3 wt% nitrogen. XRD and EDX measurements carried out on boron carbide (99+ % purity) show that it contains some Si (SiC) as impurity, while similar measurement on titanium hydride show that it contains only single phase TiH2. The average particle size distribution of commercial titanium hydride powder is d10 = 1.9 µm, d50 = 4.5 µm and d90 = 9.4 µm, while that of boron carbide based on manufacturer’s specification is d10 = 1.6 µm, d50 = 4.6 µm and d90 = 9.3 µm.
According to Equation (1) the compositions of the starting mixtures were calculated. Those materials are given in Table 1. The powders were mixed in a planetary ball mill at 200rpm with powder to ball ratio of 1:2 using hexane and alumina balls for 4hours. For comparison a pure Ti material was prepared under the same conditions using TiH2.
The blended powders were subsequently dried in a rotary evaporator and compacted in a uniaxial press using a hardened steel die of diameter of 18 mm. Cylindrical com-
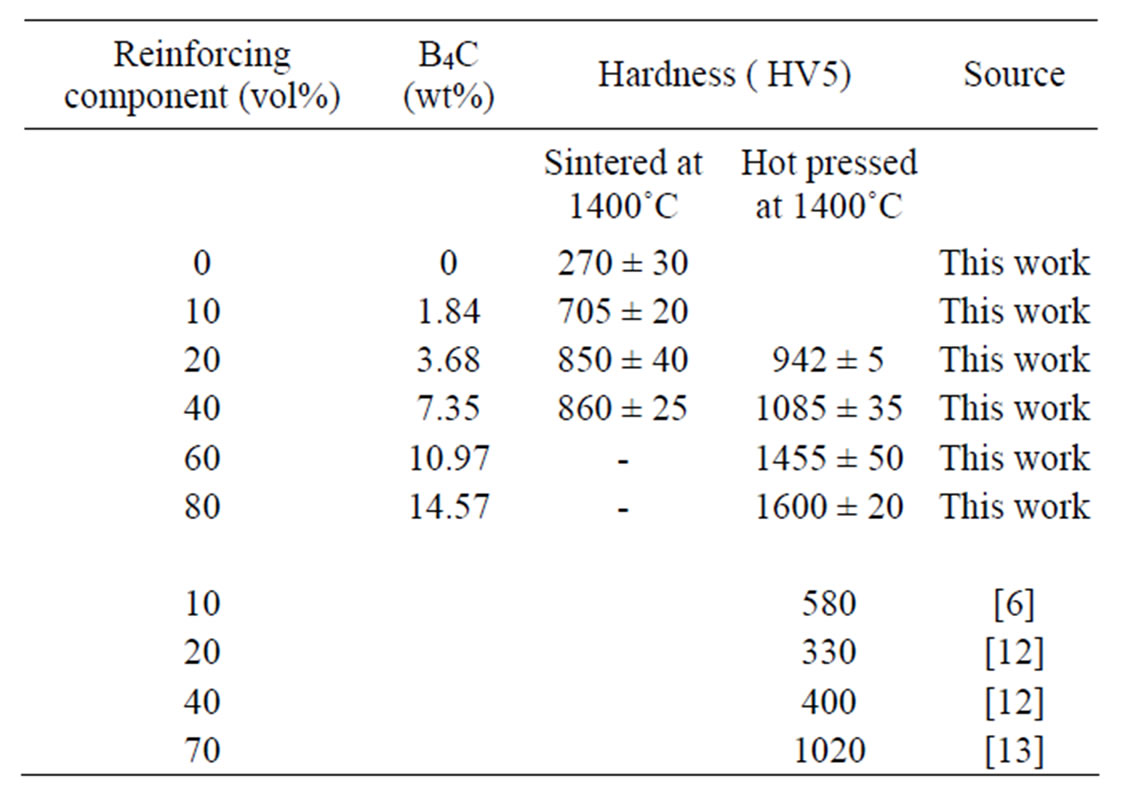
Table 1. Composition and hardness of the composites.
pacts of about 4 - 5 mm height were prepared using a pressure of 393 MPa. The powder compact exhibits green density of about 55% - 57% of theoretical density. SEM analysis of the green compacts showed a homogeneous distribution of B4C.
The compacts were subjected to a two stage heat treatment processes, dehydrogenation and sintering. The dehydrogenation process was carried out under flowing Ar at 715˚C for 2 h (heating rate—2 K/min). After dehydrogenation the samples were directly heated to the sintering temperatures (1100˚C, 1200˚C, 1300˚C, and 1400˚C) at a constant heating rate of 6 K/min. The isothermal sintering time was 90 min. The sintering was carried out in Ar purified by an oxygen getter (copper turnings at 700˚C). Additionally ceramic boats with commercially pure titanium powder were placed on both sides of the compacts in order to any avoid possible oxidation by remaining oxygen.
For comparison some of the samples were hot pressed (HP20-2560-FP20 Thermal Technology) at 30 MPa in 2 mm thick hBN lined graphite tool in Ar. The diameter of the samples was 17 mm.
X-ray diffraction (XRD) analysis of the powders and sintered samples was carried out using a Philips PW1710 diffractometer with monochromatic Cu Kα-radiation at 40 kV and 20 mA. Diffractograms were collected over a range of 2θ between 10˚ - 80˚, with a step size of 0.02˚. The phases were determined using the ICSD database.
SEM/EDS analysis of the powders and sintered samples was carried out using A LEO 1525 FE/SEM Scanning Electron Microscope (SEM) coupled with an Oxford link Pentafet Energy Dispersive X-ray (EDS) spectrometer. Sintered samples for SEM/EDS analysis were prepared using conventional grinding and mechanical polishing techniques. The polished samples were etched in Kroll’s reagent (composition: 1 - 3 ml HF, 2 - 6 ml HNO3, 100 ml water). Density was determined using the Archimedes technique, while the Vickers hardness tests were conducted using a LECO V-100-A2 hardness tester with 5 kg loads for 30 s.
3. Results and Discussion
Figure 1 shows the relative density versus the sintering temperature for the TMC produced.
The densification of the titanium is strongly reduced by the reinforcing components. The pure TiH2 powder could be sintered to dense titanium even at a temperature of 1100˚C, whereas the composite with 40 vol% inclusions even at 1400˚C has still 6.5% porosity. A Similar situation exists for the hot pressed samples (Figure 2). Materials with 80 vol% reinforcing component can be only completely densified at 1400˚C. The reaction between Ti and B4C after dehydrogenation seems to occur below 1100˚C because the SEM/EDX analysis of the sintered at 1100˚C materials showed the complete conversion of the B4C into TiB and TiC. Only trace amounts of the TiB2 were found, but these also do not disappear

Figure 1. Density of the composites sintered at different temperatures and different volume content of the reinforcing components (TiB + TiC).
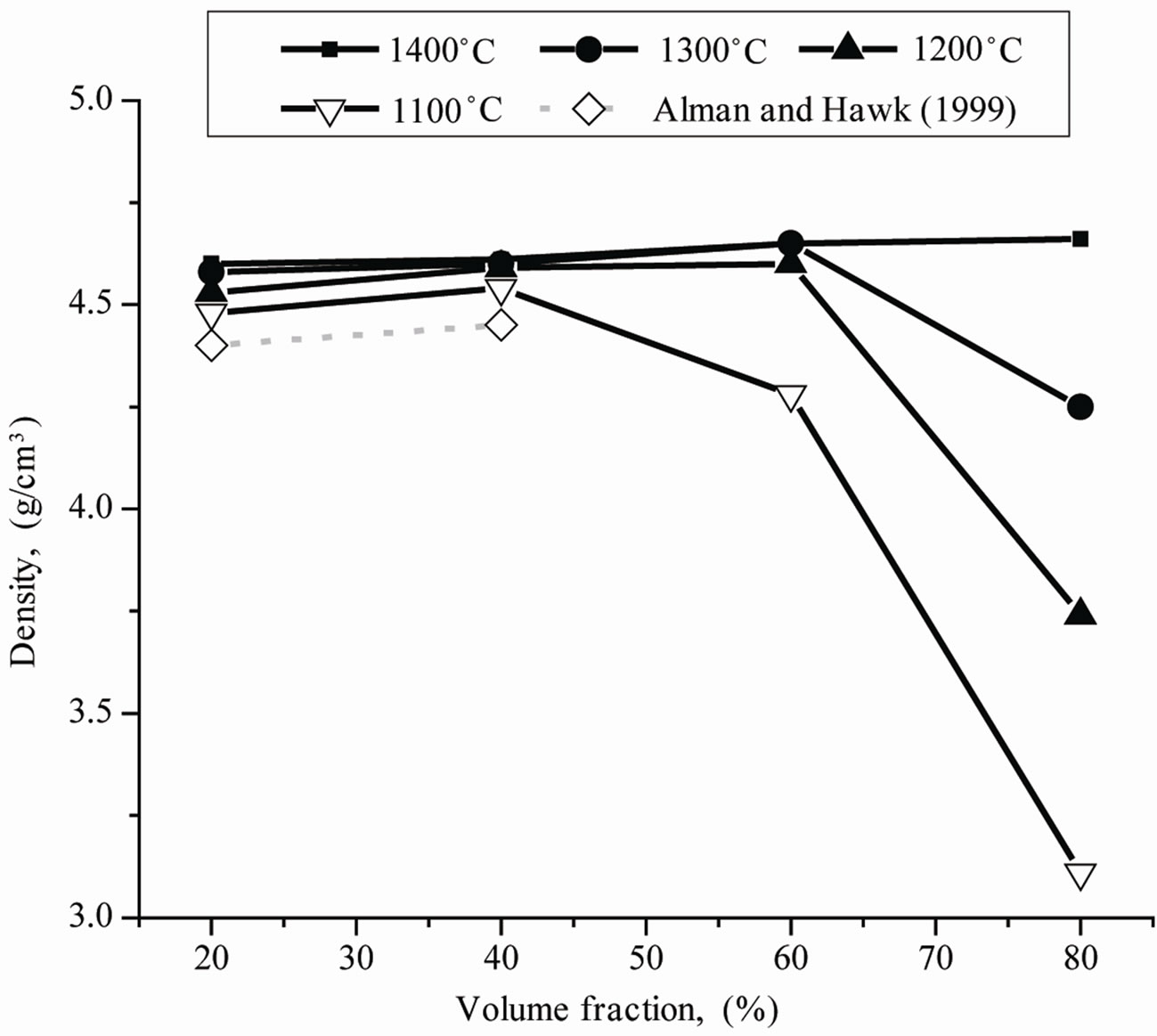
Figure 2. Density of the composites hot pressed at different temperatures and different volume fraction of the reinforcing components (TiB + TiC).
even in the 1400˚C sintered materials. This is most probably connected with kinetic reasons. According to the literature the reaction of Ti with B4C take place above 800˚C [14], 900˚C [15] or occurs only at 1093˚C (DTA analysis) [16]. However, the kinetics can be strongly influenced by the grain size of the components as is normally the case for solid state reactions. For the fine grained materials used here we expect a reaction to occur near the lower boundary of this range.
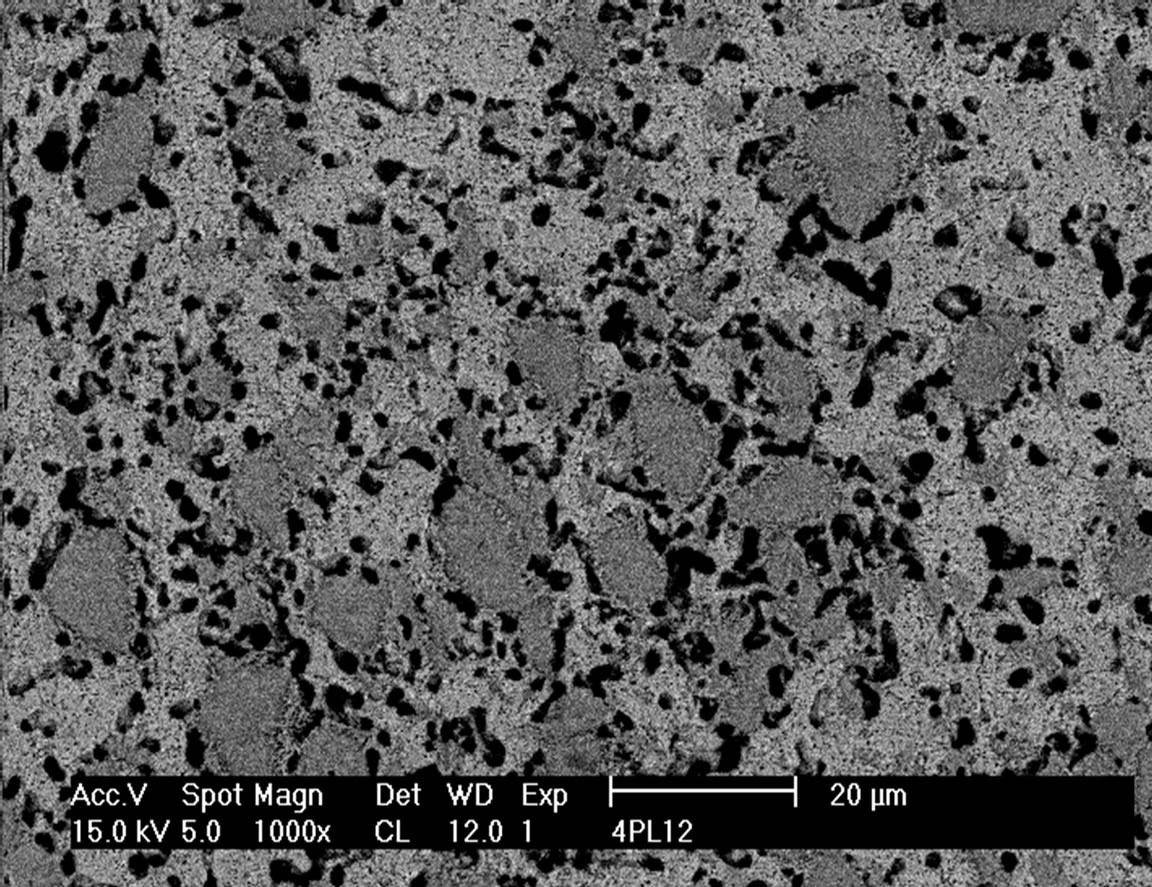
SEM micrographs of the composites sintered at 1200˚C and 1400˚C with 10 and 40 vol% are shown in Figures 3 and 4. It is clearly visible that the material with 10 vol% (TiB + TiC) does not contain residual porosity whereas in the material with 40 vol% reinforcing additives pores are still visible even when sintered at 1400˚C. The microstructure of the composite sintered at 1200˚C showed a rim of pores around the TiB grains. This is
 (a)
(a) (b)
(b)
Figure 3. SEM micrograph of 10 vol% (TiB + TiC) in-situ reinforced Ti matrix composite sintered at 1200˚C (a) and 1400˚C (b).
 (a)
(a)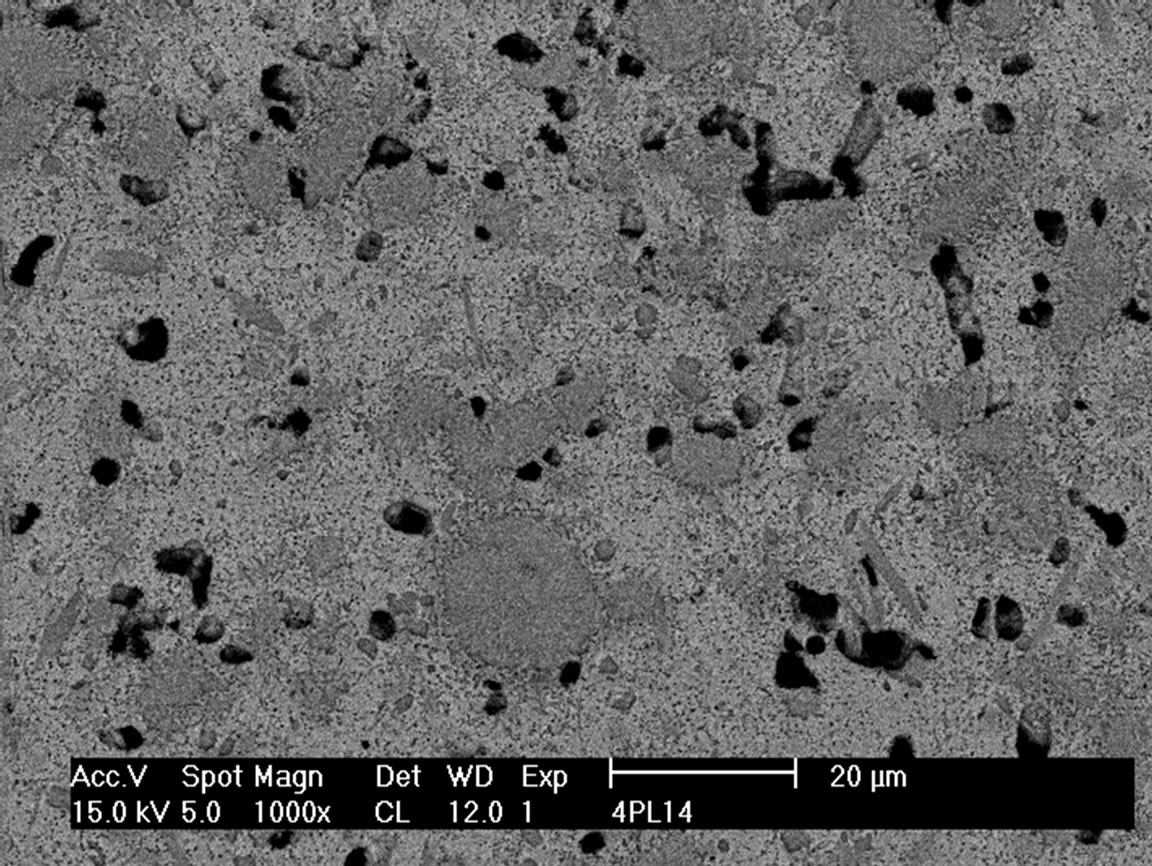 (b)
(b)
Figure 4. SEM micrograph of 40 vol% (TiB + TiC) in-situ reinforced Ti matrix composite sintered at 1200˚C (a) and 1400˚C (b).
probably caused by the volume reduction due to the conversion of B4C into TiB and TiC (app. 15%).
The micrographs of the material with 10 vol% reinforcing components showed two different morphological structures, namely needle-like shape and equiaxed-like shape that are uniformly distributed in titanium matrix. The EDS analysis of the sample shows that the needle-like shape structure corresponds to TiB; the equiaxed structure corresponds to TiC, while the light phase in the micrograph corresponds to the titanium matrix. The size and shape of the TiB grains changes with increasing sintering temperature. The TiB-grains become larger and have a higher aspect ratio (Figures 3(a)-(b)).
The morphological structure of the sample also agreed with the microstructure of materials hot pressed at 1200˚C reported by Ni [6] and Zhang [7].
The morphology strongly changes with increasing amount of reinforcing component. In the material with 40 vol% of (TiB + TiC) the TiB phase is not more needle like, but crystallizes as 5 to 10 µm large mostly equiaxed grains (Figure 4). A similar feature is found for the hot pressed samples. At 40 vol% additives in these materials there still exist few needles, while at higher content of the reinforcing elements no more needle like structures are found.
The Vickers hardness of the composites reported in Table 1 and Figure 5 is up to five times higher than that of the pure Ti. The Vickers hardness of the composites produced with 10 vol% (TiB + TiC) are independent on sintering temperature, whereas composites with higher content of reinforcing components show an increase in hardness with sintering temperature due to the presence of porosity.
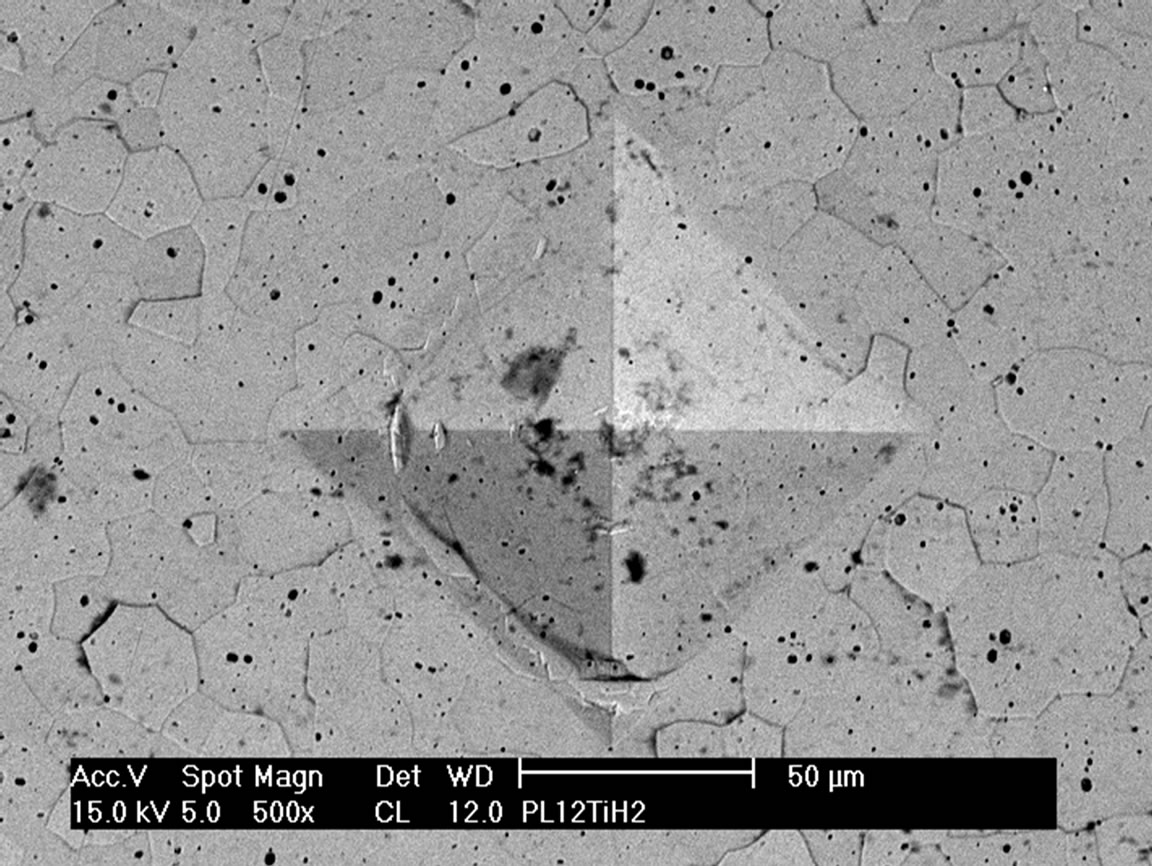
The hardness of metals depends on relative density, interstitial content and grain size [17]. The observed hardness data are higher than the values observed by Ni [6] Alman [12] and Radhakrishna Bhat [13]. The main reason for the higher hardness of the composite materials is not only the higher hardness of the reinforcing component, but the solution of some B and C into the Ti matrix. This incorporation causes in a reduced plasticity of the Ti metal resulting in higher hardness, but also much lower fracture toughness. Evidence of the strongly changed toughness is given in Figure 6. Around the indents of the pure Ti no crack formation is found indicating the plastic behavior of the Ti. This is consistent with fracture toughness values of 35 - 45 MPam1/2 observed for Ti alloys [18]. On the other hand the material with only 20 vol% of reinforcing components showed quite large cracks. Although the application of indentation fracture toughness measurements for a metal matrix composite can be not completely adequate, it gives at least an indication of the fracture toughness. The fracture toughness calculated by this way resulted in a value 4.2 MPam1/2 (10 vol%) - 5.1 MPam1/2 (40 vol%), indicating the strong reduction in fracture toughness in comparison to pure Ti alloys.
4. Conclusions
There is a good improvement in the densification of composites formed. In situ titanium Matrix composite with 10 vol% reinforcement, (TiB + TiC)-Ti, was pressureless sintered and densified fully with no residual porosity at 1200˚C using TiH2-B4C mixtures.
Composite materials with more than 20 - 30 vol% reinforcement were only completely densified by hot pressing techniques.
For Ti matrix composites with less than 20 vol% (TiB + TiC) reinforcing components, TiB grains grow into needle-like shape, while the TiC grains are equiaxed. For Ti matrix composites with more than 20 vol% (TiB + TiC) the TiB forms equiaxed grains.
Hardness values of the Ti matrix composites produced are about 5 times higher than those of the sintered pure Ti produced from TiH2. Increase in hardness was observed for composites with up to 20 vol% (TiB + TiC). Further increase of the (TiB + TiC) content improves the hardness for the completely densified hot pressed samples up to 1600 HV5.
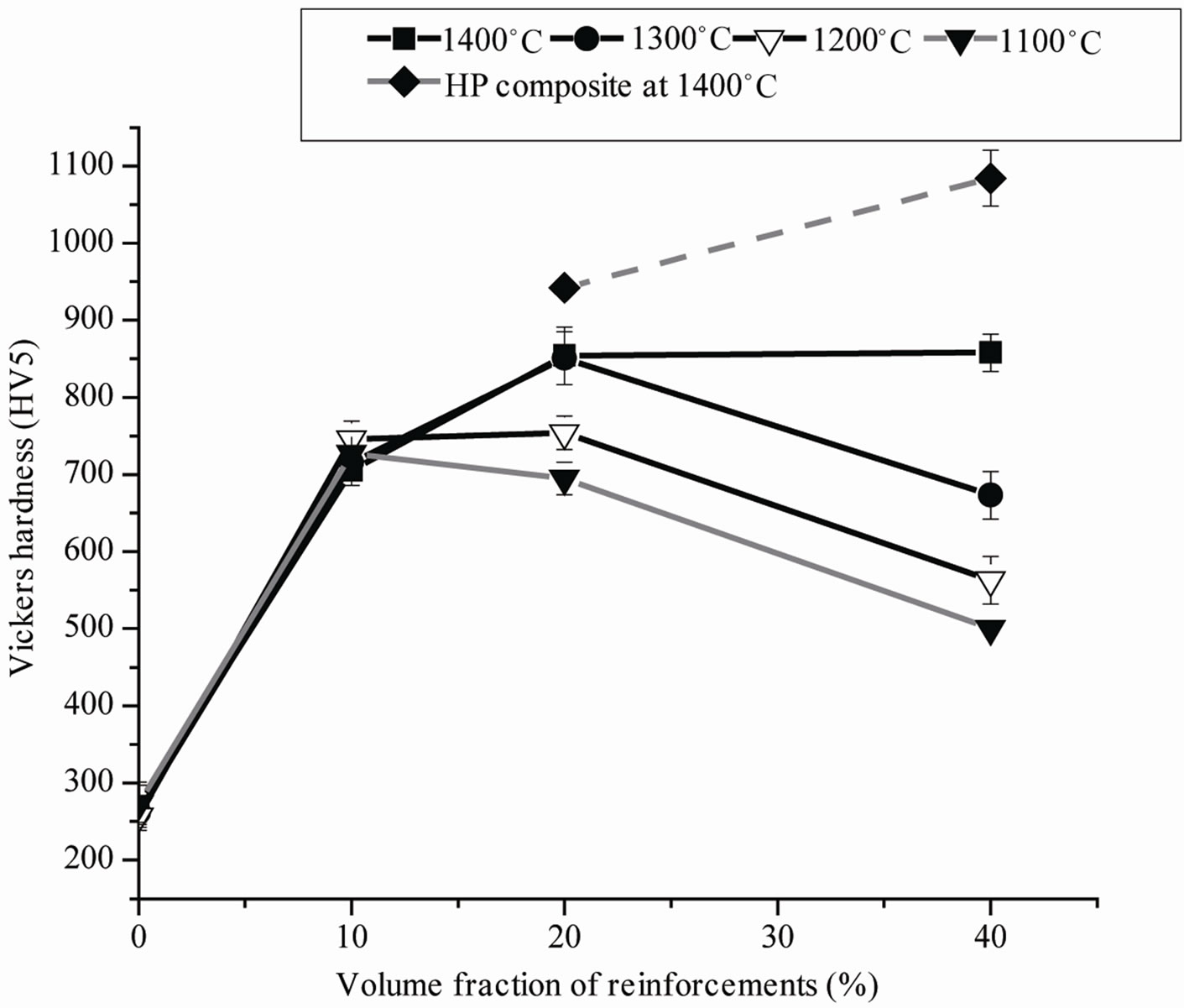
Figure 5. Vickers hardness of the sintered and hot pressed composites as a function of volume content of the reinforcing components and the sintering temperature.
 (a)
(a) (b)
(b)
Figure 6. SEM micrographs of the Vickers Indentations of the pure Ti (a) and of the Ti matrix composite with 20 vol% (TiB + TiC) sintered and 1400˚C.
REFERENCES
- Z. F. Yang, W. J. Lu, D. Xu, J. N. Qin and D. Zhang, “In Situ Synthesis of Hybrid and Multiple-Dimensioned Titanium Matrix Composites,” Journal of Alloys and Compounds, Vol. 419, No. 1-2, 2006, pp. 76-80. doi:10.1016/j.jallcom.2005.09.055
- L. Cai, Y. Zhang, L. Shi, H. Yang and M. Xi, “Research on Development of in Situ Titanium Matrix Composites and in Situ Reaction Thermodynamics of the Reaction System,” Journal of University of Science and Technology Beijing, Vol. 13, No. 6, 2006. p. 551.
- S. Ranganath, “A Review on Particulate-Reinforced Titanium Matrix Composites,” Journal of Materials Science, Vol. 32, No, 1, 1997, pp. 1-16.
- D. Vallauri, I. C. A. Adrián and A. Chrysanthou, “TiCTiB2 Composite: A Review of Phase Relationships, Processing and Properties,” Journal of the European Ceramic Society, Vol. 28, No. 8, 2008, pp. 1697-1713. doi:10.1016/j.jeurceramsoc.2007.11.011
- T. Saito, “The Automotive Application of Discontinuously Reinforced TiB-Ti Composite,” Journal of the Minerals, Metals and Materials Society, Vol. 56. No. 5, 2004, pp. 33-36. doi:10.1007/s11837-004-0125-3
- D. R. Ni, L. Geng, J. Zhang and Z. Z. Zheng, “Effect of B4C on Microstructure of in Situ Titanium Matrix Composites Prepared by Reactive Processing of Ti-B4C System,” Scripta Materialia, Vol. 55, No. 5, 2006, pp. 429- 432. doi:10.1016/j.scriptamat.2006.05.024
- X. N. Zhang, W. J. Lü, D. Zhang, R. Wu, Y. J. Bian and P. W. Fang, “In Situ Technique for Synthesizing (TiB + TiC)/Ti Composites,” Scripta Materialia, Vol. 41, No. 1, 1999, pp. 39-46.
- M. P. Dariel, N. Frage and L. Levin, “A Novel Approach for the Preparation of B4C-Based Cermets,” International Journal of Refractory Metals & Hard Materials, Vol. 18, No. 2-3, 2002, pp. 131-135.
- V. V. B. Prasad, J. Subramanyam and B. V. R. Bhat, “Preparation of Ti-TiB-TiC & Ti-TiB Composites by in-Situ Reaction Hot Pressing,” Journal of Materials Science and Engineering A, Vol. 325, No. 1-2, 2002, pp. 126-130. doi:10.1016/S0921-5093(01)01412-5
- S. Ranganath, M. Vijayakumar and J. Subrahmanyam, “Combustion-Assisted Synthesis of Ti-TiB-TiC Composite via the Casting Route.” Materials Science and Engineering A, Vol. 149, No. 2, 1992, pp. 253-257. doi:10.1016/0921-5093(92)90386-F
- A. Jimoh, “In-Situ Particulate Reinforcement of Titanium Matrix Composites with Borides,” PhD Thesis, University of Witwatersrand, Johannesburg, South Africa, 2010.
- D. E. Alman and J. A. Hawk, “The Abrasive Wear of Sintered Titanium Matrix-Ceramic Particle Reinforced Composites,” Wear, Vol. 225-229, Part 1, 1999, pp. 629-639. doi:10.1016/S0043-1648(99)00065-4
- B. V. R. Bhat, J. Subrahmanyam and V. V. B. Prasad, “Preparation of Ti-TiB-TiC & Ti-TiB Composites by in-Situ Reaction Hot Pressing,” Material Science and Engineering A, Vol. 325, No. 1-2, 2002, pp. 126-130.
- Y.-J. Kim, H. Chung and S. J. L. Kang, “In Situ Formation of Titanium Carbide in Titanium Powder Compacts by Gas-Solid Reaction,” Composites Part A: Applied Science and Manufacturing, Vol. 32, No. 5, 2001, pp. 731- 738.
- C. J. Lu and Z. Q. Li, “Structural Evolution of TiH2-B4C during Ball Milling and Subsequent Heat Treatment,” Journal of Alloys and Compounds, Vol. 448, No. 1-2, 2008, pp. 198-201. doi:10.1016/j.jallcom.2006.10.050
- Y. H. Liang, H. Y. Wang, Y. F. Yang, Y. L. Du and Q. C. Jiang, “Reaction Path of the Synthesis of TiC-TiB2 in Cu-Ti-B4C System,” International Journal of Refractory Metals and Hard Materials, Vol. 26, No. 4, 2008, pp. 383-388.
- M. Zadra, F. Casari, L. Girardini and A. Molinari, “Microstructure and Mechanical Properties of CP-Titanium Produced by Spark Plasma Sintering,” Powder Metallurgy, Vol. 51, No. 1, 2008. pp. 59-65. doi:10.1179/174329008X277000
- S. Dubey and W. O. Soboyejo, “Deformation and Fracture Properties of Damage Tolerant in-Situ Titanium Matrix Composites,” Applied Composite Materials, Vol. 4, No. 5, 1997, pp. 361-374. doi:10.1023/A:1008843504184

