Journal of Modern Physics
Vol.05 No.18(2014), Article ID:52201,7 pages
10.4236/jmp.2014.518198
De Broglie’s Velocity of Transition between Quantum Levels and the Quantum of the Magnetic Spin Moment Obtained from the Uncertainty Principle for Energy and Time
Stanisław Olszewski
Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw, Poland
Email: olsz@ichf.edu.pl
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 September 2014; revised 1 October 2014; accepted 25 October 2014
ABSTRACT
The De Broglie’s approach to the quantum theory, when combined with the conservation rule of momentum, allows one to calculate the velocity of the electron transition from a quantum state n to its neighbouring state as a function of n. The paper shows, for the case of the harmonic oscillator taken as an example, that the De Broglie’s dependence of the transition velocity on n is equal to the n-dependence of that velocity calculated with the aid of the uncertainty principle for the energy and time. In the next step the minimal distance parameter provided by the uncertainty principle is applied in calculating the magnetic moment of the electron which effectuates its orbital motion in the magnetic field. This application gives readily the electron spin magnetic moment as well as the quantum of the magnetic flux known in superconductors as its result.
Keywords:
Velocity of the Electron Transitions between Quantum Levels, De Broglie Wave Packets, Magnetic Moment of the Electron Spin, Quantum of the Magnetic Flux, The Uncertainty Principle for Energy and Time

1. Introduction
The paper has as its aim to approach two rather different problems, both on the basis of the uncertainty principle for energy and time. The first is connected with the speed of transition between two different (neighbouring) quantum levels of the harmonic oscillator. The approach is based on the fact that in the oscillator case a space difference characteristic for two quantum levels can be easily calculated on a semiclassical footing, so in order to obtain the speed of transition only the corresponding time interval  is required. This interval is provided by the uncertainty principle which couples
is required. This interval is provided by the uncertainty principle which couples 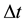 with the corresponding energy change
with the corresponding energy change . The result obtained for the seeked velocity of transition is in a satisfactory agreement with a corresponding difference of the De Broglie velocities connected with the same transition.
. The result obtained for the seeked velocity of transition is in a satisfactory agreement with a corresponding difference of the De Broglie velocities connected with the same transition.
The second problem is directed to the calculation of the electron magnetic moment. Classically this moment is an effect of the angular momentum of the electron particle moving along the orbit induced by the magnetic field. In fact the magnetic moment differs from the angular momentum only by a constant multiplier. But the uncertainty principle provides us with the result that not every size of parameters describing the classical particle motion in the magnetic field is acceptable. First the minimal intervals in space and time which should not be violated in course of the motion are presented. An application of one of these intervals, namely the spatial one, to calculation of the magnetic moment of the orbiting electron gives readily the spin magnetic moment of that electron.
2. De Broglie’s Transition Velocity between the Quantum Levels and Its Alternative Calculation
Before the outspring of the wave mechanics the De Broglie’s velocities of the wave packets were applied in calculating the action function entering the Sommerfeld rule defining the quantum levels of the old quantum theory [1] -[5] . This calculation was represented by the formula:
 (1)
(1)
Here  is the rest mass of the electron,
is the rest mass of the electron, 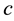 is the speed of light,
is the speed of light, 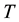 is the time period of the moving body―say an oscillator―along a closed orbit,
is the time period of the moving body―say an oscillator―along a closed orbit, 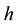 is the Planck constant,
is the Planck constant,  is the velocity of the wave packet,
is the velocity of the wave packet, 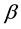 is the ratio
is the ratio
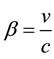 (2)
(2)
and 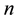 is the index of a quantum state.
is the index of a quantum state.
By neglecting the radical expression in the denominator of (1), which for small 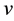 is close to unity, we obtain in (1) the formula
is close to unity, we obtain in (1) the formula
 (1a)
(1a)
or
 (3)
(3)
on condition we put
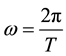 (4)
(4)
for the expression of the circular frequency, and

Formula (3) is an expression for the double kinetic energy of the harmonic oscillator calculated at position 



therefore a situation of the vanishing potential energy in (6) is attained at

An advantage of the De Broglie’s treatment is that it applies the notion of the wave packet velocity which can be next identified with the speed of a particle, for example an electron particle; see e.g. [6] .
In general the velocity observable in quantum systems is seldom discussed, though it can be of use when the electron transition between two quantum states takes place. A difficulty in calculating the transition speed between two quantum states is due to the fact that in principle we have no suitable formalism to that purpose. For example, in the framework of the old quantum theory, we can rather easily define the position change, say

of some special point of a system having the space coordinate

associated with the interval (8). For an oscillator an easily accessible change of 

due to the change of the quantum state n of the oscillator. The amplitude (10) is readily obtained from (6) because at the turning points of the oscillator we have

so

Here 


This formula is simplified to that applied in the old quantum theory, so the quantum-mechanical correction 1/2 to 
The first aim of the present paper is to calculate 


which―in calculating

The accuracy of (13a) increases as much as the speed of the examined transition approaches the velocity
3. Speed of Transitions between Quantum States Calculated on the Basis of (1a) and (13a)
For an individual quantum state 

so for 

The central oscillator point (7) in which only the kinetic energy does not vanish repeats for all quantum states, but the oscillator velocities will differ at that point according to (14) [we neglect here the result that in fact (3) gives a double kinetic energy at point (7)]. Since the motion across (7) is performed along the same direction for all






The approximation entering the last steps of (15) holds for large
The formalism of calculating the transition speed on the basis of (13a) requires some more manipulations. The aim is to obtain the transition velocity in terms of the formula

where from (12) and (6a)

On the other hand, the interval 

because

In effect of (17) and (18), the transition velocity (16) between 


The both formulae, (15) and (20), become identical on condition the constant 

The problem of the difference of transition speeds considered separately for the case of emission or absorption of energy is here neglected: in fact we can substitute only positive, or absolute, 

4. Discussion on the Speed of the Transition Wave
It can be noted that the square value of the speed entering (1a) is modified in (1) by the factor

Therefore in order to obtain with the aid of (21) roughly a full agreeement between the De Broglie velocity (15) and that of (20) we should solve the equation

Equations (2) and (22) give

which is equivalent to the bi-quadratic equation

This gives

or

from which and from (14a) we obtain the following requirement for the frequency

5. Magnetic Moment of an Electron Moving on an Orbit in a Constant Magnetic Field and Its Value Attained at the Extremal (Minimal) Radius Size Supplied by the Principle of Uncertainty
In examining the uncertainty principle for energy and time [see (13a)] two parameters concerning respectively a minimal space distance between two particles

and a minimal time interval between two events

could be derived [10] . Similar parameters characteristic for the distance in space and time, equal respectively to

and

have been proposed at a time of the outspring of quantum mechanics [5] [12] - [14] . The Formula (26a) is better known as the Compton wave length of the electron (equal to 

The task undertaken in the present paper is to apply a minimal distance (26) to calculate the magnetic moment 



Upon the action of a constant magnetic field having the induction 

where 

is the circular frequency of the motion. In the first step our task is to calculate a critical (maximal) value of 


and the smallest orbital radius 


A solution of (32) yields

The next step is to calculate the electron angular momentum with the use of (26) and (33). For a circular orbit 


A final step is to substitute the result of (34) into the expression for the electron magnetic moment. We obtain

The result in (35) is equal precisely to the magnetic moment of the electron considered as a spin magnetic moment; see [6] and [16] . A characteristic point is that no quantization process has been used in calculations: the result is based solely on the uncertainty principle (13a) and classical electrodynamics.
Let us note that 


6. Remarks on the Spin Magnetic Moment
A rather natural step can be an application of the uncertainty principle to other kinds of matter than electrons. Here one has to be cautious because 

For electrons, however, an application of the critical interval (26) can be done also in case of the electrostatics. This leads to a critical (maximal) strength 

For electrons the force 

Both forces 



independent of the particle mass
An interesting situation gives a coupling of the radius 


and 

This is a quantum of the magnetic flux which is well-known in the theory of superconductors, see e.g. [19] .
7. Summary
In the paper two rather different effects have been examined in reference to the uncertainty principle for energy and time.
The first one concerns the change of speed of the De Broglie wave in case of transition from a quantum state 

Another calculation considers the spin magnetic moment of the electron which in quantum mechanics is conventionally obtained in course of solving the Dirac’s equation; see e.g. [6] . We show that the same result for the spin magnetic moment can be attained by combining the uncertainty principle with the classical electromag- netic theory. Here a critical (minimal) length of the orbit radius 


A by-product of this calculation is a quantum of the magnetic flux well-known in the theory of super- conductors; see (40). It is obtained by multiplication of the induction 


from (33), as well as 

The ratio of 

The semi-classical model of a spinning electron developed in the present paper is as follows. The electron is gyrating with a speed close to 



which is shorter by many times than the first radius of the Bohr orbit in the hydrogen atom equal to 0.53 × 10−8 cm, but larger―also by many times―than the classical radius of the electron particle equal to [15] :

The circulation of the spinning electron is corresponding to the presence of the magnetic field 

The magnetic moment obtained due to the circulation is equal to the Bohr magneton [see (35)]. The lowering of the magnetic interaction energy being an effect of creation of a spinning electron system is

References
- De Broglie, L. (1923) Comptes Rendus, 177, 507-510.
- De Broglie, L. (1923) Comptes Rendus, 177, 548-550.
- De Broglie, L. (1923) Comptes Rendus, 177, 630-632.
- De Broglie, L. (1924) Philosophical Magazine, 47, 446-458. http://dx.doi.org/10.1080/14786442408634378
- Jammer, M. (1966) The Conceptual Development of Quantum Mechanics. McGraw-Hill, New York.
- Schiff, L.I. (1968) Quantum Mechanics. 3rd Edition, McGraw-Hill, New York.
- Olszewski, S. (2011) Journal of Modern Physics, 2, 1305-1309. http://dx.doi.org/10.4236/jmp.2011.211161
- Olszewski, S. (2012) Journal of Modern Physics, 3, 217-220. http://dx.doi.org/10.4236/jmp.2012.33030
- Olszewski, S. (2012) Quantum Matter, 1, 127-133. http://dx.doi.org/10.1166/qm.2012.1010
- Olszewski, S. (2014) Journal of Modern Physics, 5, 1264-1271. http://dx.doi.org/10.4236/jmp.2014.514127
- Olszewski, S. (2015) Quantum Matter, in Press.
- Ruark, A.E. (1928) Proceedings of the National Academy of Sciences of the United States of America, 14, 322-328. http://dx.doi.org/10.1073/pnas.14.4.322
- Flint, H.E. (1928) Proceedings of the Royal Society A, London, 117, 630-637. http://dx.doi.org/10.1098/rspa.1928.0025
- Flint, H.E. and Richardson, O.W. (1928) Proceedings of the Royal Society A, London, 117, 637-649. http://dx.doi.org/10.1098/rspa.1928.0026
- Landau, L.D. and Lifshitz, E.M. (1969) Mechanics, Electrodynamics. Izd. Nauka, Moscow.
- Landau, L.D. and Lifshitz, E.M. (1972) Quantum Mechanics. Izd. Nauka, Moscow.
- Bethe, H.A. (1947) Elementary Nuclear Theory. Wiley, New York.
- Sommerfeld, A. (1931) Atombau und Spektrallinien. Vol. 1, 5th Edition, Vieweg, Braunschweig.
- Kittel, C. (1987) Quantum Theory of Solids. 2nd Edition, Wiley, New York.

