Advances in Bioscience and Biotechnology
Vol.4 No.8(2013), Article ID:35422,7 pages DOI:10.4236/abb.2013.48109
Protecting effect of He-Ne laser on winter wheat from UV-B radiation damage by analyzing proteomic changes in leaves*
![]()
College of Life Science, Shanxi Normal University, Linfen, China
Email: #duanjiangyan123@163.com
Copyright © 2013 Zhenhu Jia, Jiangyan Duan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 7 June 2013; revised 8 July 2013; accepted 20 July 2013
Keywords: Winter Wheat; Protein; Ultraviolet-B Radiation; He-Ne Laser
ABSTRACT
The ultraviolet-B (UV-B) radiation can affect gene expression of plant in growth and development. Winter wheat (Triticum aestivum), as a kind of economic crop cultured in the Northern China, is also damaged by present-day ultraviolet-B (UV-B) radiation. It was reported that He-Ne lasers could alleviate cell damage caused by UV-B radiation in plants. To get the proteomic changes of wheat alleviated by He-Ne lasers, we studied protein expression profiles of winter wheat (Jin Mai NO.79) leaves by running 2-DE (two-dimensional gel electrophoresis), which allowed the identification of some significantly different gel spots. The spots were further verified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry, in which they were confirmed to be winter wheat proteins. The results showed: 1) the proteomic changes between the ultraviolet-B radiation group and normal light group were significantly different on sixth day; 2) The expression of some proteins such as catalase, DNA polymerase and reduced glutathione declines at the ultraviolet-B radiation group and increases at the UV-B + He-Ne laser group. This indicates UV-B could regulate genes encoding proteins in Winter wheat leaves and affect the growth of the Winter wheat. He-Ne lasers treatment could significantly alleviate UV-B-induced damage in wheat.
1. INTRODUCTION
With reduction of stratospheric ozone increasing ultraviolet-B (UV-B) radiation at the earth’s surface, UV-B radiation has been shown to be harmful to plants. An examination of more than 200 plant species reveals that roughly 20% are sensitive, 50% are mildly sensitive or tolerant and 30% are completely insensitive to UV-B radiation [1]. Winter wheat (Triticum aestivum), as a kind of economic crop cultured in the Northern China, is also affected by present-day ultraviolet-B (UV-B) radiation [2]. UV-B (280 - 320 nm) radiation is known to reduce the photosynthetic pigment and protein content of green leaves and inactivate molecular defense mechanisms. The UV-B radiation also affects gene expression for growth and development [3]. It was reported that He-Ne lasers could alleviate cell damage caused by UV-B radiation in plants. Studies have showed that He-Ne lasers play a positive role in accelerating plant growth and metabolism, and the suitable doses of He-Ne laser irradiation improved germination capacity of plant seeds, enzymatic activities and the concentration of chlorophyll. Previous studies have illustrated that a He-Ne laser pretreatment could protect plant cells from enhanced UV-B radiation [4]. Although abundant data concerning the influence of He-Ne lasers on plant have been obtained, to date, the proteomic change of He-Ne laser irradiation on wheat is not clear.
So, the aim of the present study is to investigate the proteomic change of wheat leaf alleviated by He-Ne lasers. For this purpose, we employed an instrument to emit He-Ne lasers (650 nm) that was transformed from the semiconductor laser to test the optical effect of laser protection on wheat damaged by UV-B irradiation.
2. MATERIALS AND METHODS
2.1. Plant Materials and Growth Conditions
Winter wheat (Triticum aestivum, cv JinMai NO.79) seeds were obtained from Shanxi Wheat Research Institute of Agricultural Sciences. Seeds were selected for uniform size and sterilized for 10 min with 0.1% HgCl2 and were washed for 50 min by flowing water. The seeds were grown in Petri plates (diameter 18 cm) on cotton soaked with distilled water under continuous white fluorescent light at a fluence rate of 200 µmol/m2 at 25˚C without any external nutrient. Three replications of 30 pure seeds were used for each of the different treatment. The treatment groups were divided into normal light group (CK group), UV-B Radiation group (UV-B group) and He-Ne Radiation repairing group (UV-B + He-Ne laser group).
2.2. Establishment of Different Treatment Groups
When the seedlings began germination, Supplemental UV-B radiation was provided by filtered Tai brand (Shanxi, China) 30 W sunlamps. Lamps were suspended above. A semiconductor laser (wavelength 650 nm, power density 3.97 mW∙mm−2) was directly irradiated the wheat seeds for 2 min every day [5] (Table 1).
2.3. Preparation of Protein Extract [6]
At 12 day after treatment, the wheat leaf was collected. A portion (200 mg) of leaf was homogenized in 1 mL of lysis buffer containing 8 M urea, 2% NP-40, 0.8% ampholine (pH 3.5 to 10), 5% 2-mercapthoethanol and 5% Polyvinyl pyrrolidne-40, using a glass mortar and pestle on ice. The homogenates were centrifuged in a RA-50JS rotor (Kubota, Tokyo, Japan) for 5 min. The supernatant was centrifuged at 20,000 g for 5 min and subjected to electrophoresis.
2.4. 2-DE [7]
Prepared samples were separated by 2-DE in the first dimension by IEF (Isoelectro focusing) tube gels (Daiichi pure Chemicals, Tokyo, Japan) and in the second dimension by SDS-PAGE. An IEF tube gel of 11 cm length and 3 mm diameter was prepared. IEF gel solution consisted of 8 M urea, 3.5% acrylamide, 2% NP-40, 2% carrier ampholines (pH 3.5 - 10.0), 10% ammonium persulfate and TEMED. Electrophoresis was carried out at 200 V for 30 min, followed by 400 V for 16 h and 600 V for 1 h. After IEF, SDS-PAGE in the second dimension was performed using 15% polyacrylamide gels with 5% stacking gels. The gels were stained with CBB (Coomassie brilliant blue), and image analysis was performed. 2-DE images were synthesized and the position of individual proteins on gels was evaluated automatically with Image Master 2D Elite software (Amersham Biosciences, Uppsala, Sweden). The pI and Mr (molecular weight) of each protein was determined using 2-DE markers (Bio-Rad, Richmond, CA, USA).
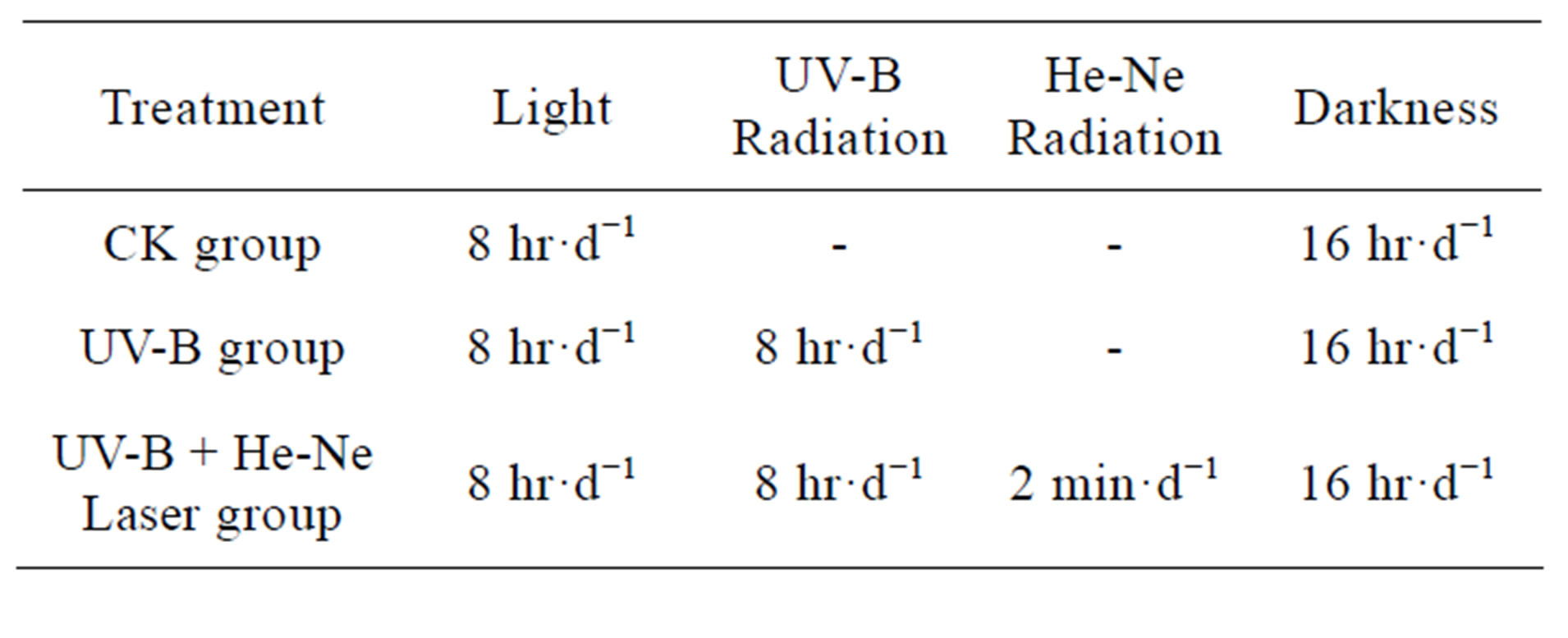
Table 1. The establishment and procedure of different treatment groups.
2.5. Data Analysis
Different 2-DE gel spots were further verified by matrixassisted laser desorption ionization-time of flight mass spectrometry. The amino acid sequences obtained were compared with those of known proteins in the Swiss-Prot, PIR, GenPept and PDB databases with the Web-accessible search program FastA (http://www.dna.affre.go.jpl).
2.6. Identification of Rice Leaf Proteins by MALDI-TOF MS
The digestions were desalted by Poros R2 and eluted with 0.6 mL matrix solution consisted of CHCA (12 mg/mL) in 70% ACN with 0.1% TFA. The eluted solution was applied onto the target well, dried at room temperature and introduced into a Bruker AutoFlex MALDITOF MS. The mass spectrometer was operated under 19 kV accelerating voltage in the reflectron mode and a m/z range of 600 - 4000. The monoisotopic peptide masses obtained from MALDI-TOF MS were analyzed by m/z software, and interpreted with MASCOT (Matrix Science) against the NCBInr database and the nonredundant databases of the rice genome generated by the Beijing Genomics Institute. Some digestive products from 2-DE spots were carried out by LC-MS/MS using a LCQ Deca IT mass spectrometer (Thermo Finnigan, Ringoes, NJ, USA) for further confirmation of amino acid sequences. After capillary reverse phase HPLC, the separated peptides were subjected into IT MS with 3.2 kV spray voltage and 150˚C at the heated desolvation capillary. The m/z range from 400 - 2000 was scanned in 1.2 s, and the ions were detected with a high energy Conversion Dynode detector. The LC-MS/MS data were converted into DTA-format files which were further searched for proteins with MASCOT.
3. RESULTS
3.1. Protein Content of Wheat Leaf on Different Treatment Days
Figure 1 show levels of proteins with molecular weight of 100, 60, 40, 25 and 20 KDa polypeptides of wheat leaves after the UV-B on different treatment days. These alterations ranged in molecular weight from as low as 20 KDa to as high as 100 KDa. From the general picture of leaves proteins emerging from this work, one point is noteworthy, more protein alterations were scored in 66 KDa after enhanced UV-B radiation. The synthesis of 66 KDa polypeptides was decreased on the 6 and 9 treatment days. The synthesis of 21 - 31 KDa polypeptides was increased on the 6 and 9 treatment days. The total protein of content control group (CK) and experimental group(E) were significantly different on sixth day after enhanced UV-B radiation (Figure 2).
3.2. The Proteomic Profiles of Wheat Leaves Analyzed by 2-DE
Figures 3-5 show representative images of 2-DE gels of wheat leaves proteins on the six day. To test whether the proteins of wheat leaves could be effectively separated by 2-DE, the protein extracts were first examined in a pH range of 3 - 10 followed by a separation on a gradient polyacryamide gel (8% - 20%). Most leaf proteins were located within a narrow pH range of 5 - 7 and only a few proteins existed at the extreme pH regions of 3 - 4 or 9 - 10.
Figures 6-8 show representative images analysis of 2-DE gels by software calculations. On the basis of these
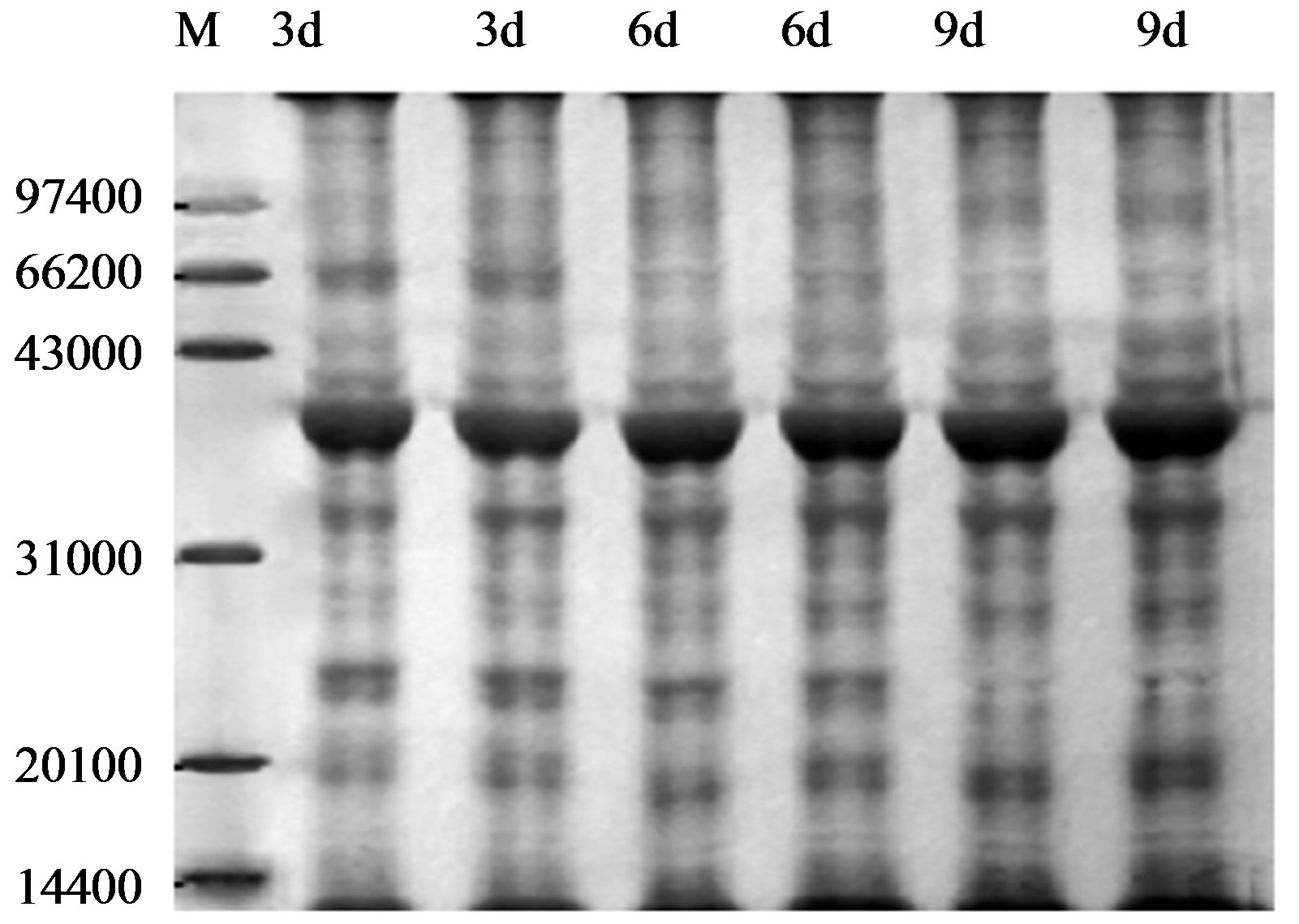
Figure 1. Protein content of SDS-PAGE in wheat leaves after UV-B radiation. M: Marker; 3d, 6d, 9d refer to 3d, 6d and 9d after UV-B radiation, respectively.
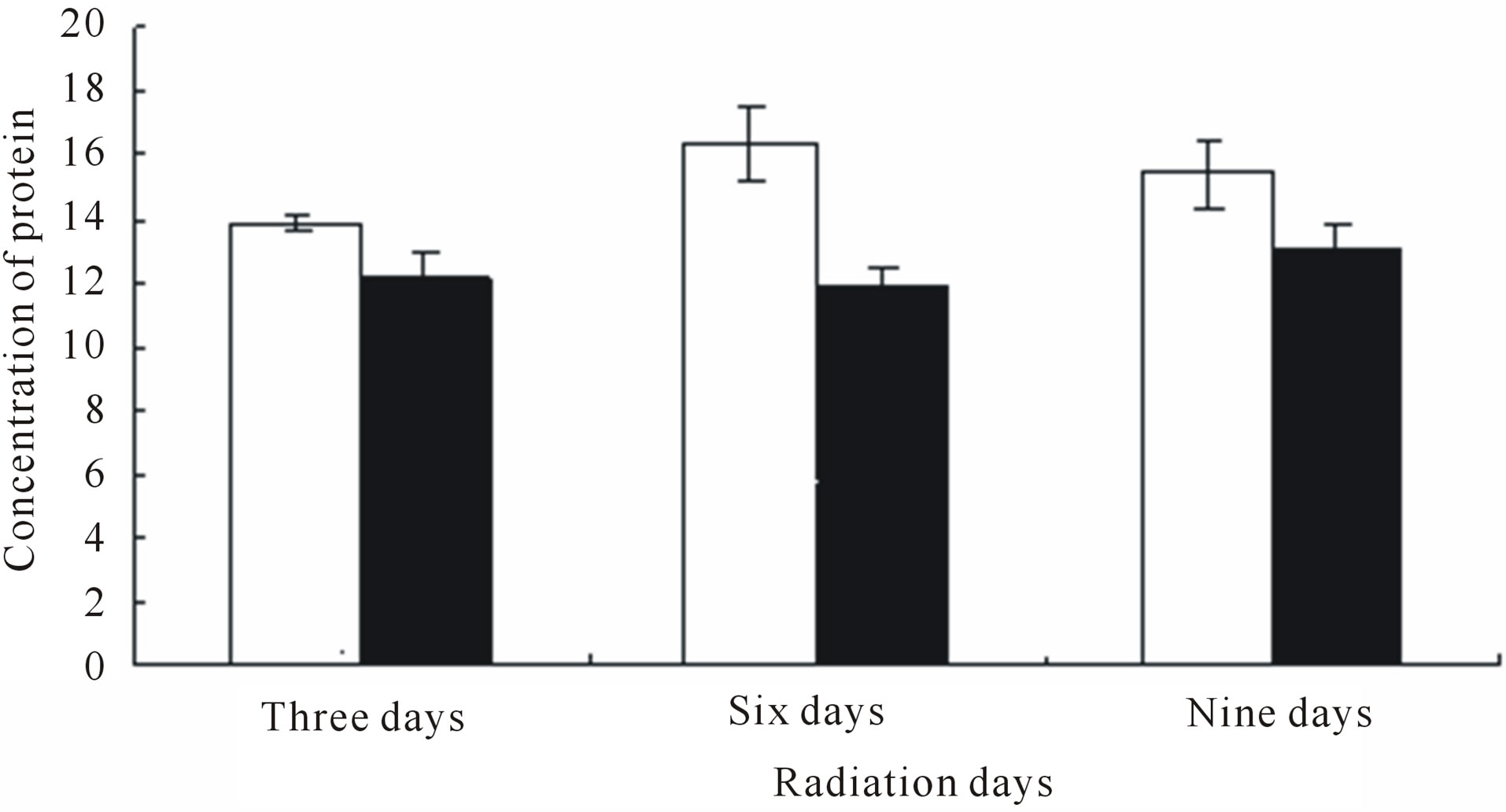
Figure 2. Changes in protein content exposed to different treatment days.
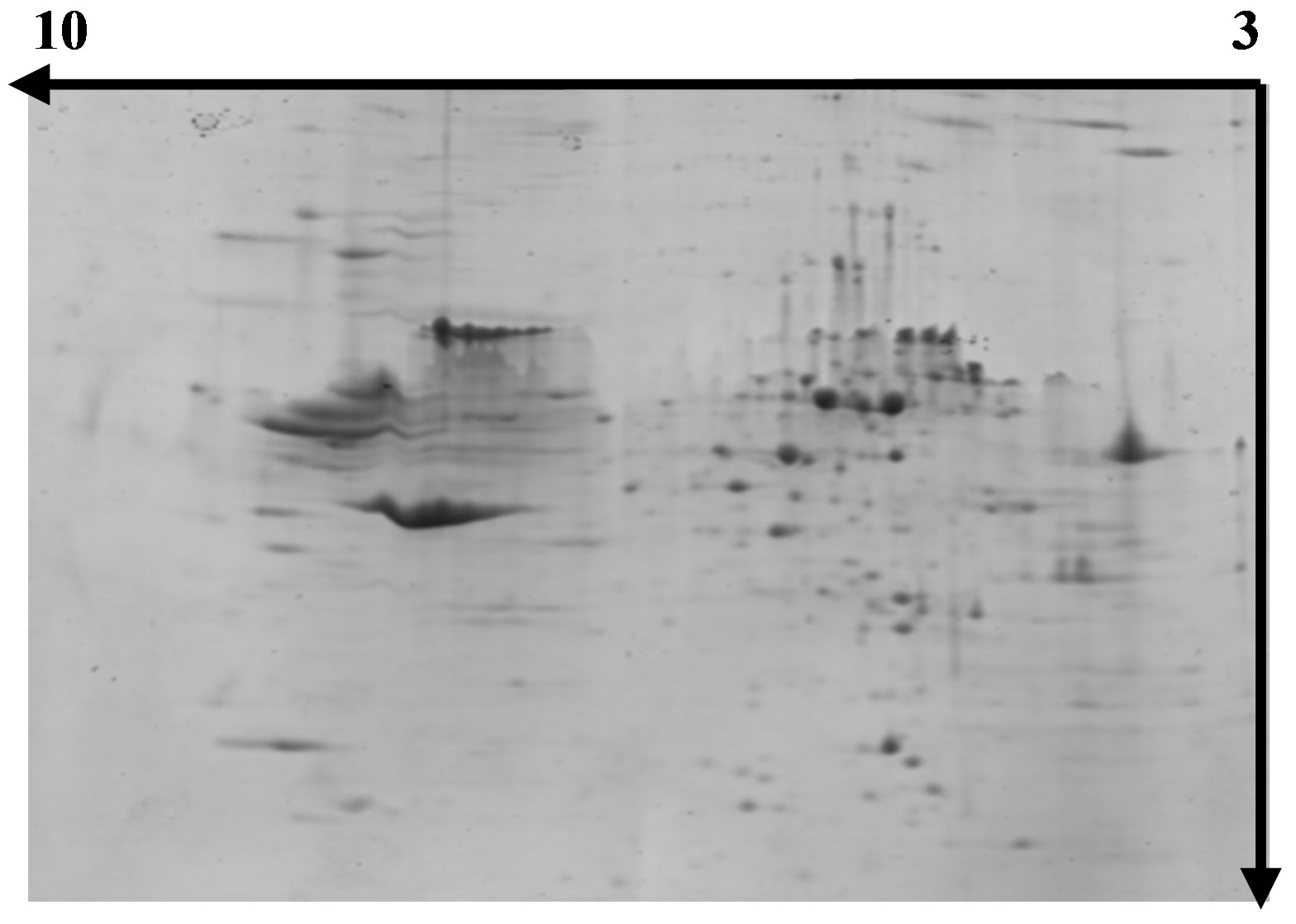
Figure 3. Separation of wheat leaf proteins by 2- DE under normal light.
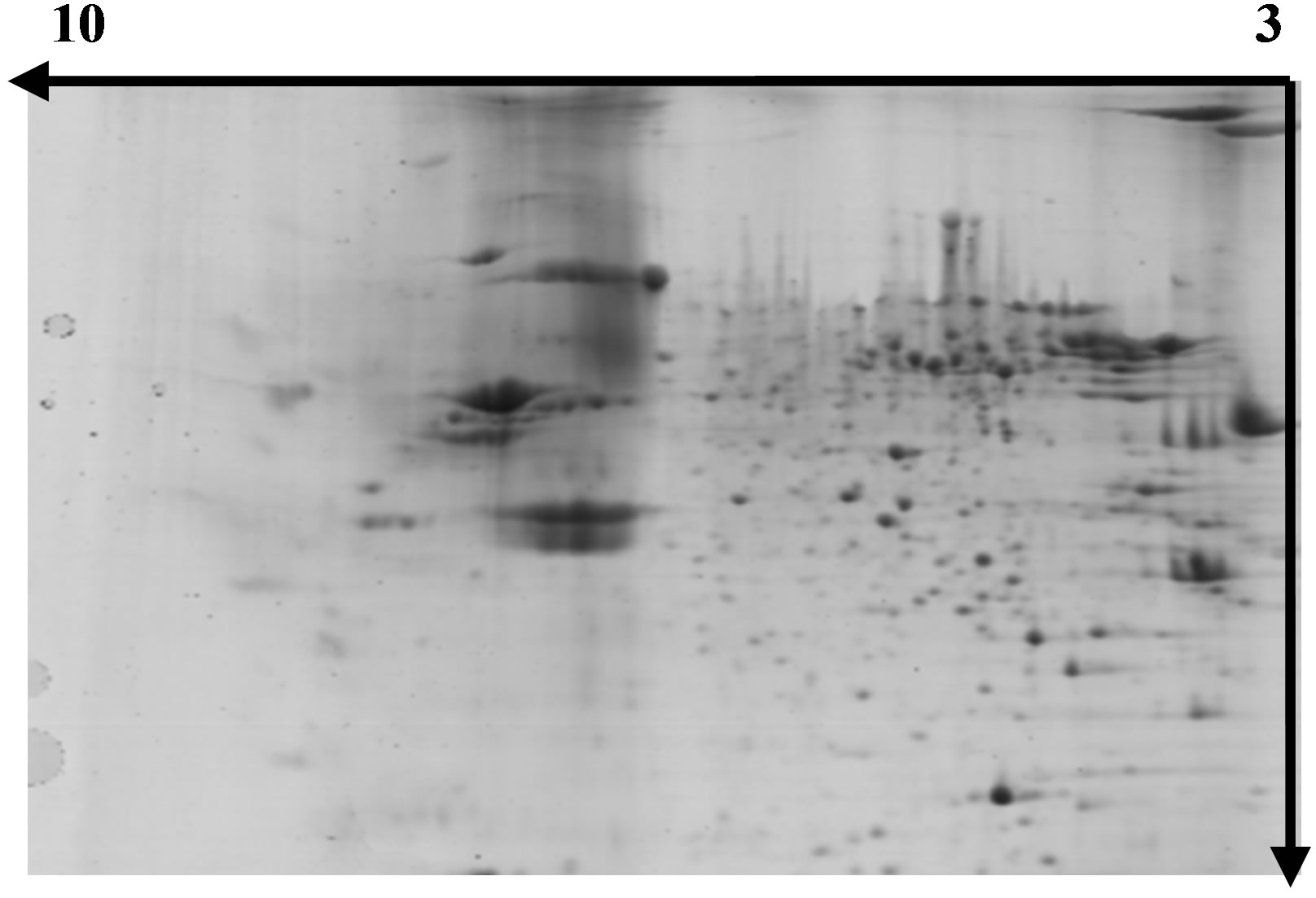
Figure 4. Separation of wheat leaf proteins by 2-DE under UV-B radiation.
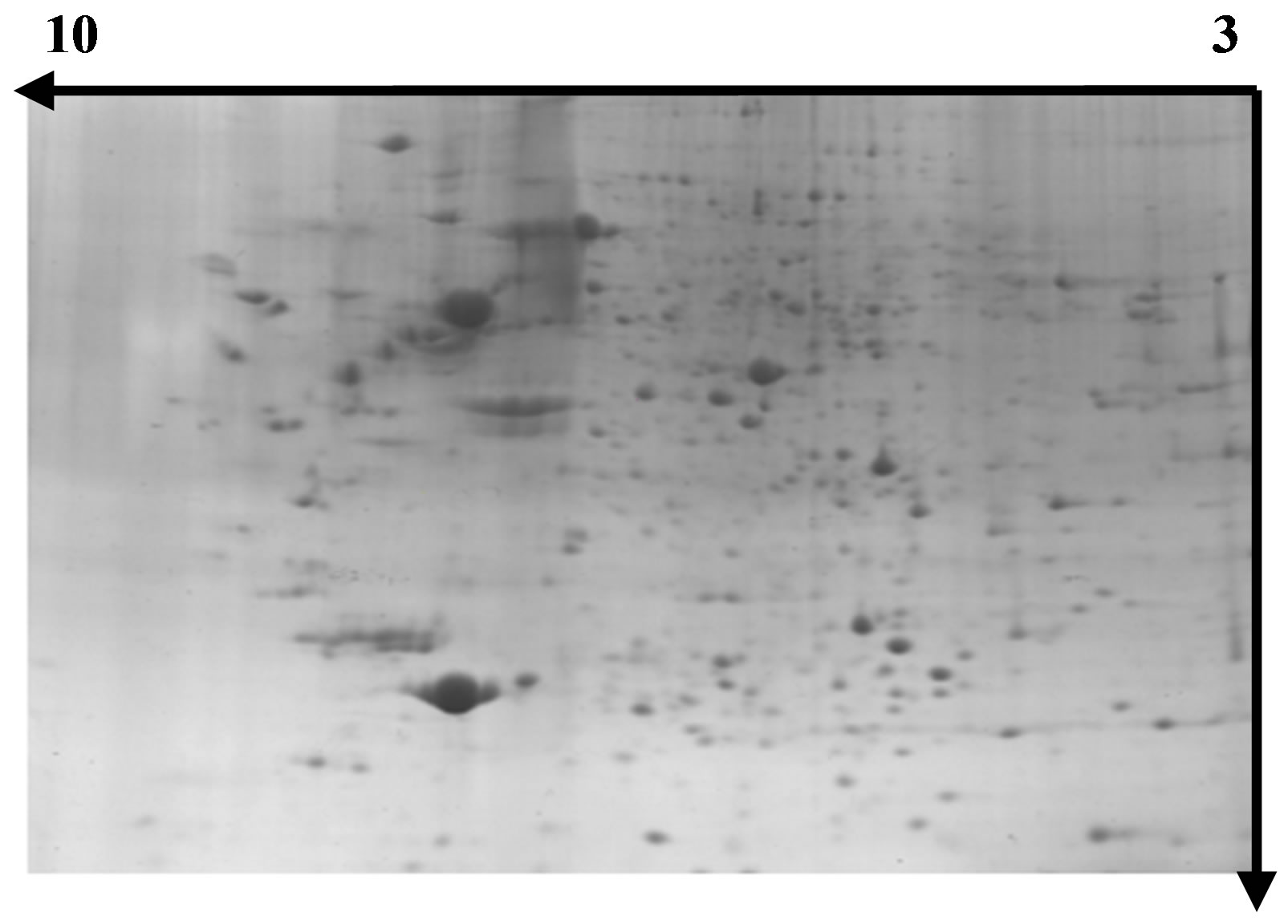
Figure 5. Separation of wheat leaf proteins by 2- DE under UV-B + He-Ne laser.
images, we have an outline of the expression profile for the relatively abundant and soluble proteins in wheat leaves. The distribution patterns of most gel spots are similar on all groups of the 2-DE images indicating that the protein components in the wheat leaves are similar from the CK group and UV-B group through UV-B + He-Ne Laster group, at least for proteins with middle and high abundance. Figure 9" target="_self"> Figure 9 show the images analysis of 2-DE gels of three groups by software calculations. The UV-B group had the lowest number of leaf protein spots. Since the leaves at UV-B group are believed to be at the
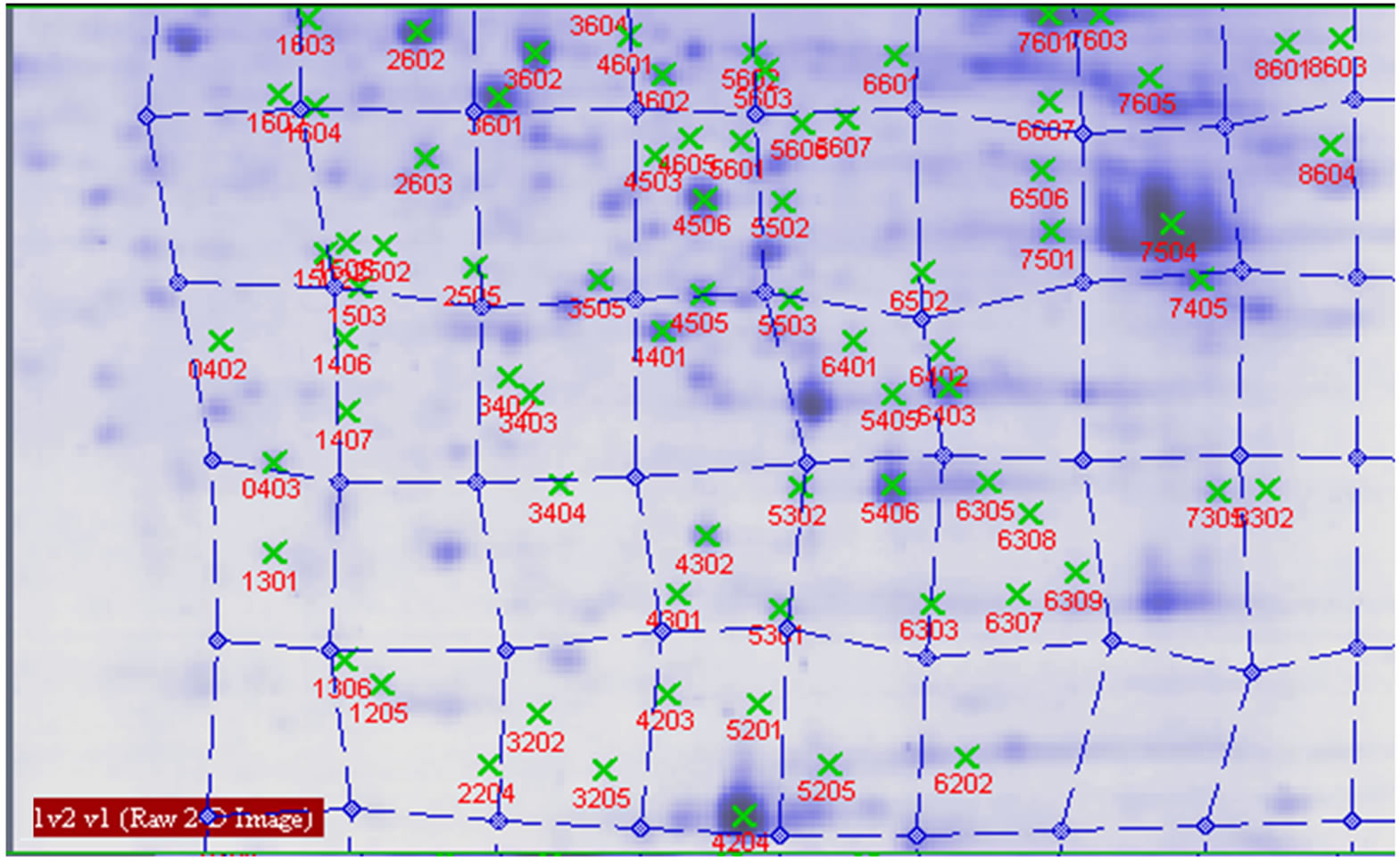
Figure 6. Proteins analysed by 2-DE in wheat leaves under normal light.
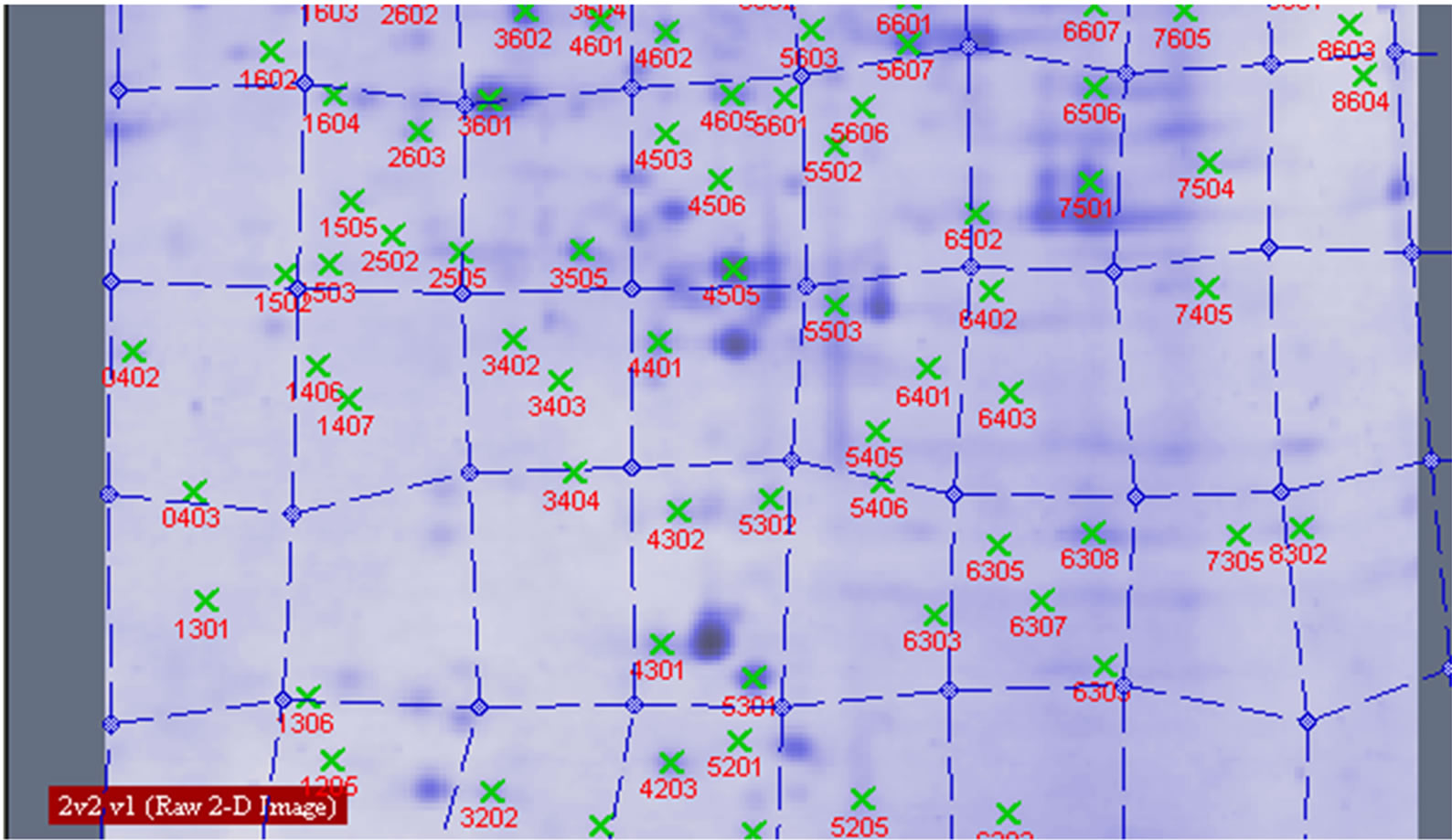
Figure 7. Proteins analysed by 2-DE in wheat leaves under UV-B.
UV-B radiation stage, the rate of synthesis of protein is believed to be negatively affected. The UV-B with HeNe radiation group had the same number of leaf protein spots. Since He-Ne lasers could alleviate cell damage caused by UV-B radiation in plants, it leads to up-regulation of the some initial responsive proteins.
3.3. Protein Spots of Wheat Leaves on Differential Treatment
Protein spots with differential expression among three groups are listed in the inset of Figures 10 and 11. Figure 10 shows there are 21 different protein spots between CK group and UV-B group. 6 of protein spots show protein expression increase during UV-B radiation. Such as 1301, 3505, 3602, 5201, 7305, 7504. 12 of spots show protein expression decrease Such as 1603, 2505, 2603, 3202, 3404, 4203, 4401, 5606, 5803, 6307, 6402, 8603. There are 3 differential individual protein plots which are expressed after UV-B radiation. Such as 2502, 4802, 5503. The results reveal that the number of upregulated proteins on 2-DE gels gradually declines during UV-B. 3 UV-B-responsive proteins appear.
Figure 11 shows there are 24 different protein spots
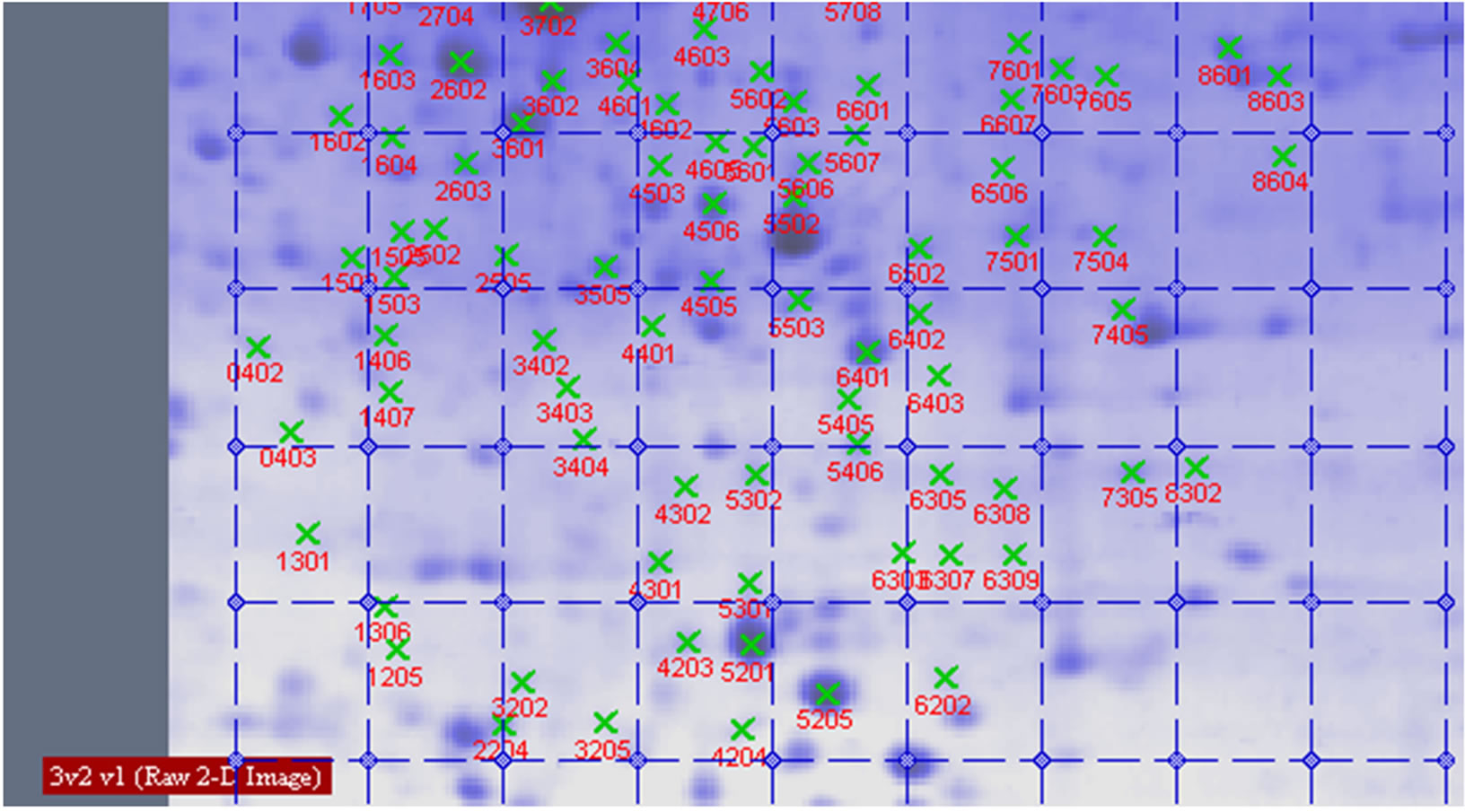
Figure 8. Proteins analysed by 2-DE in wheat leaves under UV-B + He-Ne laser.
 (a)
(a)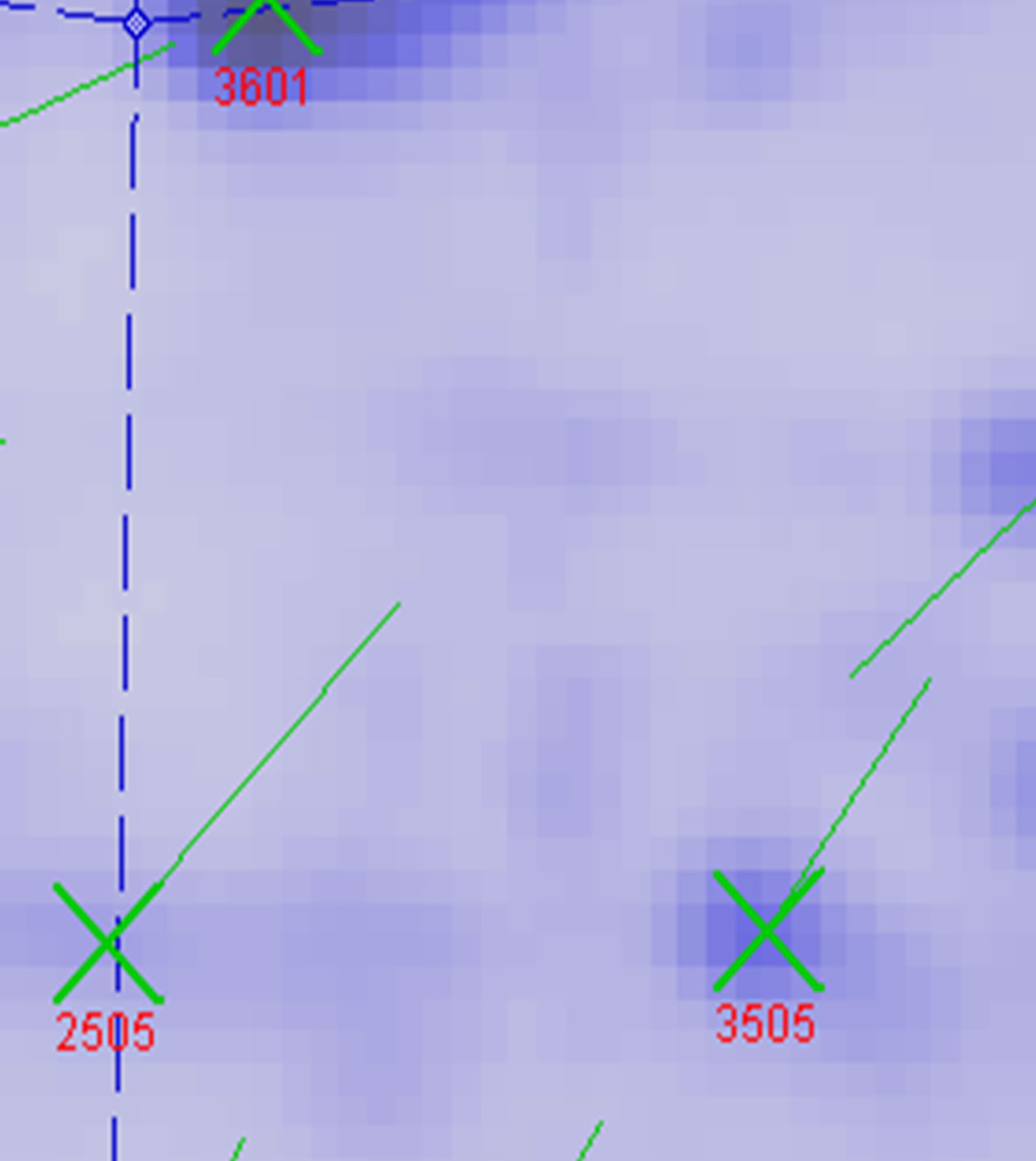 (b)
(b)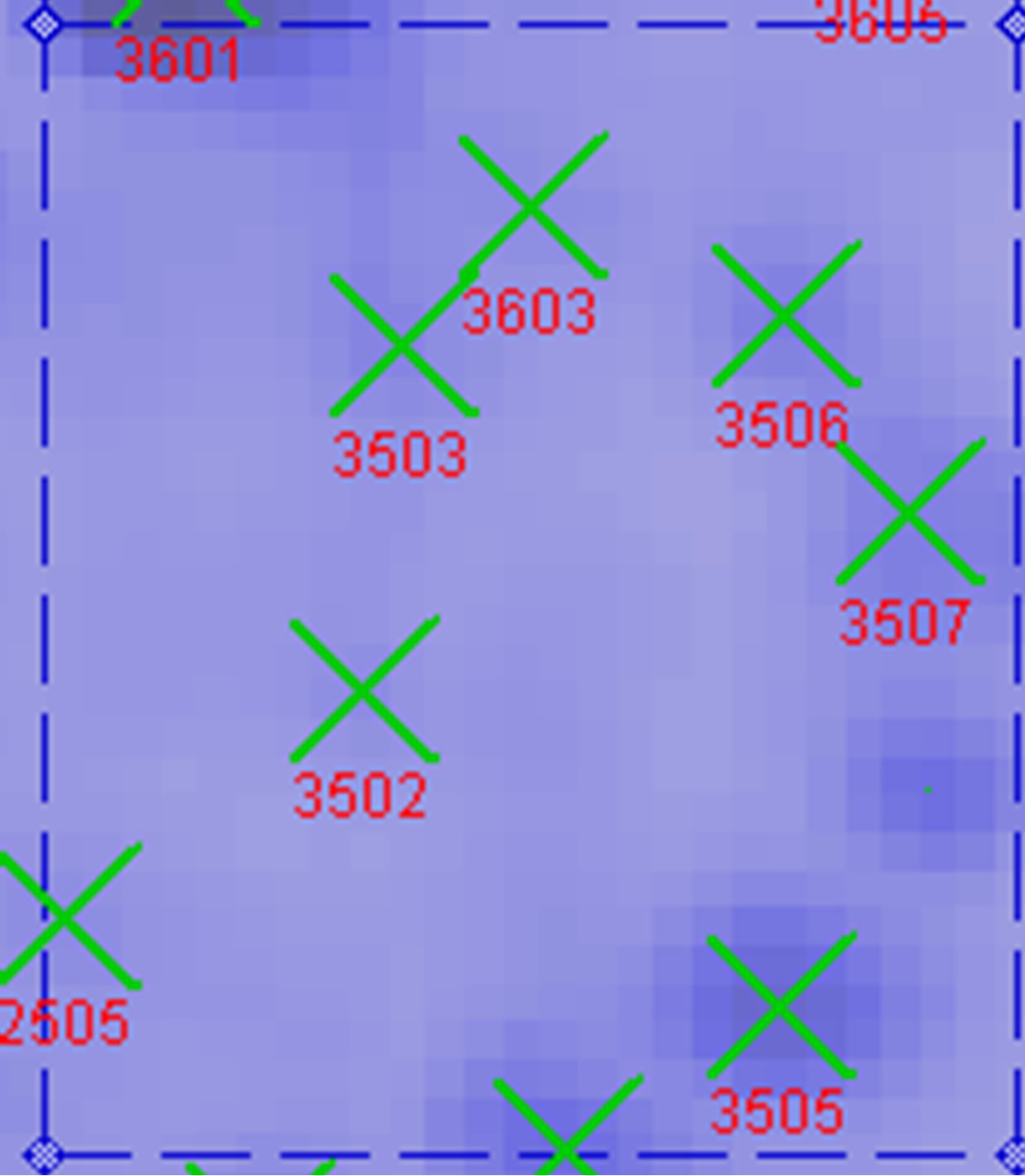 (c)
(c)
Figure 9. Proteins analysed by PD Quest 8.0 in wheat leaves on six day. (a) CK group; (b) UV-B group; (c) UV-B + He-Ne laser group.
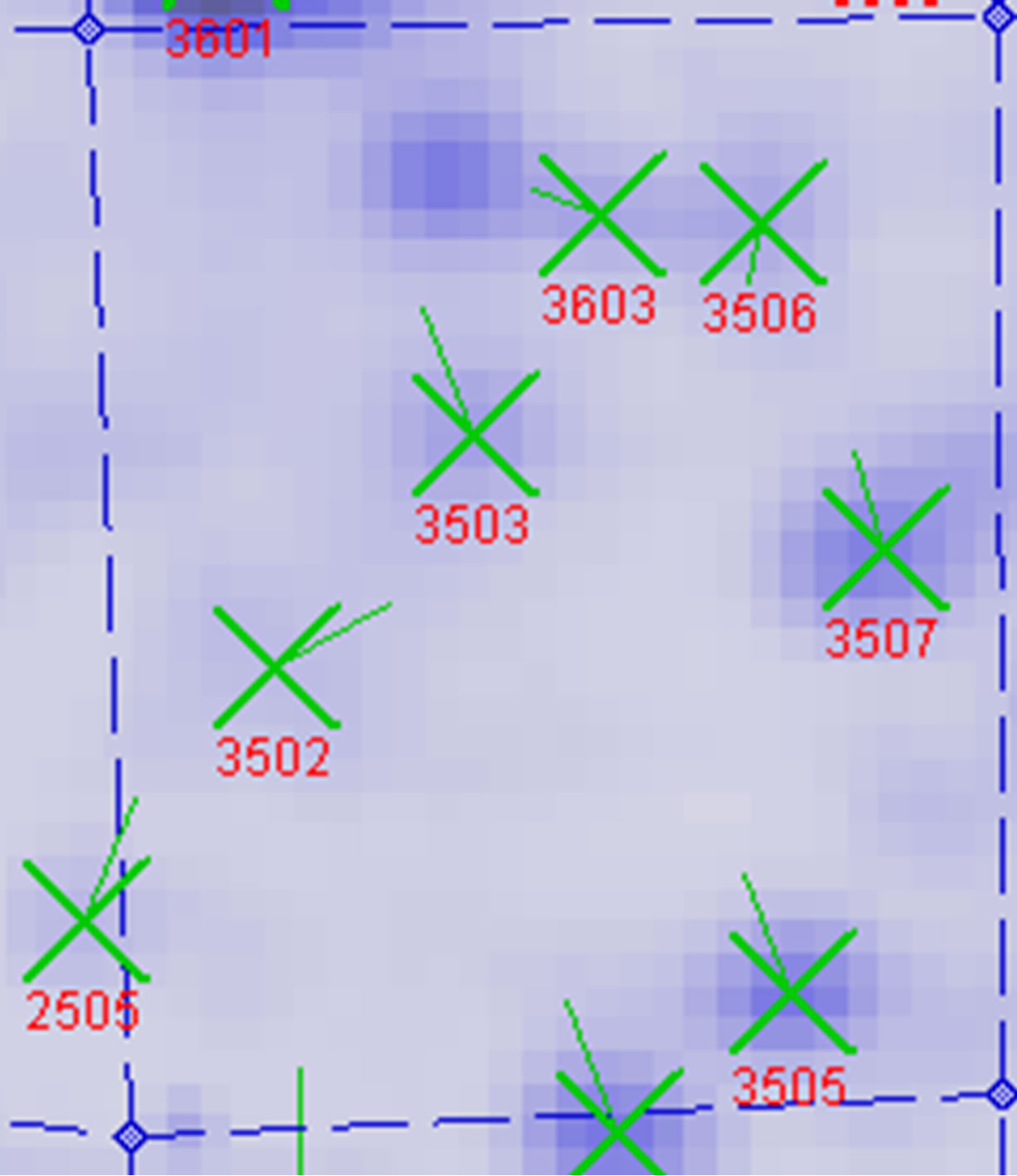
between UV-B group and UV-B + He-Ne laser group. 14 of protein spots show protein expression increase after He-Ne radiation. Such as 1602, 2204, 2502, 3205, 3502, 3503, 3506, 3507, 4505, 5405, 5503, 6202, 6401, 8302. 6 of spots show protein expression decrease Such as 1301, 5201, 6305, 6307, 6309, 6507. There are 4 differential individual protein plots which are expressed after He-Ne radiation such as 4204, 4603, 7305, 8601.
The results reveal that the number of up-regulated proteins on 2-DE gels gradually increases during UV-B + HeNe laser group. 4 He-Ne laser-responsive proteins appear.
3.4. The Analysis of Differential Individual Protein Spots
A total of 5 dependent spots including 3205, 3502, 3503, 3506, 3507 were picked from differential individual protein spots. 3 spots matched the correct proteins. The identification results from MALDI-TOF MS are summarized in Table 2" target="_self"> Table 2.
4. DISCUSSION
Proteomics is a recent addition to the molecular tools used to analyze ultraviolet-B affected plants. To monitor the proteomic changes of wheat leaves under ultraviolet-B radiation, a fundamental consideration is reliable quantitative measurements for their proteomes. 2-DE possesses advantages that deem the technique essential for proteomic research [8]. First, 2-DE allows the separation and identification of intact proteins rather than peptides; second, it can be used for analysis of most soluble
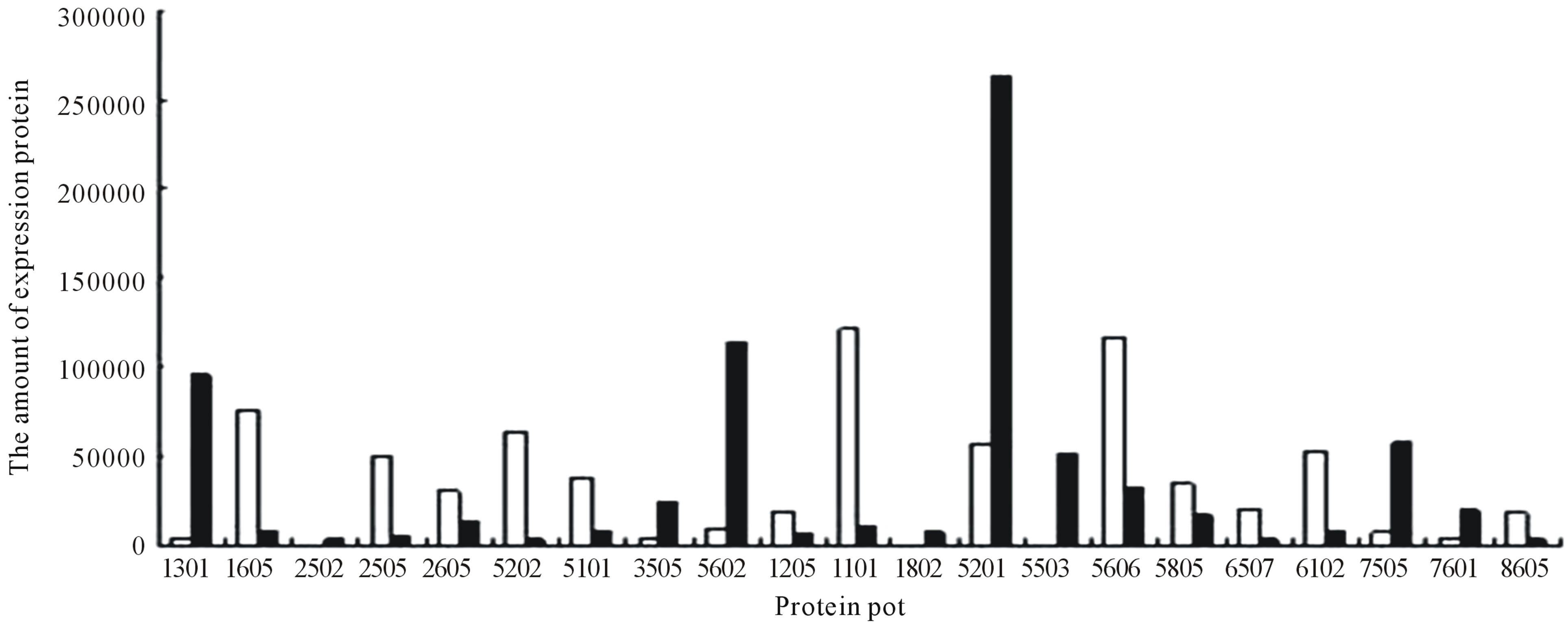
Figure 10. Changes in the relative spot volumes on wheat leaves between UV-B radiation and CK.
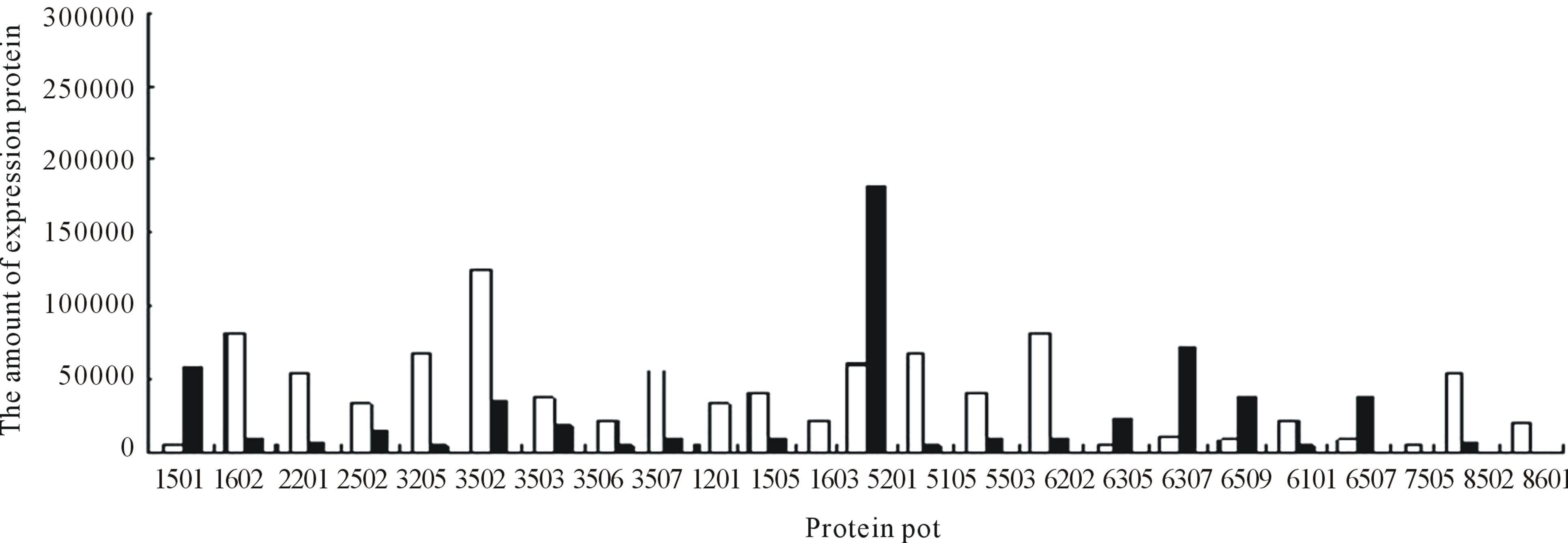
Figure 11. Changes in the relative spot volumes on wheat leaves between UV-B + He-Ne laser and CK.
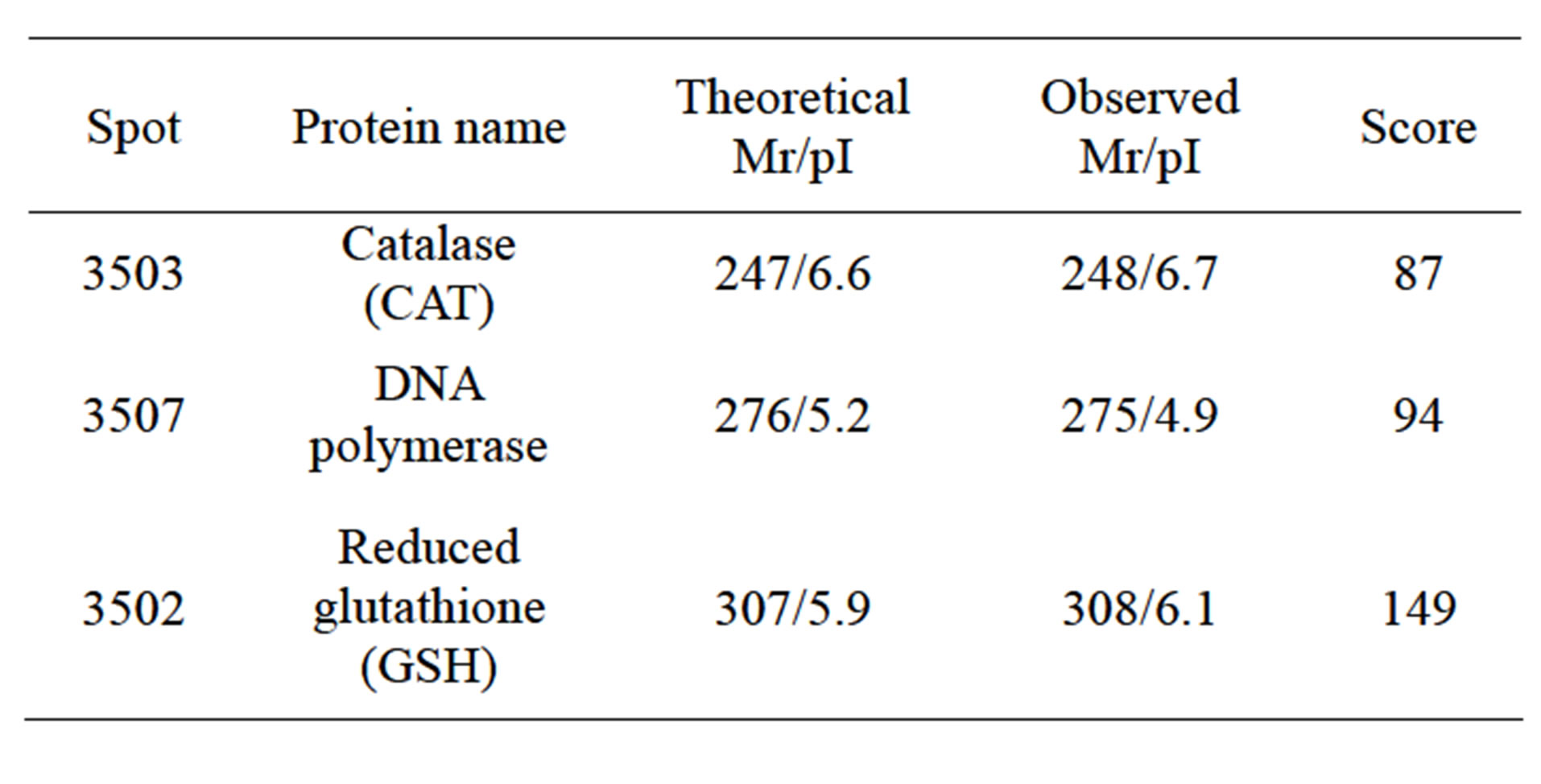
Table 2. Identification of differential protein spots by ESI-Q MS/MS and database searching.
proteins based upon their Mr and pI values; third, it can provide direct information regarding protein modifications; and fourth, the experimental cost of 2-DE is feasible for most research laboratories. In this study, to achieve reliable quantification of 2-DE gels, we have adopted average values of spot density for quantitative comparisons. All of the samples were paralleled quadruply and the average of the spot density was estimated at least in triplicate. For image analysis: although software analysis for 2-DE imaging is not labor intensive, the results are often not dependable. As described in Section 3.2, we analyzed 2-DE images using Image Master Elite and then by manual confirmation. Through these efforts, we achieved a reliable quantification analysis for the 2-DE images.
Enhanced UV-B radiation may exert an adverse influence on physiological and biochemical processes of plants. Potential key targets for UV-B damage are membranes, DNA and other macromolecules. Because lasers could alleviate cell damage caused by UV-B radiation in plants [9]. Our results indicate that specific proteins enhanced in wheat leaves show a coordinated response to UV-B radiation. UV-B radiation responsive proteins accumulated when plants are subjected to UV-B radiation. This initial response of wheat to UV-B radiation was transient. After UV-B radiation exerts an adverse influence on wheat 6 days, it leads to down-regulation of the some initial responsive proteins. But He-Ne lasers could alleviate cell damage caused by UV-B radiation in plants, it leads to up-regulation of the some initial responsive proteins.
The identification of protein spots by MS has revealed that CAT exists in wheat leaves after He-Ne radiation. As is well known, SOD, POD and CAT could eliminate accumulation of poisonous free radicals and prevent lipid peroxidation. Because of the low activity of SOD, POD and CAT in wheat, poisonous free radical production would not be eliminated quickly. As a result, they could not prevent lipid peroxidation production [10]. Our results suggest that enhanced expression of SOD, POD and CAT evolving complex protein and aldolases are some of the protective responses of wheat to He-Ne lasers. When wheat was exposed to He-Ne lasers, enhanced expression of CAT was consistently detected.
UV-B can not only damage plant cellular membranes and free radical elimination systems, but can also affect plant DNA, which results in heritable mutation if damaged DNA is not repaired before replication [11]. Direct absorption of UV-B radiation induces the formation of pyrimidine dimmers in DNA, which are the most predominant and biologically significant UV-B-induced lesions in DNA [12]. Usually, plants possess some mechanisms and pathways to repair UV-B induced DNA damages. The identification of protein spots by MS has revealed that DNA polymerase exists in wheat leaves after He-Ne radiation Our present results will be more reliable that laser irradiance is capable of protecting wheat from UV-B-induced DNA damage, and that the potential mechanism is up-regulation of the DNA polymerase.
5. CONCLUSION
In this work we suggested that He-Ne lasers treatment could significantly alleviate UV-B-induced damage in winter wheat.
REFERENCES
- Qiu, Z.B., Zhu, X.J., Li, F.M., Liu, X. and Yue, M. (2007) The optical effect of a semiconductor laser on protecting wheat from UV-B radiation damage. Photochemical & Photobiological Sciences, 6, 788-793. doi:10.1039/b618131g
- Zu, Y., Li, Y., Chen, J. and Chen, H. (2004) Intraspecific responses in grain quality of 10 wheat cultivars to enhanced UV-B radiation under field conditions. Journal of Photochemistry and Photobiology B, 74, 95-100. doi:10.1016/j.jphotobiol.2004.01.006
- Jiang, J.Y., Niu, C.P., Hu, Z.H., Yang, Y.F. and Huang, Y. (2006) Study on mechanism of enhanced UV-B radiation influencing on N2O emission from soil-winter wheat system. Huan Jing Ke Xue, 27, 1712-1716.
- Uchid, A., Higa, K., Shiba, T., Yoshimori, S., Kuwashima, F. and Iwasawa, H. (2003) Generalized synchronization of chaos in He-Ne lasers. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics, 68, 016215. doi:10.1103/PhysRevE.68.016215
- Qi, Z., Yue, M. and Wang, X.L. (2002) Protect effect of He-Ne laser irradiation on the damage and repair of wheat seeding by enhanceUV-B radiation. Chinese Journal of Lasers, 29, 863-869. doi:10.1364/AO.7.000559
- Agrawal, G.K. and Thelen, J.J. (2005) Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics, 5, 4684-4688. doi:10.1002/pmic.200500021
- Abbasi, F.M. and Komatsu, S. (2004) A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics, 4, 2072-2081. doi:10.1002/pmic.200300741
- Miura, K. (2003) Imaging technologies for the detection of multiple stains in proteomics. Proteomics, 3, 1097- 1108. doi:10.1002/pmic.200300428
- Reddy, G.K. (2003) Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers in Surgery and Medicine, 33, 344-351. doi:10.1002/lsm.10227
- Pradhan, M.K., Joshi, P.N., Nair, J.S., Ramaswamy, N.K., Iyer, R.K., Biswal, B. and Biswal, U.C. (2006) UV-B exposure enhances senescence of wheat leaves: Modulation by photosynthetically active radiation. Radiation and Environmental Biophysics, 45, 221-229. doi:10.1007/s00411-006-0055-2
- Yarosh, D.B. (2002) Enhanced DNA repair of cyclobutane pyrimidine dimers changes the biological response to UV-B radiation. Mutation Research, 509, 221-226.
- Li, Y., He, Y. and Zu, Y. (2006) Effects of enhanced UV-B radiation on physiological metabolism, DNA and protein of crops: a review. Ying Yong Sheng Tai Xue Bao, 17, 123-126.
NOTES
*The study was supported by the Natural Science Foundation of Shanxi Province (2009011044-2).
#Corresponding author.

