Green and Sustainable Chemistry
Vol.3 No.1(2013), Article ID:28071,5 pages DOI:10.4236/gsc.2013.31005
An Efficient FeCl3 Catalyzed Synthesis of N,N’-Diarylformamidines
Department of Organic Chemistry, Indian Association for the Cultivation of Science, Jadavpur Kolkata, India
Email: ocscr@iacs.res.in
Received November 23, 2012; revised December 25, 2012; accepted January 8, 2013
Keywords: Fe(III) Chloride; Diarylformamidines; Aryl Amines; Triethylorthoformate
ABSTRACT
An efficient FeCl3 catalyzed synthesis of N,N’-diarylformamidines using triethylorthoformate (1 equivalent) and primary aryl amines (2 equivalents) at ambient temperature has been described. This methodology provides an ecofriendly and simple procedure without using any hazardous and expensive chemicals.
1. Introduction
Formamidines have structural similarity to the imidazole ring, a part of the histamine molecule, are supposed to possess enormous biological activities. The biochemical aims of formamidines include monoamine oxidase inhibitor [1,2], adrenergic, neurochemical receptors [3-8] and prostaglandin E2 synthesis [9]. Formamidines are also noted for their complexation with transition metals [10,11] and usage as auxiliaries in asymmetric synthesis [12,13], electrophiles [14]. The utility of formamidines as support linkers in solid phase synthesis [15] is now well established in the field of organic synthesis. Formamidines are now vastly used for the preparation of imidazolium salts which are the precursor for the synthesis of N-Heterocyclic carbenes [16]. Moreover, formamidines are useful subject of interest to the physical chemists for dynamic NMR study [17]. There have also been reported some cryoscopic molecular weight determination experiments utilizing the molecular association property of diarylformamidines in benzene solution [18].
2. Results and Discussion
There are only a few reports [16,19-26] in the literature for the synthesis of formamidines specially using triethyl orthoformate and amines. However, there is still scope for further improvement in this field since most of the reported methods suffer from long reaction times, elevated temperature or use of toxic and expensive reagents. Very recently, Sadek et al. [26] reported the synthesis of diarylformamidines using ceric ammonium nitrate (CAN) in water. But it is well known that CAN is a toxic and strong oxidizing reagent and especially in water it shows strong acidic property to affect many sensitive functional groups. So, a mild and efficient method is still desirable. We report herein an efficient FeCl3 catalyzed synthesis of N,N’-diarylformamidines using triethylorthoformate (1 equivalent) and primary aryl amines (2 equivalents) at ambient temperature. Compared to other methods this method is much more environment friendly due to not using any toxic chemicals. In a preliminary experiment, a solution of aniline (1a) (2 mmol) and triethyl orthoformate (1 mmol) in the presence of a catalytic amount of FeCl3 (10 mol%) in toluene (10 mL) was stirred for 3 h at room temperature. Solvent was removed and the solid mass obtained was purified by column chromatography over silica gel to afford pure formamidine 1b in excellent yield (Scheme 1).
Thus, a series of diarylformamidines have been synthesized using the reaction conditions and the results are summarized in Table 1. All the products were characterized by spectral and analytical studies and were compared with the reported data (10,13b,13d,13f-h). The probable mechanism of the formation of the product may be suggested with the line of the report by Sadek et al. [26] (Scheme 2). It is proposed that FeCl3 as a Lewis acid activates ethoxy groups and enhances the C-O bond cleavage to generate a stable carbocation which facilitates the subsequent nucleophilic displacements by aromatic amines.
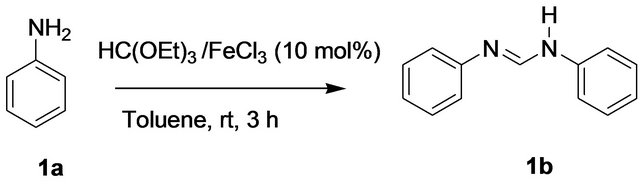
Scheme 1. Synthesis of diarylformamidines.

Scheme 2. Plausible mechanism for the formation of diarylformamidines.
Table 1. Synthesis of N,N’-diarylformamidines.
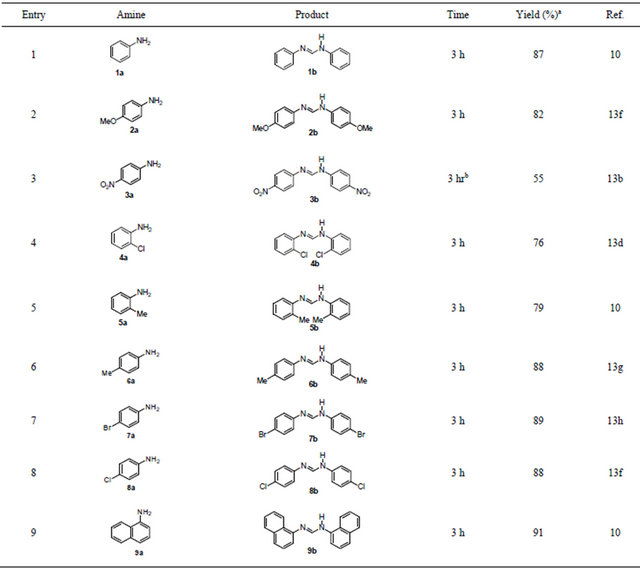
aYields refer to pure isolated products. bRefluxed in toluene.
3. Conclusion
In conclusion, we have developed a mild and efficient method for the direct conversion of primary aryl amine to N,N’-diarylformamidines using a catalytic amount of FeCl3. This provides an eco-friendly and simple method without using any toxic and expensive reagents.
4. Experimental Section
4.1. General Procedures
All melting points were taken on a Gallenkamp melting point apparatus and are uncorrected. The 1H and 13C NMR were recorded in CDCl3 using TMS as an internal standard on 300 and 75 MHz spectrometer (Bruker) respectively and IR were recorded using a Shimadzu FT IR-8300 instrument. High-resolution mass spectra were obtained using a Qt of Micro YA263 instrument. Toluene was dried over sodium. Chloroform was freshly distilled from phosphorus pentoxide. Petroleum ether of boiling range 60˚C - 80˚C and silica gel of 60 - 120 mesh were used for column chromatography.
4.2. Experimental Section
4.2.1. Representative Procedure for the Synthesis of Diarylformamidines
To a well stirred a solution of aniline (1a) (186 mg, 2 mmol) and triethyl orthoformate (148 mg, 1 mmol) in the presence of a catalytic amount of FeCl3 (10 mol%) in toluene (10 mL) was stirred for 3 h at room temperature. Solvent was removed under reduced pressure and the solid mass was dissolved in chloroform (30 mL) and then filtered through a Whatmann filter paper. The solvent was removed under reduced pressure and the crude residue obtained was purified by column chromatography over silica gel (100 - 200 mesh) (40% ethyl acetate in petroleum ether) to afford N,N’-diphenylformamidine (1b) as a pale yellow solid; m.p.139˚C -140˚C. IR (KBr): 3356, 3037, 2875, 1685, 1600, 1498, 1442, 1313, 1174, 1028, 752, 692 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.04-7.18 (m, 6H), 7.29 - 7.34 (m, 4H), 8.19 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 118.9, 120.2, 124.8, 125.3, 129.1, 129.8, 136.8, 137.0, 159.5 ; HRMS: calcd. for C13H13N2 [M+H]+: 197.1073; found: 197.1072.
4.2.2. N,N’-Bis(4-methoxyphenyl) formamidine (2b)
Colourless solid; m.p. 85˚C - 86˚C. IR (KBr): 2835, 1672, 1504, 1319, 1242, 1109, 1033, 825, 719, 580 cm−1; 1H NMR (300 MHz, CDCl3): δ 3.81 (s, 6H), 6.89 - 6.92 (d, J = 8.8 Hz, 4H), 7.09 - 7.12 (d, J = 8.8 Hz, 4H), 8.06 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 55.5, 55.6, 114.3, 115.0, 121.7, 121.9, 129.7, 130.1, 156.8, 157.7, 163.3; HRMS: calcd. for C15H17N2O2 [M+H]+: 257.1285; found: 257.1283.
4.2.3. N,N’-Bis(4-nitrophenyl) formamidine (3b)
Pale yellow solid; m.p. 239˚C - 240˚C (decomposed). IR (KBr): 3086, 2885, 1687, 1562, 1500, 1411, 1330, 1271, 1111, 854, 752, 688, 540 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 7.82 - 7.97 (m, 5H), 8.22 - 8.25 (m, 3H), 8.42 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 112.9, 119.5, 125.6, 126.9, 136.2, 143.1, 144.7, 156.2, 161.1; HRMS: calcd. for C13H10N4O4 [M+H]+: 286.0775; found: 286.0776.
4.2.4. N,N’-Bis(2-chlorophenyl) formamidine (4b)
Pale yellow solid; m.p. 141˚C -142˚C. IR (KBr): 3010, 2981, 1685, 1503, 1363, 1091, 821 cm−1; 1H NMR (300 MHz, CDCl3): δ 6.96 - 7.35 (m, 8H), 8.33 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 115.9, 119.0, 119.3, 124.2, 127.6, 127.8, 129.4, 129.9, 143.0; HRMS: calcd. for C13H11N2Cl2 [M+H]+: 265.0294; found: 265.0292.
4.2.5. N,N’-Di-O-tolylformamidine (5b)
Light brown solid; m.p. 152˚C -153˚C IR (KBr): 3010, 2806, 1662, 1580, 1480, 1465, 1310, 1210, 1187, 996, 780, 744, 723, 613 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.25 (s, 6H), 6.92 - 6.97 (m, 4H), 7.08 - 7.18 (m, 4H), 7.98 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 18.0, 117.6, 123.8, 127.1,128.8, 130.5, 130.9, 147.8; HRMS: calcd. for C15H17N2 [M+H]+: 225.1386; found: 225.1386.
4.2.6. N,N’-Di-P-tolylformamidine (6b)
Brownish solid; m.p. 140˚C - 141˚C. IR (KBr): 3342, 2922, 1693, 1518, 1356, 1215, 1037, 817, 669, 509 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 2.24 (brs, 6H), 7.18 - 7.28 (m, 8H), 8.76 (brs, 1H); HRMS: calcd. for C15H17N2 [M+H]+: 225.1386; found: 225.1385.
4.2.7. N,N’-Bis(4-bromophenyl) formamidine (7b)
White solid; m.p. 19˚C - 192˚C. IR (KBr): 3003, 2976, 1697, 1491, 1352, 1076, 819, 634, 495 cm−1; 1H NMR (300 MHz, DMSO-d6): δ 7.51 - 7.59 (m, 4H), 7.65 - 7.68 (m, 4H), 8.98 (brs, 1H); 13C NMR (75 MHz, CDCl3): δ 117.6, 118.4, 120.4, 121.6, 132.2, 132.9, 135.9, 136.0, 159.0; HRMS: calcd. for C13H11N2Br2 [M+H]+: 352.9283; found: 352.9283.
4.2.8. N,N’-Bis(4-chlorophenyl) formamidine (8b)
Light brown solid; 179˚C - 180˚C. IR (KBr): 3013, 2974, 1691, 1501, 1367, 1100, 823, 656, 476 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.02 - 7.10 (m, 2H), 7.26 - 7.33 (m, 3H), 7.43 - 7.58 (m, 3H), 8.35 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 120.2, 121.4, 129.2, 129.9, 130.9, 135.4, 135.5, 159.3; HRMS: calcd. for C13H11Cl2N2 [M+H]: 265.0294 ; found: 265.0295.
4.2.9. N,N’-Dinaphthalen-1-Yl-formamidine (9b)
Light purple solid; m.p. 200˚C - 201˚C. IR (KBr): 3047, 1660, 1573, 1394, 1300, 1263, 993, 788, 765 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.48 - 7.68 (m, 10H), 7.90 (s, 2H), 8.30 (s, 2H), 8.50 (brs, 1H); HRMS: calcd. for C22H16N2 [M+H]+: 297.1386; found: 297.1386.
5. Acknowledgements
The authors thank the Department of Science and Technology, New Delhi for financial assistance. P C thanks CSIR, New Delhi for awarding the research fellowships.
REFERENCES
- R. W. Beeman and F. Matsumura, “Chlordimeform: A Pesticide Acting upon Amine Regulatory Mechanisms,” Nature, Vol. 242, No. 5395, 1973, pp. 273-274. doi:10.1038/242273a0
- S. A. Aziz and C. O. Knowles, “Inhibition of Monoamine Oxidase by the Pesticides Chlordimeform and Related Compounds,” Nature, Vol. 242, No. 5397, 1973, pp. 417- 418. doi:10.1038/242417a0
- V. K. S. Leung, T. Y. K. Chan and V. T. F. Yeung, “AmiTraz Poisining in Humans,” Clinical Toxicology, Vol. 37, No. 4, 1999, pp. 513-514. doi:10.1081/CLT-100102523
- A. Nakayama, M. Sukekawa and Y. Eguchi, “Stereochemistry and Active Conformation of a Novel Insecticide, Acetamiprid,” Pesticide Science, Vol. 51, No. 2, 1997, pp. 157-164. doi:10.1002/(SICI)1096-9063(199710)51:2<157::AID-PS620>3.0.CO;2-C
- G. D. Baxter and S. C. Barker, “Isolation of a cDNA for an Octopamine-Like, G-Protein Coupled Receptor from the Cattle Tick, Boophilus microplus,” Insect Biochemistry and Molecular Biology, Vol. 29, No. 5, 1999, pp. 461- 467. doi:10.1016/S0965-1748(99)00023-5
- M. Gall, J. M. McCall, R. E. TenBrink, P. F. VonVoigtlander and J. S. Mohrland, “Arylformamidines with Antinociceptive Properties,” Journal of Medicinal Chemistry, Vol. 31, No. 9, 1988, pp. 1816-1820. doi:10.1021/jm00117a023
- A. Donetti, E. Cereda, E. Bellora, A. Gallazzi, C. Bazzano, P. Vanoni, P. D. Soldato, R. Michelett, F. Pagani and A. Giachetti, “(Lmidazolylphenyl)Formamidines. A Structurally Novel Class of Potent Histamine H2 Receptor Antagonists,” Journal of Medicinal Chemistry, Vol. 27, No. 3, 1984, pp. 380-386. doi:10.1021/jm00369a025
- T. Goto, H. Sakashita, K. Murakami, M. Sugiura, T. Kondo and C. Fukaya, “Novel Histamine H3 Receptor Antagonists: Synthesis and Evaluation of Formamidine and S-Methylisothiourea Derivatives,” Chemical & Pharmaceutical Bulletin, Vol. 45, No. 2, 1997, pp. 305-311. doi:10.1248/cpb.45.305
- G. K. W. Yim, M. P. Holsapple, W. R. Pfister and R. M. Hollingworth, “Prostaglandin Synthesis Inhebited by Formamidine Pesticides,” Life Sciences, Vol. 23, No. 25, 1978, pp. 2509-2515. doi:10.1016/0024-3205(78)90176-5
- D. I. Arnold, F. A. Cotton, J. H. Matonic and C. A. Murillo, “Bis(N,N’-diphenylformamidine)silver(l) Triflate: A Three-Coordinate Silver Formamidine Compound Stabilized by Intramolecular Hydrogen Bonds,” Polyhedron, Vol. 16, No. 11, 1997, pp. 1837-1841. doi:10.1016/S0277-5387(96)00496-2
- D. B. Mitzi and K. Liang, “Synthesis, Resistivity, and Thermal Properties of the Cubic Perovskite NH2CH= NH2SnI3 and Related Systems,” Journal of Solid State Chemistry, Vol. 134, No. 2, 1997, pp. 376-381. doi:10.1006/jssc.1997.7593
- A. I. Meyers and R. Hutchings, “Asymmetric Dialkylation of Chiral 2-Benzazepine Formamidines,” Heterocycles Vol. 42, No. 2, 1996, pp. 475-478. doi:10.3987/COM-95-S58
- M. Matulenko and A. I. Meyers, “Total Synthesis of (-)-Tetrahydropalmatine via Chiral Formamidine Carbanions: Unexpected Behavior with Certain Ortho-Substituted Electrophiles,” The Journal of Organic Chemistry, Vol. 61, No. 2, 1996, pp. 573-580. doi:10.1021/jo951611q
- S. J. Benkovic, T. H. Barrows and P. R. Farina, “Studies on Models for Tetrahydrofolic Acid. IV. Reactions of Amines with Formamidinium Tetrahydroquinoxaline Analogs,” Journal of the American Chemical Society, Vol. 95, No. 25, 1973, pp. 8414-8420.
- P. S. Furth, M. S. Reitman and A. F. Cook, “A Novel Formamidine Linker for Use in Soid-Phase Synthesis,” Tetrahedron Letters, Vol. 38, No. 31, 1997, pp. 5403- 5406. doi:10.1016/S0040-4039(97)01200-8
- K. Hirano, S. Urban, C. Wang and F. Glorius, “A Modular Synthesis of Highly Substituted Imidazolium Salts,” Organic Letters, Vol. 11, No. 4, 2009, pp. 1019-1022. doi:10.1021/ol8029609
- L. Meschede and H-H Limbach, “Dynamic NMR Study of the Kinetic HH/HD/DD Isotope Effects on the Double Proton Transfer in Cyclic Bis(p-fluorophenyl)formamidine Dimers,” The Journal of Physical Chemistry, Vol. 95, No. 25, 1991, pp. 10267-10280. doi:10.1021/j100178a009
- R. M. Roberts, “The Molecular Association of Diarylformamidines. II. Effects of Oand P-Methyl Groups,” Journal of the American Chemical Society, Vol. 78, No. 11, 1956, pp. 2606-2608. doi:10.1021/ja01592a076
- K. M. Kuhn and R. H. Grubbs, “A Facile Preparation of Imidazolinium Chlorides,“ Organic Letters, Vol. 10, No. 10, 2008, pp. 2075-2077. doi:10.1021/ol800628a
- H. G. Mandel and A. J. Hill, “The Conversion of Formamides into Formamidines,” Journal of the American Chemical Society, Vol. 76, No. 15, 1954, pp. 3978-3982. doi:10.1021/ja01644a034
- R. M. Roberts, R. H. DeWolfe and J. H. Ross, “Ortho Esters, Imidic Esters and Amidines. II. Disproportionation Reactions of Nitrophenyl-, Chlorophenyland Tolylsubstituted Formimidates and Formamidines,” Journal of the American Chemical Society, Vol. 73, No. 5, 1951, pp. 2277-2281. doi:10.1021/ja01149a105
- C. D. Lewis, R. G. Krupp, H Tieckelmann and H. W. Post, “The Action of Ethyl Orthoformate on Aniline and Certain of Its Derivatives,” The Journal of Organic Chemistry, Vol. 12, No. 2, 1947, pp. 303-307. doi:10.1021/jo01166a016
- C. Lin, J. D. Protasiewicz, E. T. Smith and T. Ren, “Linear Free Energy Relationships in Dinuclear Compounds. 2. Inductive Redox Tuning via Remote Substituents in Quadruply Bonded Dimolybdenum Compounds,” Inorganic Chemistry, Vol. 35, No. 22, 1996, pp. 6422-6428. doi:10.1021/ic960555o
- M. L. Cole, P. C. Junk and L. M. Louis, “Synthesis and Structural Characterisation of Some Novel Lithium and Sodium N,N’-di(para-tolyl)formamidinate Complexes,” Journal of the Chemical Society, Dalton Transacions, No. 20, 2002, pp. 3906-3914. doi:10.1039/b204047f
- L.-J. Han, “Redetermination of (E)-N,N’-bis(4-bromophenyl)formamidine,” Acta Crystallographica section E, Vol. 67, No. 5, 2011, p. 1159. doi:10.1107/S1600536811013419
- K. U. Sadek, A. Alnajjar, R. A. Mekheimer, N. K. Mohamed and H. A. Mohamed, “Cerium (IV) Ammonium Nitrate (CAN) Mediated Reactions IV. A Highly Efficient Synthesis of N,N’-Diarylsubstituted Formamidines in Water at Ambient Temperature,” Green and Sustainable Chemistry, Vol. 1, No. 3, 2011, pp. 92-97. doi:10.4236/gsc.2011.13015

