Journal of Water Resource and Protection
Vol. 2 No. 3 (2010) , Article ID: 1493 , 5 pages DOI:10.4236/jwarp.2010.23025
Treatment of Acetonitrile by Catalytic Supercritical Water Oxidation in Compact-Sized Reactor
1Fuels Research Center, Department of Chemical Technology, Faculty of Science,
Chulalongkorn University, Bangkok, Thailand
2Center for Petroleum, Petrochemicals and Advance Materials, Chulalongkorn University, Bangkok, Thailand
E-mail: somkiat.n@chula.ac.th
Received November 20, 2009; revised December 18, 2009; accepted January 11, 2010
Keywords: Acetonitrile, Supercritical Water Oxidation, Compact-Sized Reactor
ABSTRACT
The objective of this research was to study the treatment of acetonitrile by catalytic supercritical water oxidation in a compact-sized tubular reactor, with an internal volume of 4.71 mL. Manganese dioxide was used as the catalyst and H2O2 was used as the oxidant. The oxidation of acetonitrile in supercritical water was studied at 400-500 oC, 25-35 MPa, the flow rate of 2-4 mL/min, the initial concentration of acetonitrile 0.077-0.121 M and the %excess O2 of 50-200%. As a result, the products were mainly N2, CO2 and CO and acetonitrile can be decomposed > 93 % within a very short contact time (1.45-6.19 s). The oxidation process was carried out with respect to the conversion of acetonitrile by 25 factorial design. Regression models were obtained for correlating the conversion of acetonitrile with temperature and flow rate. The complete oxidation can be achieved at a condition as moderate as 400 oC, 25 MPa with the flow rate of 2 mL/min.
1. Introduction
Laboratory wastes, which contain various types of hazardous chemicals generated from scientific research, have become a serious problem both financially and environmentally. Thus, two important and fundamental concepts of “treatment at the origin” and “self-responsibility for waste”, brings about a development of a compact-sized reactor for an on-site laboratory wastewater treatment. Supercritical water oxidation (SCWO) has been proposed as a promising candidate for the treatment of various organic wastes. Organic compounds and permanent gases (such as oxygen) are completely miscible in supercritical water, so the oxidation occurs in a single-phase aqueous environment. It was reported that, above the critical point of water (374 oC, 22.1 MPa), most organic compounds can be converted into CO2 and H2O by SCWO in a very short residence time [1–3]. Recently, there has been increasing interests in the use of heterogeneous catalysts in SCWO. Many catalysts can increase the oxidation rate, reduce the residence time and temperature requirement, and possibly provide a better selectivity for competing reaction pathways, which is difficult to achieve in noncatalytic processes [4].
Acetonitrile (CH3CN) is widely used as a solvent for the synthesis of many chemicals such as perfumesacrylic nail removers, pharmaceuticals, rubber products, pesticides and in the manufacturing of photographic film. It is also used as a solvent in various extraction processes and HPLC analysis technique [5]. In recent years, there have been a number of studies in literature demonstrating processes for the treatment of acetonitrile wastewater [6–8]. For incineration of nitrogen-containing organic compounds, the byproducts may include NO and NO2. At lower temperatures as required in SCWO, however, thermodynamics favor nitrogen conversion into N2 and N2O, rather than NO or NOx. In addition, any NOx formed would remain in the supercritical water, rather than being emitted to the atmosphere [9].
Thus, we have examined the oxidation of CH3CN in supercritical water using MnO2 as a catalyst in a compact-sized reactor [10]. The reaction is of great interest since nitrile groups are commonly found in many commercial products and they are major constituents of waste streams.
2. Methods
2.1. Apparatus and Procedure
The SCWO experiments were performed in a custommade tubular reactor system schematically shown in Figure 1. It was designed to be a compact reaction system with a similar size to a gas chromatograph, so that it can be installed in a limited space of a general laboratory. The experimental apparatus consists of a tubular reactor (0.1 m x 3/8” I.D. with an internal volume of 4.71 mL), which is heated by a 200 W vial heater. A commercial catalyst, bulk MnO2 was packed in the reactor. The wastewater and oxidant pre-heating line (180 cm x 1/8” I.D.) is heated by a 100 W electrical heater. All wetted parts (tubing, fittings, etc.) from the feeding pumps to the gas–liquid separator, were made of SUS316 stainless steel. The oxidant feed stream was prepared by dissolving hydrogen peroxide with deionized water in a feed tank. Another feed tank was loaded with a mixture of hydrogen peroxide (oxidant) and acetonitrile solution as a simulated wastewater. The two feed streams were fed in different lines using two HPLC pumps (Jasco) and were brought together at a SUS316 stainless steel mixing tee, prior to entering the pre-heater section. Hydrogen peroxide decomposes into oxygen and water completely when it is heated in the pre-heater section (150-200 oC) as verified by previous experiments [11]. After preheating, the solution was fed into the reactor. The reactor inlet and outlet temperature were measured using thermocouples. The experiments were carried out at 400- 500 oC, 25-35 MPa, the initial concentration of acetonitrile 0.077-0.121 M and the % excess O2 of 50-200 %. Upon exiting the reactor, the effluent was cooled rapidly by passing through a heat exchanger. Then, the effluent was flowed through a 0.5 µm inline filter, before being depressurized by a back-pressure regulator (GO REGULATOR Inc.). The product stream was then separated into liquid and vapor phases. The liquid products were collected in a graduated cylinder. It was assumed that the liquid and vapor phases were in equilibrium in the phase separator, and Henry’s law was used to calculate the amount of each gas dissolved in the liquid. The flow rates of both the gas and liquid phases were measured at the ambient laboratory condition.
2.2. Materials and Analytical Methods
The oxidant is oxygen which was produced by the thermal decomposition of hydrogen peroxide (Fisher, 30 %, w/v aqueous solution). Acetonitrile (Lab scan, > 99 % purity) was used as the organic compound to be oxidized in the catalytic SCWO experiments.
The effluent liquid phase was collected and then analyzed by a Shimadzu 7AG gas chromatograph with a flame ionization detector. The on-line gas samples were analyzed using a Shimadzu 2014 gas chromatograph with a thermal conductivity detector.
2.3. Experimental Design
A full 25 factorial design was performed to evaluate the
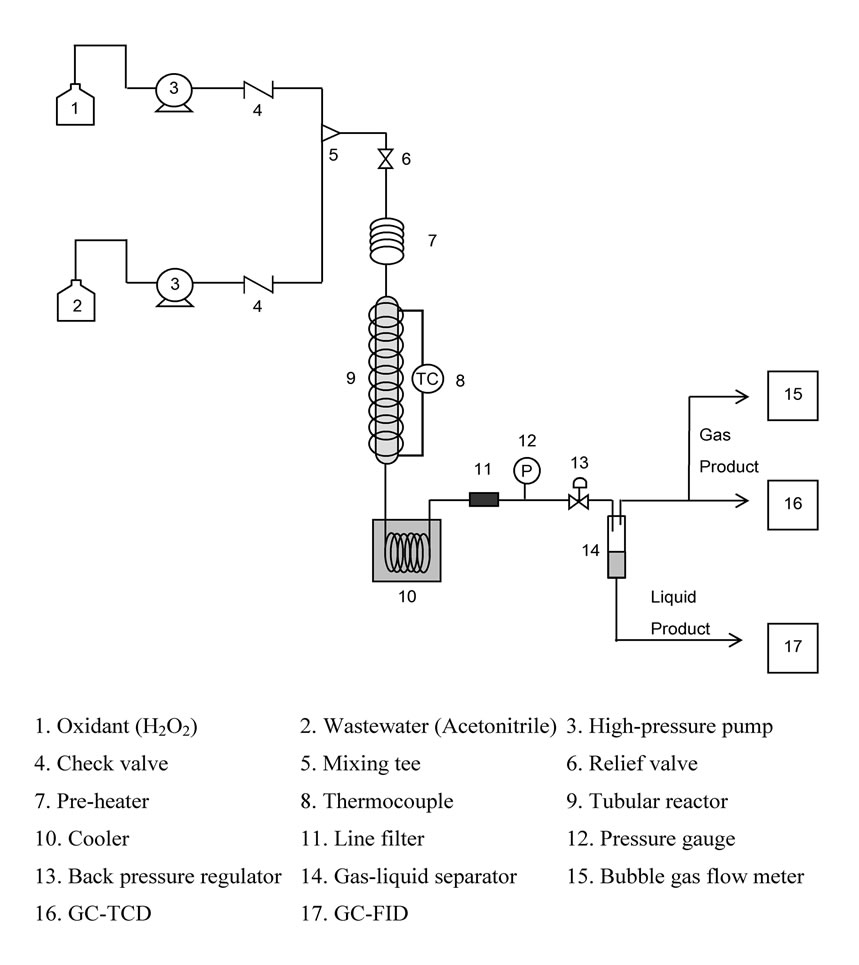
Figure 1. Schematic diagram of the compact-sized reactor.
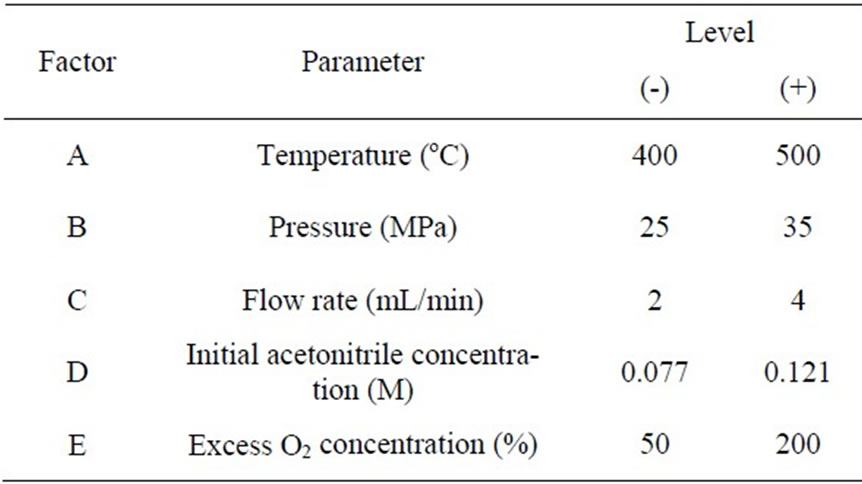
Table 1. Factors and levels used in the 25 factorial design.
effects of 5 factors; temperature, pressure, flow rate, initial acetonitrile concentration and % excess O2 concentration on conversion of acetonitrile. Table 1 summarizes these factors and their respective levels. The statistical analysis of variance, ANOVA, was carried out to determine the significance and impact of each factor as well as their interactions on the conversion. The relationships linking the main factors and interactions with the conversion were determined and presented as a quadratic equation of the general form in the following equation.
Y = mean + ∑main effects + ∑interactions(1)
The equation coefficients were calculated using the coded values, thus the effects of various terms can be compared directly regardless of their actual magnitude. The coding used throughout the statistical analysis denotes that −1 was taken as the lower level and +1 as the upper level. Therefore, a positive coefficient of a parameter indicates that the conversion increases with increasing level of the parameter, while a negative coefficient indicates that the conversion increases with decreasing level of the parameter.
The conversions of acetonitrile were calculated using the following equation.
X = (1 − Ct / Co) × 100(2)
where: X = acetonitrile conversion (%)
Ct = concentration of acetonitrile at a given time t (M)
Co = initial concentration of acetonitrile (M)
3. Results and Discussion
The experimental data using full 25 factorial design were listed in Table 2, 32 factorial points and 4 central points included. The experiments were executed in a random order to correctly evaluate experimental errors [12]. The conversions were obtained to be between 93.63 % and 100 % at the contact times ranging from 1.45 to 6.19 s under various reaction conditions. The gas phase products were detected as CO, CO2, CH4, N2O, N2, H2 and O2 by GC analysis. The liquid phase products were clear and colorless. The analysis of gas and liquid products showed that acetonitrile was completely degraded and transformed into intermediate products, CO2, H2O and N2. The decomposition of CH3CN in the SCWO can be expected to follow the oxidation reaction,
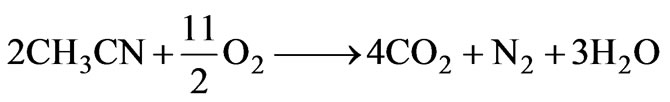 (3)
(3)
Although catalytic SCWO was carried out at high pressures and temperatures, its advantages of short reaction time and environmental friendliness overcame its shortcomings. In comparison with other treatment techniques such as nitrile-degrading microorganisms [6,8], which needed more than 10 h of reaction time, catalytic SCWO required only in the order of seconds with the
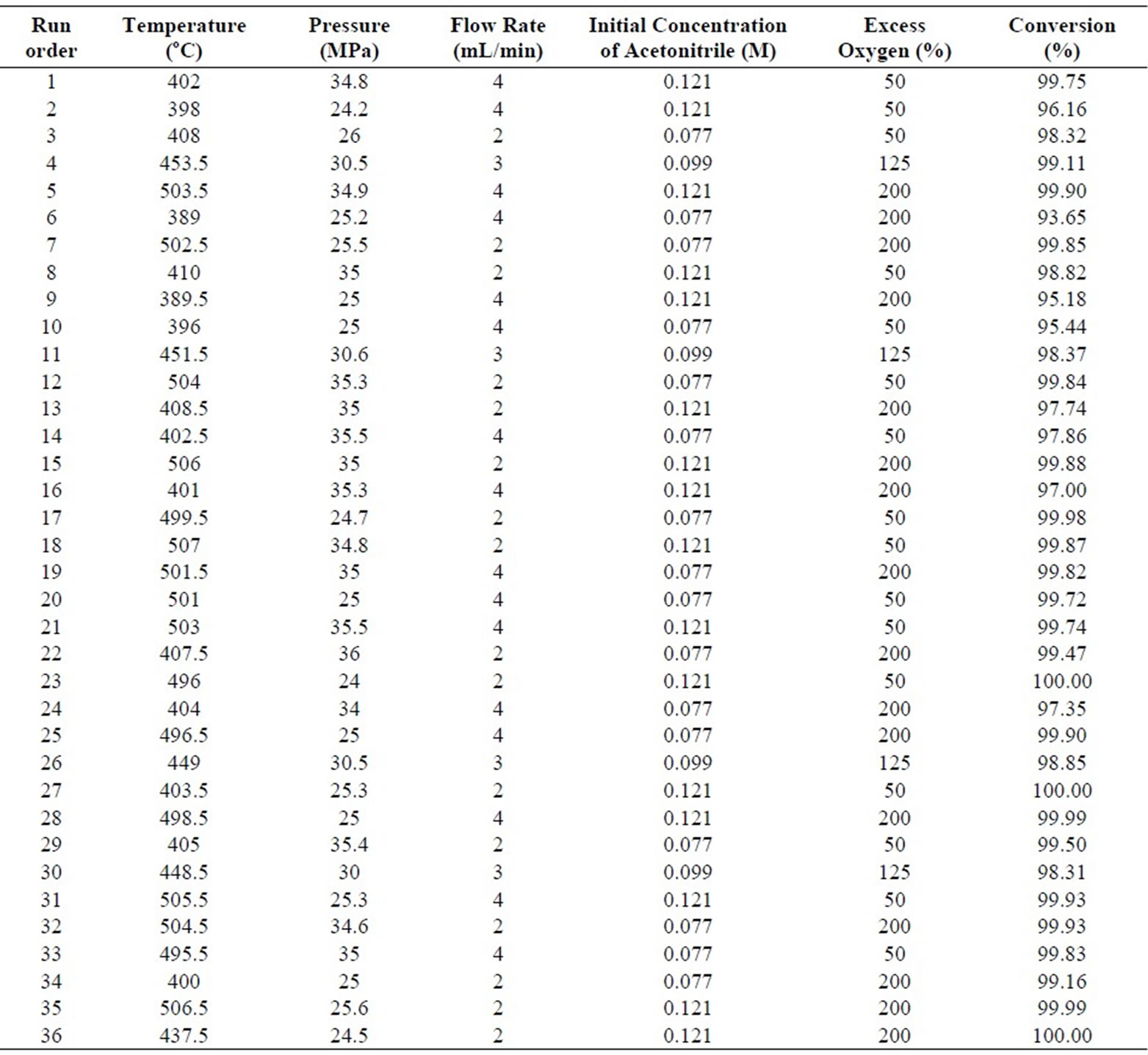
Table 2. Results and reaction parameters for SCWO of acetonitrile.
same conversion. In terms of environmental impact, catalytic SCWO generated less toxic components as supposed to NO or NOx emitted from incineration [7].
The 25 factorial study revealed that the conversion was not significantly influenced by the initial acetonitrile concentration, pressure and % excess oxygen, while temperature, and flow rate influenced the conversions at 95 % confidence interval. A mathematical model for the conversion of acetonitrile can be written in terms of the statistically significant effects on conversion in (4) and (5) [12,13].
%Conversion = 98.9+0.989T'–0.529F'+0.545T'F'(4)
where: T' = coded value of temperature
F' = coded value of flow rate
%Conversion = 106–0.013T–5.48F+0.011TF(5)
where: T = actual value of temperature (oC)
F = actual value of flow rate (mL/min)
A positive sign of the coefficient represents a synergistic effect, while a negative sign indicates an antagonistic effect. The temperature and flow rate have a negative relationship with the conversion, so with an increase of those factors, there will be a decrease in the conversion. However, the interaction term has a positive sign indicating that with a change in temperature and flow rate in the same direction from the central point, there will be an increase in the conversion. Figure 2 shows a comparison between the experiment and predicted conversion for acetonitrile decomposition. The high value of the coefficient of determination, R2 = 0.8 indicates a good correlation between the observed and the predicted values of conversion.
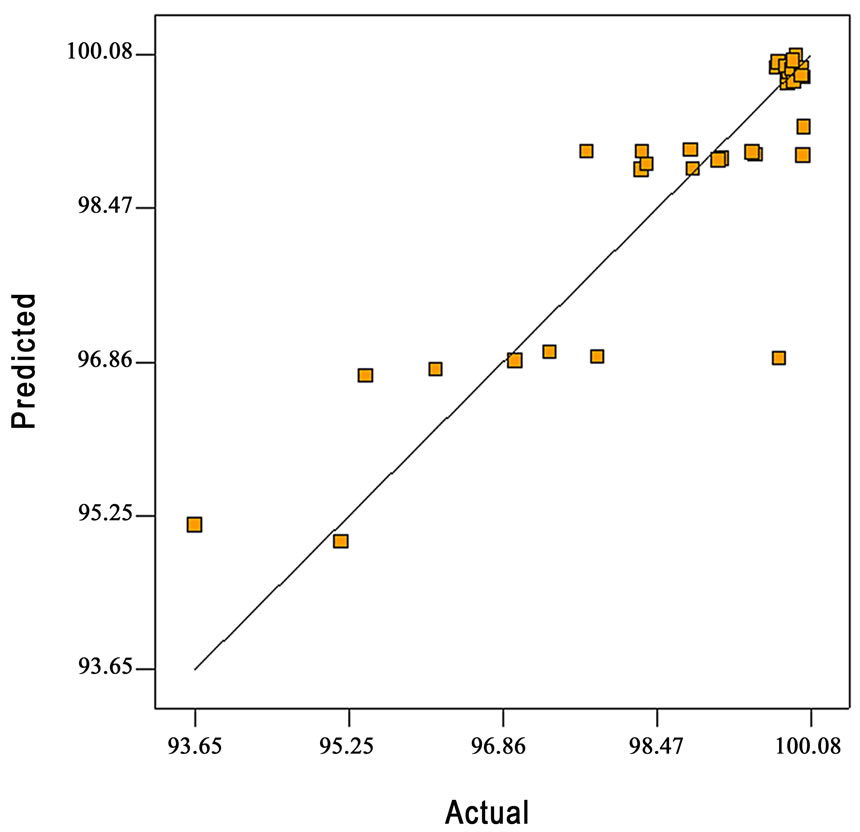
Figure 2. Comparison of the experimental and the predicted conversion.
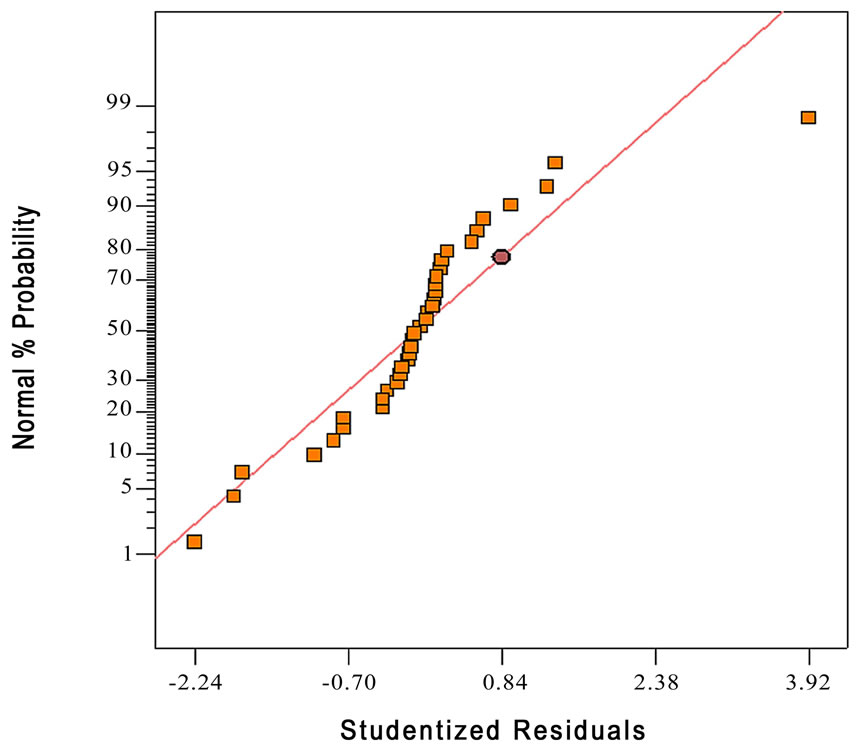
Figure 3. Normal probability plot of the difference between the observed and the predicted value.
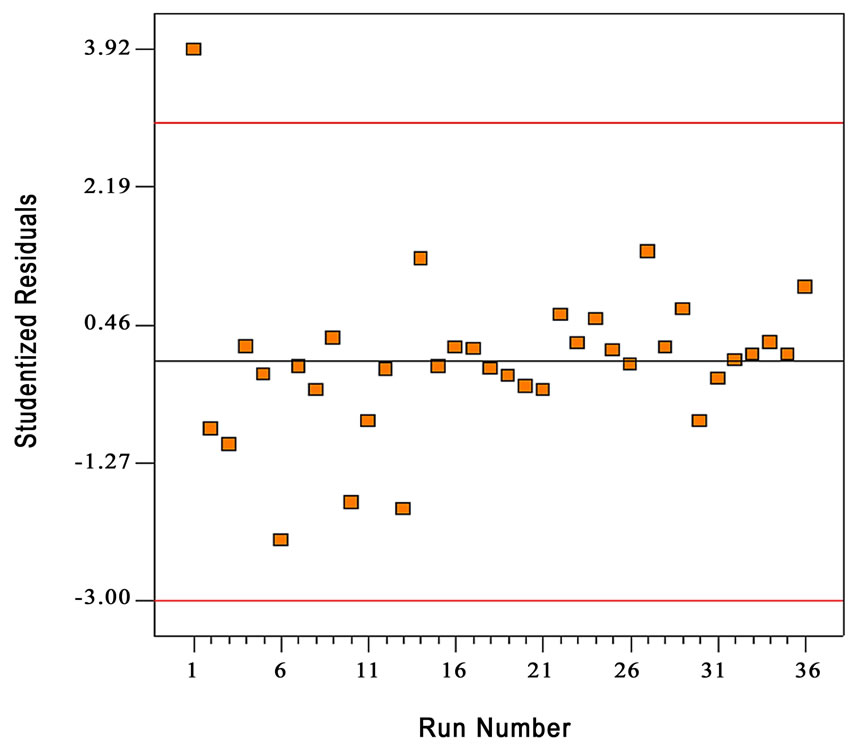
Figure 4. The residuals versus the run number when the variance is constant.
The normality of the data can be checked by plotting the normal probability plot (NPP) of the residuals. The normal probability plot is a graphical technique for assessing whether or not a data set is approximately normally distributed [14]. The residual is the difference between the observed and the predicted values (or the fitted values from the regression). Figure 3 shows the normal probability plot of residuals. The residuals fall fairly close to the diagonal line, so the data are normally distributed. Figure 4 illustrates the residuals for each run number. The residuals scatter randomly about zero with no specific trend, confirming that the residuals are the random error that have a normal distribution.
4. Conclusions
A compact-sized tubular reactor was designed to treat the aqueous solution of acetonitrile at the origin using catalytic supercritical water oxidation. The oxidation of acetonitrile was studied using 2k factorial experimental design. The significant factors were found to be temperature and flow rate. The mathematical models correlating the conversion to temperature and flow rate were formulated and evaluated. The results demonstrated that the SCWO process can treat acetonitrile efficiently, with the contact times between 1.45 and 6.19 s, and the acetonitrile concentration between 0.077 and 0.121 M. It was found that acetonitrile was completely degraded and transformed into its intermediate products, CO2, H2O and N2. The liquid phase products were clear and colorless. The advantage of catalytic supercritical water oxidation is the rapid oxidation of organics and the nitrogen conversion into N2 and N2O rather than NO or NOx.
5. Acknowledgment
The authors would like to thank the Fuels Research Center, Department of Chemical Technology and Center for Petroleum, Petrochemicals and Advanced Materials, Chulalongkorn University, Thailand, for the financial and instrumental supports of this work. The supports are gratefully acknowledged.
REFERENCES
- P. E. Savage, “Organic chemical reactions in supercritical water,” Chemical Reviews, Vol. 99, pp. 603–621, February 1999.
- M. Watanabe, T. Sato, H. Inomata, R. L. J. Smith, K. Arai, A. Kruse, and E. Dinjus, “Chemical reactions of C1 compounds in near-critical and supercritical water,” Chemical Reviews, Vol. 104, pp. 5803–5821, December 2004.
- Y. Arai, T. Sato, and Y. Takebayashi, “Supercritical fluids: Molecular interaction, physical properties, and new applications,” Springer-Verlag, Berlin, 2002.
- Z. Y. Ding, M. A. Frisch, L. Li, and E. F. Gloyna, “Catalytic oxidation in supercritical water,” Industrial and Engineering Chemistry Research, Vol. 35, pp. 3257–3279, October 1996.
- http://www.michigan.gov/documents/MDCH_Acetonitrile_fast_sheet_approved_4–19–05_122749_7.pdf.
- T. Li, J. Liu, R. Bai, and F. S. Wong, “Membrane-aerated biofilm reactor for the treatment of acetonitrile wastewater,” Environmental Science and Technology, Vol. 42, pp. 2099–2104, March 2008.
- P. Braos-García, D. Durán-Martín, A. Infantes-Molina, D. Eliche-Quesada, E. Rodríguez-Castellón, and A. JiménezLópez, “The effect of thermal treatment under different atmospheric conditions on the catalytic performance of nickel supported on porous silica in the gas-phase hydrogenation of acetonitrile,” Adsorption Science and Technology, Vol. 25, pp. 185–198, April 2007.
- E. Kohyama, A. Yoshimura, D. Aoshima, T. Yoshida, H. Kawamoto, and T. Nagasawa, “Convenient treatment of acetonitrile-containing wastes using the tandem combination of nitrile hydratase and amidase-producing microorganisms,” Applied Microbiology and Biotechnology, Vol. 72, pp. 600–606, September 2006.
- W. R. Killilea, K. C. Swallow, and G. T. Hong, “The fate of nitrogen in supercritical-water oxidation,” The Journal of Supercritical Fluids, Vol. 5, pp. 72–78, March 1992.
- T. Ruamchat, R. Hayashi, S. Ngamprasertsith, and Y. Oshima, “A novel on-site system for the treatment of pharmaceutical laboratory wastewater by supercritical water oxidation,” Environmental Sciences, Vol. 13, pp. 297–304, 2006.
- B. D. Phenix, J. L. Dinaro, J. W. Tester, J. B. Howard, and K. A. Smith, “The effects of mixing and oxidant choice on laboratory-scale measurements of supercritical water oxidation kinetics,” Industrial and Engineering Chemistry Research, Vol. 41, pp. 624–631, February 2002.
- G. E. P. Box, J. S. Hunter, and W. G. Hunter, “Statistics for experimenters: Design, innovation and discovery,” 2nd ed., Hoboken, Wiley-Interscience, NJ, 2005.
- R. E. Bruns, I. S. Scarminio, and B. De Barros Neto, “Statistical design: Chemometrics,” Elsevier, Amsterdam, 2006.
- D. Montgomery, “Design and analysis of experiments,“ 5th ed., Wiley, New York, 2001.

