World Journal of Vaccines
Vol.3 No.2(2013), Article ID:31507,8 pages DOI:10.4236/wjv.2013.32009
Evaluation of “Indigenous Vaccine” Developed Using “Indian Bison Type” Genotype of Mycobacterium avium subspecies paratuberculosis Strain “S5” of Goat Origin in a Sheep Flock Endemic for Johne’s Disease: A Three Years Trial in India*
![]()
1Central Institute for Research on Goats, Mathura, India; 2Southern Regional Research Centre, Central Sheep and Wool Research Institute, Kodaikanal, India.
Email: #shoorvir.singh@gmail.com, #shoorvir_singh@rediffmail.com
Copyright © 2013 Shoor Vir Singh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 6th, 2012; revised January 7th, 2013; accepted January 15th, 2013
Keywords: Paratuberculosis; Indigenous Vaccine; Indian Bison Type; Bharat Merino Sheep; India
ABSTRACT
“Indigenous vaccine” developed from native “Indian Bison Type” strain (“S5”) of Mycobacterium avium subspecies paratuberculosis (MAP) of “goat origin” was first time evaluated in a sheep flock of Bharat Merino breed located in Mannavanur town of Tamil Nadu in South India. Therapeutic efficacy of the vaccine was evaluated for 3 years between 2008 and 2010, on the basis of improvements in productivity (body weights, reproductive efficiency and survivability), physical condition, clinical symptoms (weakness, diarrhea, wool quality), immune response (sero-conversion) and infection load in feces (shedding). After immunization of the flock in 2008, the successive progenies of 112 and 53 lambs born in 2009 and 2010, respectively were vaccinated. Whereas, 40 lambs born to control animals were kept as unvaccinated controls. Though gain in body weights in vaccinated versus controls were not significant in 2008, growth rates were distinctly superior in Ist and IInd generations of vaccinated lambs. Reproductive performance (tupping percent) and survivability of lambs and adult sheep improved significantly. There was overall reduction in yearly morbidity (diarrhea) and mortality rates of the flock in post vaccination years. Shedding of MAP in feces was reduced in vaccinated sheep by 6.2%, 14.3% and 27.3% in 2008, 2009 and 2010 respectively, whereas shedding increased in the control sheep. Seromonitoring of the animals by “indigenous ELISA kit” showed enhanced “flock immunity” in successive generations. “Indigenous vaccine” reduced clinical disease and shedding and improved immunity and productivity of Bharat Merino flock, endemic for Johne’s disease.
1. Introduction
Paratuberculosis or Johne’s disease (JD), caused by Mycobacterium avium subspecies paratuberculosis (MAP), is the most important disease of domestic ruminants and is responsible for substantial economic losses to the livestock industry world-over [1-3]. Reduced milk production, loss of body condition, progressive weight loss, diarrhea, emaciation and death, negatively affect productivity. In recent years, MAP attracted renewed interest due to increasing evidences of association with Inflammatory Bowel Disease (IBD) or Crohn’s disease (CD) in human beings [4-6]. In absence of effective control measures, MAP infection has slowly spreaded in the livestock population throughout the world. The 70% of US dairy herds are infected with JD, costing $200 million to $1.5 billion per year to the dairy industry [2]. Despite high prevalence of disease in the domestic ruminant population of the country [7-10], disease has not received priority for control and eradication. Production losses have neither been estimated nor given cognizance outside developed countries. Different workers [11-13] showed low to very high prevalence of JD in some of the native sheep flocks. Sheep population in the country has shown to be exclusively infected with “Indian Bison Type’ biotype of MAP [14].
Perez [15] and Corpa [16] showed the efficacy of vaccination in sheep, by evaluating number of clinical cases and level of fecal excretion. Both killed or live vaccines induce cellular and humoral immune responses [16,17] and fewer animals develop clinical disease and also reduce excretion of MAP [18]. Hygienic measures and culling of shedders add to the efficacy of vaccination and reports on vaccine failure are rare [19]. In young sheep, vaccine showed reduction in mortality and fecal shedding. Since 2003, Australian sheep industry increasing relied on vaccination for the control of ovine JD [20]. More recently, killed vaccines have shown “therapeutic potential” [21-23]. In India, first “indigenous inactivated vaccine” was developed in 2005 at Central Institute for Research on Goats (CIRG), Makhdoom using native strain “S5” of MAP of goat origin [24]. Strain “S5” of MAP has since been characterized as “Indian Bison Type”—a new biotype of MAP not reported so far in the literature [25]. Evaluation of this “indigenous vaccine” showed high efficacy both as “preventive and therapeutic” vaccine in herds endemic for Johne’s disease [23,24]. However, “indigenous vaccine” has not been evaluated in sheep flocks. This study evaluated “indigenous vaccine” in a sheep flock endemic for ovine Johne’s disease (OJD). Indigenous vaccine was developed using “S5” strain of “Indian Bison type” biotype of MAP of goat origin. Production performance, status of clinical disease, concentration (+4 to +1) of MAP bacilli/gm of feces (shedders), immunological parameters between vaccinated and nonvaccinated control groups of Bharat Merino sheep located down south in the hills of Nilgiris, and Mannavanur block of Tamil Nadu was compared.
2. Materials and Methods
2.1. Experimental Animals and Monitoring Parameters
A flock of 332 sheep (Bharat Merino breed) located at the Southern Regional Research Centre (SRRC) of Central Sheep and Wool Research Institute (CSWRI) at Mannavanur (Tamil Nadu), was vaccinated against Johne’s disease for the first time in January 2008. Bharat Merino is a strain of sheep having 75% of exotic inheritance of Rambouillet/Russian Merino and 12.5% of Nalli/ Chokla and 12.5% of Malpura/Jaisalmere sheep. This sheep strain was evolved at CSWRI, Avikanagar (Rajasthan). During 1987, this strain was introduced in southern sub-temperate climate Kodaikanal in the Nilgiri hills of Tamil Nadu to study their performance. Since establishment of this farm in 1965, animals were provided concentrate ration and grazing in lush green pasture under semi-intensive management on the slopes of Kodaikanal (Palani) hills. Cases of weakness and diarrhea have been frequently reported and confirmed for Johne’s diseases [26]. In this study lambs born to vaccinated ewes were subsequently vaccinated in second (2009) and third (2010) year of vaccination trial. Second and third generation of lambs were taken in the vaccination trial in effort to build the “flock immunity”. Sheep flocks were monitored on health (mortality, morbidity etc.), production (birth weights, body weights, reproductive efficiency etc.), physical condition (diarrhea, weakness, etc), immunological parameters (ELISA titer) and status of shedding of MAP in vaccinated and control groups. Since population of sheep flock in this research station is dynamic the morbidity and mortality due to Johne’s disease was calculated in terms of EADR (Equivalent Average Death rate) [27].
2.2. Vaccine
“Indigenous vaccine” was developed using native “S5” strain and characterized as “Indian Bison type” (a new biotype) of Mycobacterium subsp. paratuberculosis (MAP) of “goat origin”. This biotype has not been reported outside India [25]. “S5” strain was recovered in 1999 from a terminally sick goat which subsequently died of JD in the farm herd of Jamunapari goat breed at Central Institute for Research on Goats (CIRG), Mathura. JD is endemic in the farm herds of the goats of the institute. “Therapeutic potential” of “indigenous vaccine” was evaluated in naturally infected spontaneous cases of JD in goats [23] and as a preventive vaccine in a classical vaccination and challenge trial in young goats [24]. One dose of vaccine contained 2.5 mg dry weight of S5” MAP culture containing 5 × 109 bacilli/ml, in aluminium hydro-oxide gel as adjuvant [24]. Inactivation of MAP bacilli was carried-out in water bath at 72°C for 2 h.
2.3. Vaccination
A total of 262 sheep of Bharat Merino breed were vaccinated and monitored whereas 70 sheep were kept as nonvaccinated control during trial period (2008-2010). In 2008, 97 adult Bharat Merino sheep were vaccinated with 1 ml (2.5 mg/ml) of “indigenous vaccine” subcutaneously in the neck region against Johne’s disease and 30 were kept controls. In 2009, second generation 112 lambs (3 to 6 months) were vaccinated and 25 were left controls and in 2010, 53 third generation lambs (3 to 6 months), were vaccinated and 15 lambs were kept as controls.
2.4. Live Body Weights
Average gain in body weights of the vaccinated and control group of sheep were statistically analyzed using unpaired “t Test” with Welch correction by GraphPad InStat 3.0 software.
2.5. Shedding of MAP in Feces
Shedding of MAP was monitored in fecal samples of serially sampled cohort by microscopic examination of each of samples collected on the day of vaccination (zero day) and after vaccination. Approximately, 2 gram of fecal sample was finely grounded in sterilized pestle and mortar with sterilized distilled water (10 - 12 ml). Finely grounded fecal samples were centrifuged at 4500 RPM for 45 min at room temperature (RT). The supernatant was discarded and middle layer was used to prepare smears for Ziehl Neelsen (ZN) staining for acid-fast bacilli (AFB) indistinguishable to MAP. Fecal samples were graded as positive or negative on the basis of presence and absence of acid fast bacilli indistinguishable to MAP.
2.6. MAP Bacteraemia and Genotyping
2.6.1. DNA Isolation and IS900 PCR
DNA was isolated from the blood samples and subjected to specific IS900 PCR [28]. Briefly, PCR was set in volume of 50 µl, using 1.0 - 5.0 ng template DNA, 5 µl of 10X PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 10 pmol of each primer and 5 U Taq DNA Polymerase. Thermal cycling conditions were: initial denaturation at 94˚C for 3 min, followed by 37 cycles of denaturation at 94˚C for 30 sec, annealing at 64˚C for 30 sec, extension at 72˚C for 1 min, and final extension at 72˚C for 7 min. Presence and yield of the specific PCR product (413 bp) was analyzed by 2% agarose ethidium bromide gel electrophoresis.
2.6.2. IS1311 PCR REA
IS1311 PCR was carried out using M56 and M119 primers as per [29]. Briefly, PCR was be set in volume of 25 µl, using 0.5 - 1.0 ng template DNA, 2.5 µl of 10X PCR buffer, 2.5 mM MgCl2, 0.2 mM dNTPs, 0.6 µmol of each primer and 1 U Taq DNA Polymerase. Thermal cycling conditions were as follows: initial denaturation at 94˚C for 3 min, followed by 37 cycles of denaturation at 94˚C for 30 s, annealing at 62˚C for 30s, extension at 72˚C for 1 min, and final extension at 72˚C for 10 min. Amplicon sizes of 608 bp were considered positive, after separation on 2% agarose gel stained with ethidium bromide. IS1311 PCR REA reaction was carried out in a volume of 30 µl, containing 20 µl positive IS1311 PCR product, 3 µl reaction 10X buffer, and 2 U of each endonuclease HinfI and MseI [29]. The reaction mixture was incubated at 37˚C for 2 hrs. Band patterns will be visualized after electrophoresis on 4% agarose gel with ethidium bromide. Genotype profiles were interpreted as per [30].
2.7. Vaccine Mediated Sero-Conversion
Each of the vaccinated sheep serum samples collected during the study were screened by using “Indigenous ELISA kit”, developed for screening of goats and sheep against JD Infection. Indigenous ELISA test uses semipurified soluble protoplasmic antigen (PPA) prepared from the native isolates of MAP (“S5”) recovered from a terminal case of JD in a Jamunapari goat (CIRG) and has since been characterized as “Indian Bison type” [25], OD values were transformed to S/P ratio [31] and sheep in strong positive and positive category were considered as positive for MAP infection and as sero-converts.
3. Results and Discussion
Of the total 332 sheep for which the post vaccination data was collected from the flock of Bharat Merino sheep, 6, 2 and 4 were suspected of death due to JD in 2008, 2009 and 2010, respectively (Table 1). Monitoring of the flock for Johne’s disease exhibited that though the disease existed in the flock but was under control mainly due to the JD vaccination program which continued for three years (2008 to 2010). This vaccination study was continued for 3 long years using “indigenous vaccine” in either goat or sheep since development of “indigenous vaccine” against JD in 2005. The morbidity due to Johne’s disease was also reduced substantially after vaccination of flock. Other causes of mortality were severe anemia and debility, chronic hepatitis, pneumonia, pulmonary abscess, recurring bloat, pregnancy toxemia, etc., The Equivalent Average Death rate in JD was 0.00234/1000 animal days at risk.
Mean of body weights gained over the period of one year (in year 2008) were males-12.86; females-5.42 and males-12.0; females-5.84 in vaccinated and control groups, respectively (Table 2). Mean of body weights gained over the period of one year (year 2009 in progeny of 2008) were males-9.88; females-4.44 and males-6.0; females-3.91 in vaccinated and control groups, respectively (Table 3). Mean of weight gained up to 120 days
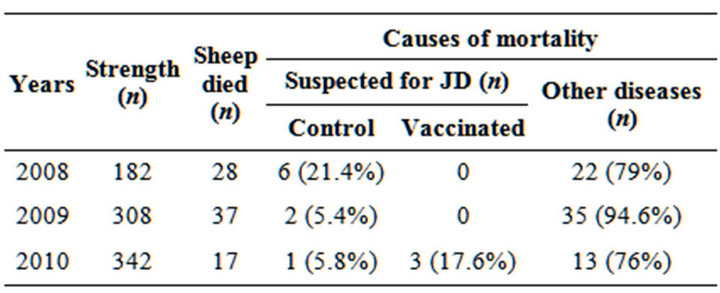
Table 1. Livestock profile and mortality pattern of sheep flocks at SRRC after vaccination.
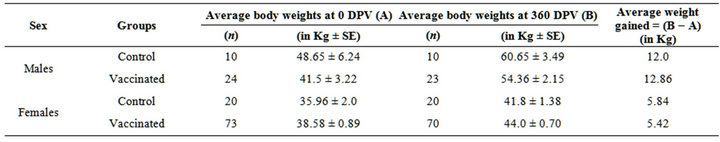
Table 2. Average body weights gained in the year 2008.
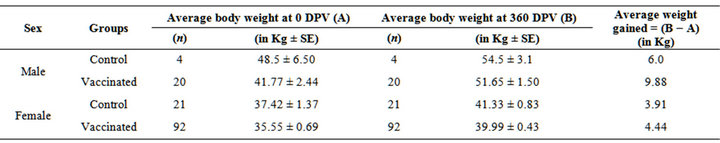
Table 3. Average body weights gained in the year 2009 in the progeny of 2008 vaccinated sheep.
post vaccination (DPV), year 2010 in progeny of 2009 were males-2.06 and females-1.38 and males-0.87 and females-0.67 in vaccinated and control groups, respectively (Table 4). However, there was decline in the body weights in few sheep under both groups which might be due to the unfavorable weather (heavy rainfall) and were under stress. These observations are in agreement to the findings of [24], when they used the same vaccine in goats. However, sheep in vaccinated groups gained more in body weights as compared to control group in terms of body weights and body condition.
Fecal samples profile with respect to shedding of MAP at zero day and 360 DPV showed decreasing trend in shedding of MAP in vaccinated groups. Shedding of MAP was reduced in vaccinated sheep by 6.2%, 14.3% and 27.3% in 2008, 2009 and 2010, respectively; whereas it increased in control sheep (Table 5). Improvement in the control group of sheep was due to reduced contamination of the environment (soil and pasture). Due to good nutrition the control group of animals also started improving as the daily dose of MAP was reduced in these animals. This was clear in the table which showed reduction in shedders with respect to intensity of infection +4 to +1. Reduced environmental shedding by large number of vaccinated sheep helped to improve the environment for other sheep in the control group.
Blood samples collected from vaccinated and control sheep group at pre and post vaccination intervals were screened by IS900 PCR (Figure 1). MAP bacteremia in vaccinated group was reduced to 4.5% and 4.6% in 2009 and 2010, respectively (Table 6). Typing of the IS900 PCR positive DNA samples using IS1311 PCR-REA, from Bharat Merino sheep at Mannavanur showed that animals were infected with “Indian Bison type” biotype (Figure 2), which also justified the use of this “indigenous vaccine” of goat origin against ovine JD, since vaccine strain of MAP was based on “S5” strain, an “Indian Bison Type” biotype of MAP of goat origin. Earlier studies reported presence of “Indian Bison type” genotype of MAP shared by different species, breeds and agro-climatic regions of the country due to high pathogenic nature [14,32].
Due to long distance, remoteness of the location and non-availability of the health scientist at this farm, proper monitoring of serological titers could not be taken up at this farm under the present trial. Of the 137 and 68 sheep screened at pre-vaccination, 26 (18.9%) and 21 (30.8%) were positive for MAP antibody by “indigenous ELISA kit” in 2009 and 2010, respectively (Table 7). However all the sheeps in vaccinated group became sero-converted at different post vaccination intervals (Figure 3).
An ideal vaccine would either generate immunity or help out the animals to contain the infection so there is no horizontal spread. Currently, most of the MAP vaccines use mineral oil adjuvants to evoke more active immune responses [33]. Most of the sheep studies used strain 316F, strain 18 and virulent field strains with oily adjuvant as killed vaccines for the control of Johne’s disease [19]. Vaccination with these strong adjuvants often lead to development of lesions at the site of vaccination [34]. While vaccine invariably result in a strong cellular immune response, it is overlayed with an equally strong humoral response [35].
Vaccine trials showed that there was overall improvement (cessation of passing cow like faeces, reduced morbidity, mortality, 100% tupping percentage, reduced fecal shedding of MAP, all vaccinated sheep were sero-converted (Figure 3), there was no animal reported in sick-
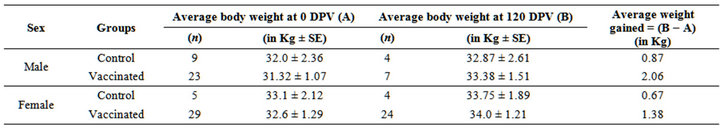
Table 4. Average body weights gained in the year 2010 in the progeny of 2009 vaccinated sheep.
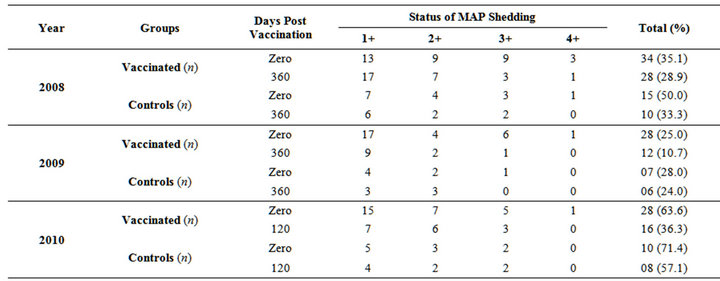
Table 5. Status of MAP shedding in fecal samples of Bharat Merino sheep at pre and post days vaccination (year 2008, 2009 and 2010).
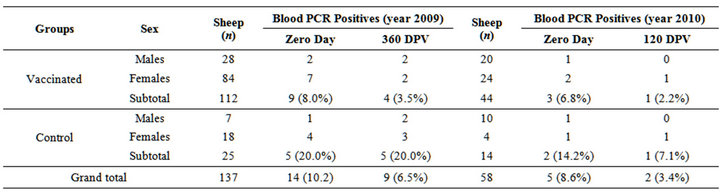
Table 6. Restriction of presence of MAP in the blood samples of Bharat Merino sheep at pre and post days vaccination at SRRC, mannavanur (year 2009 and 2010).
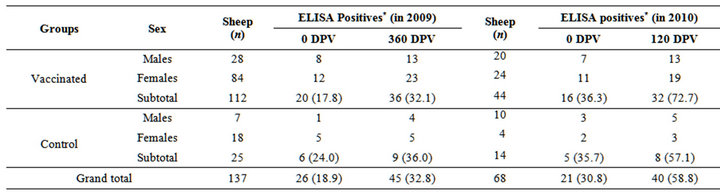
Table 7. Sero-monitoring by indigenous ELISA at different DPV in the year 2009 and 2010.
*Positive and strong positive were taken as positive.
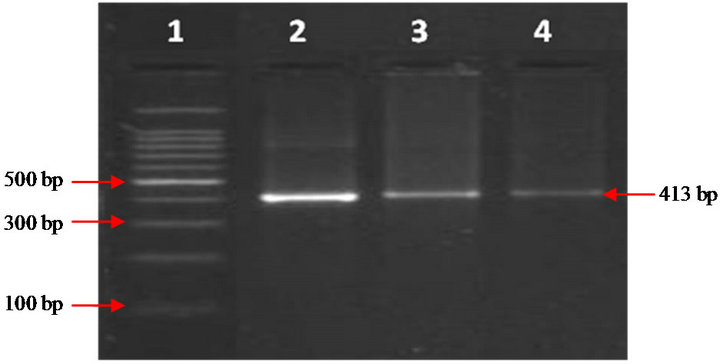
Figure 1. MAP specific amplicons (413bp) by PCR using IS900 specific primers. Lane 1: 100bp ladder, lane 2: Positive control (MAP DNA), lane 3 and lane 4: DNA samples isolated from blood.
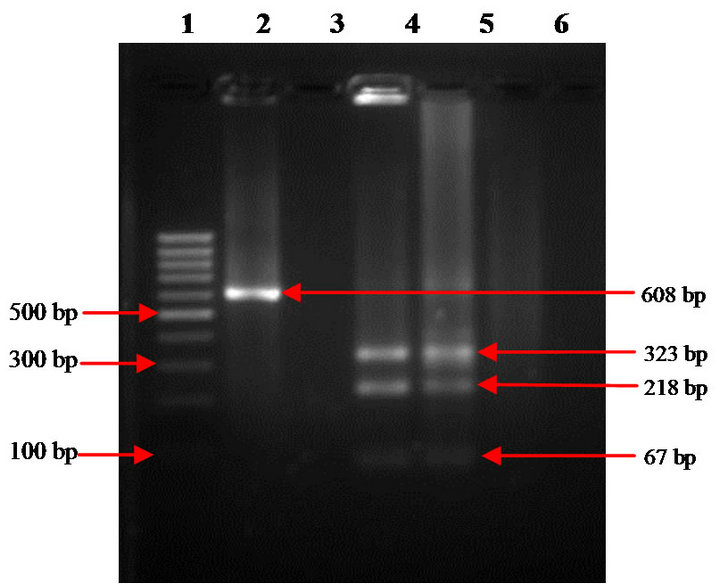
Figure 2. IS 1311 PCR-REA analysis of IS900 positive MAP DNA. Lane 1: 100bp DNA ladder; lane 2: undigested IS1311 PCR product (608 bp); lane 3: Negative control; lane 4: Positive control (MAP DNA); lane 5: Digested DNA sample (Indian Bison Type).
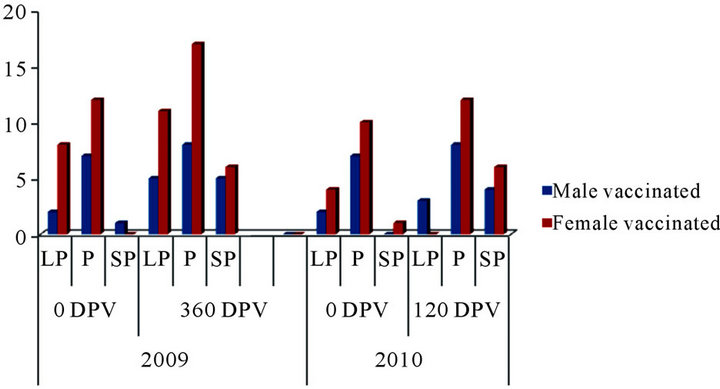
Figure 3. Sero-conversion at different days post vaccination (year 2009 and 2010).
ness, despite inclement weather conditions. Body weights of animals showed increasing trend and improvement in condition of animals after vaccination. Since both “vaccines strain” and MAP biotype prevalent at Mannavanur in Bharat Merino sheep were homologous, along with better nutrition status of flock may be reasons for “long time effect of the vaccine”. The “indigenous vaccine” made from strain of goat origin was equally effective in sheep flock that is located in the Kodai hills of Tamil Nadu and infected with same (Indian Bison Type) genotype. The positive effect of vaccine was seen up to the 1 year and beyond, though monitoring of the body weights on monthly basis was suspended. This is the first flock where lambs born to second generation ewes have been vaccinated. Earlier attempt was also made to save the Jamunapari herd from culling due to JD by treating the spontaneous cases of sub-clinical, clinical and advanced clinical JD with “indigenous vaccine” [23]. After the success of indigenous vaccine trials in goatherds and cattle herd located in the different agro-climatic region of the country, the therapeutic efficacy of a new “indigenous vaccine” prepared from native highly pathogenic “Indian Bison Type” biotype of Mycobacterium avium subspecies paratuberculosis (MAP) of goat origin has been evaluated with respect to control groups of clinical Johne’s disease (JD) in naturally infected Bharat Merino sheep at Mannavanur, Tamil Nadu.
Infection of MAP usually occurs soon after the birth, thus traditionally vaccination has been practiced during the first few weeks of animal life on the basis that protection would be conferred for the first contact with mycobacteria [16]. Due to vaccination, no allergic reactions and abscess formation were observed in sheep whereas vaccinated sheep showed nodule formation (“Take”) at the vaccination site just after 24 - 48 hrs of vaccination. Similar observations have been made by [36], who used same vaccine in goats and cattle, respectively, in North India. Earlier study observed similar vaccination reaction after site (3 - 45 mm) of vaccination as seen in the vaccinated Bharat Merino sheep but in his study the diameter of “take” did not decline with time [37]. Difference in the composition of the vaccines with respect to strain and the adjuvant used varied from vaccine to vaccine in previous studies and use of Aluminium hydroxide gel alone might have apparently contributed to the reduction in size of vaccine nodule formation in the present study.
4. Conclusion
“Indigenous vaccine” made from strain “S5” (Indian Bison Type) of goat origin was equally effective as it significantly reduced morbidity, mortality and shedding of MAP, reduced clinical disease (diarrhoea) and the burden of MAP and enhanced flock immunity and productivity (100% tupping) in the sheep flock naturally infected and endemic for JD. Therefore, indigenous vaccine against Johne’s disease developed at Central Institute for Research on Goats was both “therapeutic vaccine” and “preventive” and helped to cure cases of clinical Johne’s disease in endemically infected sheep and reduced number of cases of clinical JD in vaccinated young lambs.
5. Acknowledgements
Authors are thankful to CSIR, New Delhi for providing the funds and Director, CIRG, Makhdoom for providing the facilities.
REFERENCES
- N. B. Harris and R. G. Barletta, “Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine,” Clinical Microbiology Review, Vol. 14, No. 3, 2001, pp. 489-512. doi:10.1128/CMR.14.3.489-512.2001
- S. L. Ott, S. J. Wells and B. A. Wagner, “Herd-Level Economic Losses Associated with Johnes Disease on US Dairy Operations,” Preventative Veterinary Medicine, Vol. 40, No. 3-4, 1999, pp. 179-192. doi:10.1016/S0167-5877(99)00037-9
- R. D. Bush, P. A. Windsor and J. A. Toribio, “Losses of Adult Sheep Due to Ovine Johne’s Disease in 12 Infected Flocks over a 3-Year Period,” Australian Veterinary Journal, Vol. 84, No. 7, 2006 pp. 246-253. doi:10.1111/j.1751-0813.2006.00001.x
- R. J. Greenstein and M. T. Collin, “Emerging Pathogens: Is Mycobacterium avium subspecies Paratuberculosis Zoonotic,” Lancet, Vol. 364, No. 9432, 2004, pp. 396- 397. doi:10.1016/S0140-6736(04)16781-0
- M. Feller, K. Huwiler, R. Stephan, E. Altpeter, A. Shang, H. Furrer, G. E. Pfyffer, T. Jemmi, A. Baumgartner and M. Egger, “Mycobacterium avium subspecies paratuberculosis and Crohn’s Disease: A Systematic Review and Meta-Analysis,” Lancet Infectious Diseases, Vol. 7, No. 9, 2007, pp. 607-613. doi:10.1016/S1473-3099(07)70211-6
- A. V. Singh, S. V. Singh, P. K. Singh, J. S Sohal and M. K. Singh, “High Prevalence of Mycobacterium avium subspecies paratuberculosis (Indian Bison Type’) in Animal Attendants Suffering from Gastrointestinal Complaints Who Work with Goat Herds Endemic for Johne’s Disease in India,” International Journal of Infectious Diseases, Vol. 15, No. 10, 2011, pp. e677-e683. doi:10.1016/j.ijid.2011.04.013
- G. L. Koul, S. Somvanshi and J. C. Biswas, “Mortality Pattern in Pashmina Goats,” Indian Veterinary Journal, Vol. 65, 1989, pp. 847-49.
- M. M. Sharma, P. S. Loonkar, C. P. Srivastava, A. Maru and S. C. Dubey, “Prevalence and Johne’s Disease (JD), in Sheep and Goats,” Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, Vol. 8, No. 1, 1987, pp. 48-49.
- S. V. Singh, M. Solanki, A. Kumar, P. K. Singh, A. V. Singh, B. Singh and J. S. Sohal, “Comparative Evaluation of Improved ‘Modified Microscopic Test’ with Traditional Microscopy, Indigenous ELISA Kit, Fecal and Blood PCR for the Diagnosis of Mycobacterium avium subs paratuberculosis in Goatherd Endemic for Johne’s Disease,” Research & Reviews: A Journal of Life Sciences, Vol. 1, No. 1, 2011, pp. 8-15.
- P. Kaur, G. Filia, S. V. Singh, P. K. Patil, G. V. P. P. S. Ravi Kumar and K. S. Sandhu, “Molecular Epidemiology of Mycobacterium avium subspecies paratuberculosis: IS900 PCR Identification and IS1311 Polymorphism Analysis from Ruminants in the Punjab Region of India,” Comparative Immunology, Microbiology and Infectious Diseases, Vol. 34, No. 2, 2011, pp. 163-169. doi:10.1016/j.cimid.2010.09.002
- B. N. Tripathi, K. Rajukumar, N. P. Kurade and N. S. Parihar, “Incidence of Mycobacterium paratuberculosis Infection in Goats and Sheep Based on the Fecal Culture,” Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, Vol. 21, No. 1, 1999, pp.72-75.
- T. K. Goswami, V. Tiwari, A. Gupta, R. Mall and G. C. Ram, “Seroprevalence of Mycobacterium paratuberculosis in an Organized Pashmina Goat Farm,” Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, Vol. 21, No. 2, 2000, pp. 132-135.
- P. K. Singh, S. V. Singh, A. V. Singh and J. S. Sohal, “Screening of Tissues and Serum by Culture, PCR and ELISA for the Detection of Mycobacterium avium subspecies paratuberculosis from Cases of Clinical Ovine Johne’s Disease in Farmer’s Flocks,” Indian Journal of Animal Sciences, Vol. 78, No. 10, 2008, pp. 1052-1056.
- A. V. Singh, S. V. Singh, P. K. Singh and J. S Sohal, “Genotype Diversity in Indian Isolates of Mycobacterium avium subspecies paratuberculosis Recovered from Domestic and Wild Ruminants from Different Agro-Climatic Regions,” Comparative Immunology, Microbiology and Infectious Diseases, Vol. 33, No. 6, 2010, pp. e127-e131. doi:10.1016/j.cimid.2010.08.001
- V. Perez, J. F. Garcia Marin and J. J. Badiola, “Description and Classification of Different Types of Lesion Associated with Natural Paratuberculosis Infection in Sheep,” Journal of Comparative Pathology, Vol. 114, No. 2, 1996, pp. 107-122. doi:10.1016/S0021-9975(96)80001-6
- J. M. Corpa, V. Peerez and J. F. Garcıa Marın, “Differences in the Immune Responses in Lambs and Kids Vaccinated against Paratuberculosis, According to the Age of Vaccination,” Veterinary Microbiology, Vol. 77, No. 3-4, 2000, pp. 475-485. doi:10.1016/S0378-1135(00)00332-1
- R. A. Juste, J. F. Garcia-Marin, B. Peris, C. S. de Ocariz and J. J. Badiola, “Experimental Infection of Vaccinated and Non-Vaccinated Lambs with Mycobacterium paratuberculosis,” Journal of Comparative Pathology, Vol. 110, No. 2, 1994, pp. 185-194. doi:10.1016/S0021-9975(08)80189-2
- R. S. Merkal, “Paratuberculosis: Advances in Cultural, Serologic and Vaccination Methods,” Journal of the American Veterinary Medical Association, Vol. 184, No. 8, 1984, pp. 939-943.
- F. Bastida and R. A. Juste, “Paratuberculosis Control: A Review with a Focus on Vaccination,” Journal of Immune Based Therapies and Vaccines, Vol. 9, No. 1, 2011, p. 8. doi:10.1186/1476-8518-9-8
- J. Eppleston, P. A. Windsor and R. Whittington, “The Impact of Leaving Merino Wether Lambs Unvaccinated on Shedding of Mycobacterium avium subsp. paratuberculosis in Flocks Vaccinated for Ovine Johne’s Disease,” Australian Veterinary Journal, Vol. 89, No. 1, 2011, pp. 38-40. doi:10.1111/j.1751-0813.2010.00651.x
- E. Spangler, L. E. Heider, S. Bech-Nielsen and C. R. Dorn, “Serologic Enzyme-Linked Immunosorbent Assay Responses of Calves Vaccinated with a Killed Mycobacterium paratuberculosis Vaccine,” American Journal of Veterinary Research, Vol. 52, No. 8, 1991, pp. 1197- 1200.
- R. M. Pitchappan, V. Brahmajothi, K. Rajaram, P. T. Subramanyam, K. Balakrishnan and R. Muthuveeralakshmi, “Spectrum of Immune Reactivity to Mycobacterial (BCG) Antigens in Healthy Hospital Contacts in South India,” Tubercle, Vol. 72, No. 2, 1991, pp. 133-139. doi:10.1016/0041-3879(91)90040-Y
- S. V. Singh, P. K. Singh, A. V. Singh, J. S. Sohal and M. C. Sharma, “Therapeutic Effects of a New ‘Indigenous Vaccine’ Developed Using Novel Native ‘Indian Bison Type’ Genotype of Mycobacterium avium subspecies paratuberculosis for the Control of Clinical Johne’s Disease in Naturally Infected Goatherds in India,” Veterinary Medicine International, 2010, Article ID: 351846.
- S. V. Singh, P. K. Singh, A. V. Singh, J. S. Sohal, V. K. Gupta and V. S. Vihan, “Comparative Efficacy of an Indigenous ‘Inactivated Vaccine’ Using Highly Pathogenic Field Strain of Mycobacterium avium subspecies paratuberculosis ‘Bison Type’ with a Commercial Vaccine for the Control of Capri-Paratuberculosis in India,” Vaccine, Vol. 25, No. 41, 2007, pp. 7102-7110. doi:10.1016/j.vaccine.2007.07.054
- J. S. Sohal, N. Sheoran, K. Narayansamy, V. Brahmachari, S. V. Singh and S. Subhod, “Genomic Analysis of Local Isolate of Mycobacterium avium subspecies paratuberculosis,” Veterinary Microbiology, Vol. 134, No. 3-4, 2009, pp. 375-382. doi:10.1016/j.vetmic.2008.08.027
- O. R. VinodhKumar, N. Swain, S. Rajapandi and S. Parthasarathy, “Paratuberculosis in Bharat Merino and Avikalin Sheep Farm of Kodai Hills,” Indian Journal of Small Ruminants, Vol. 13, No. 1, 2007, pp. 98-99.
- O. R. VinodhKumar, C. P. Swarnkar, A. K. Shindae and D. Singh, “Clinical and Haemato-Biochemical Studies of Urolithiasis in Weaner Lambs,” African Journal of Agricultural Research, Vol. 5, No. 15, 2010, pp. 2045-2050.
- P. K. Singh, S. V. Singh, H. Kumar, J. S. Sohal and A. V. Singh, “Diagnostic Application of IS900 PCR Using Blood as a Source Sample for the Detection of Mycobacterium avium subspecies paratuberculosis in Early and Subclinical Cases of Caprine Paratuberculosis,” Veterinary Medicine International, Vol. 2010, 2010, Article ID: 748621. doi:10.4061/2010/748621
- I. Sevilla, S. V. Singh, J. M. Garrido, G. Aduriz, S. Rodriguez, M. V. Geijo, R. J. Whittington, V. Saunders, R. H. Whitlock and R. A. Juste, “Molecular Typing of Mycobacterium avium subspecies paratuberculosis Strains from Different Hosts and Regions,” Scientific and Technical Review (OIE), Vol. 24, No. 3, 2005, pp. 1061-1066.
- R. J. Whittington and E. S. Sergeant, “Progress towards Understanding the Spread, Detection and Control of Mycobacterium avium subsp paratuberculosis in Animal Populations,” Australian Veterinary Journal, Vol. 79, No. 4, 2001, pp. 267-278. doi:10.1111/j.1751-0813.2001.tb11980.x
- M. T. Collins, “Interpretation of a Commercial Bovine Paratuberculosis Enzyme-Linked Immunosorbent Assay by Using Likelihood Ratios,” Clinical and Diagnostic Laboratory Immunology, Vol. 9, No. 6, 2002, pp. 1367- 1371.
- S. V. Singh, A. V. Singh, S. Gupta, A. S. Rajindran, N. Swain, P. K. Singh, H. Singh, J. S. Sohal and N. Kumar, “Interspecies Sharing of ‘Indian Bison Type’ a Novel Predominant Genotype of Mycobacterium avium subspecies paratuberculosis between Naturally Infected and Endemic Flocks of Bharat Merino Sheep and a Colony of Rabbits (Oryctolagus cuniculus) Raised on the Same Ecosystem in South India,” Research and Reviews: A Journal of Life Sciences, Vol. 2, No. 3, 2012, pp. 1-8.
- D. J. Begg and J. F. T. Griffin, “Vaccination of Sheep against M. paratuberculosis: Immune Parameters and Protective Efficacy,” Vaccine, Vol. 23, No. 42, 2005, pp. 4999-5008. doi:10.1016/j.vaccine.2005.05.031
- S. V. Singh, P. K. Singh, M. K. Singh, A. V. Singh and J. S. Sohal, “Therapeutic Potential of Johne’s Disease Vaccine: A Follow up Post Vaccination Study in a Goatherd of Endangered Jamunapari Breed, Naturally Infected with Mycobacterium avium subspecies paratuberculosis,” International Journal of Livestock Production, Vol. 1, No. 2, 2011, pp. 192-204.
- J. F. Garcia Marin, J. Tellechea, M. Gutierrez, J. M. Corpa and V. Perez, “Evaluation of Two Vaccines (Killed and Attenuated) against Small Ruminant Paratuberculosis,” 6th International Colloquium on Paratuberculosis, Melbourne, 14-18 February 1999, pp. 234-241.
- A. K. Srivastav, “Prophylactic and Therapeutic Effect of Johne’s Disease Vaccine in Cattle,” M.V.Sc. Thesis, Pandit Deen Dayal Upadhyaya Pashu Chikitsa Vigya Vishwavidyalya Evam Go Anusandhan Sansthan, Mathura, 2010, pp. 1-89.
- L. Reddacliff, J. Eppleston, P. Windsor, R. Whittington and S. Jones, “Efficacy of Killed Vaccine for the Control of Paratuberculosis in Australian Sheep Flocks,” Veterinary Microbiology, Vol. 115, No. 1-3, 2006, pp. 77-90. doi:10.1016/j.vetmic.2005.12.021
NOTES
*Conflict of interest: No conflict of interest to declare.
#Corresponding author.

