World Journal of Vaccines
Vol.2 No.1(2012), Article ID:17224,10 pages DOI:10.4236/wjv.2012.21005
Rabies DNA Vaccines: Current Status and Future
![]()
Department of Neurovirology, National Institute of Mental Health & Neurosciences, Bangalore, India.
Email: *mshampur@gmail.com
Received November 16th, 2011; revised January 8th, 2012; accepted January 25th, 2012
Keywords: Rabies; Plasmid; DNA Vaccine; Glycoprotein; Prophylaxis
ABSTRACT
Rabies continues to be a significant cause of human and animal mortality, despite the availability of safe and effective prophylactics. Apart from limited access, the cost and complex schedules of rabies biologics often impact on the success of post-exposure prophylaxis in humans in the endemic countries. Mass vaccination of dogs, critical in rabies control, often fails to achieve its goal in rabies-endemic countries due to logistic, animal and vaccine-related issues. DNA vaccination has been proposed as a cheaper and efficient strategy for rabies prophylaxis, and its feasibility has been demonstrated in a number of animal models including companion animals, since 1994. Despite the proven efficacy, the technology suffers from a few drawbacks that limit its large-scale application, such as delayed and weaker immune responses in larger animals. Recent advances in the field of vector design and delivery hold promise for enhancement of rabies DNA vaccine efficacy. The present article provides an overview of developments in the field of DNA rabies vaccination and its future prospects.
1. Introduction
With an estimated global mortality of about 50,000 per year, rabies has been identified as one of the major causes of human death from infectious diseases. According to estimates [1], one person dies due to rabies every 15 minute, and 300 people are exposed to the risk, during the same period. More than 99% rabies deaths occur in the developing countries of Asia and Africa. The disease manifests itself as a progressive fatal encephalomyelitis, and results from infection with viruses of the genus Lyssavirus in the family Rhabdoviridae. Infection is usually acquired from transcutaneous or mucosal exposure to virus-laden saliva of a rabid animal. All warmblooded animals are susceptible, though companion animals, especially dogs, constitute the major vector in most developing countries [2].
Limited access to healthcare facilities and the high costs and complex schedules of rabies biologics often hamper human rabies prophylaxis in the developing countries. The cell culture vaccines, though 100% effective when combined with appropriate wound care and use of immunoglobulins, are expensive, require multiple doses over at least 3 weeks, and demand cold chain maintenance. Canine rabies control is critical in prevention of human rabies in the endemic countries; but faces greater challeges—logistics of mass vaccination drivesever-expanding population of unowned dogs, difficulties in locating the dogs for repeat doses, disrespect of vaccine cold chain, poor immune responses in malnourished and sick animals etc. [3]. A cheaper, easily producible vaccine that requires single or a few doses, and reasonably stable at room temperature would be highly desirable in endemic countries, at least for veterinary vaccination. In this context, a DNA vaccine could be a suitable option.
2. DNA Vaccines—The Beginnings
DNA vaccines are bacterial plasmids constructed to express an encoded protein following in vivo administration and subsequent transfection of cells [4]. Tang et al. first reported the immunogenic use of DNA, by demonstrating the production of a human growth hormone (hGH) and human α-1 antitrypsin (hAAT)-specific antibodies following injection of hGH DNA into mouse skin [5]. Since then, DNA vaccines have shown promising results in a number of trials for prophylaxis of bacterial, viral, parasitic, autoimmune and neoplastic diseases. DNA vaccines have made significant strides in veterinary practice, wherein four DNA vaccines have been licensed recently, including those against West Nile virus infection in horses (licensed in USA), Infectious Hematopoietic Necrosis Virus in Salmon (licensed in Canada), melanoma cancer of dogs (conditionally licensed in USA) and Growth Hormone Releasing Factor therapy for pigs(licensed in Australia) [4].
Several facts explain the interest in a DNA-based rabies vaccine approach:
1) Protection from rabies is commensurate with the presence of adequate amounts of virus neutralizing antibodies, principally targeted against the rabies virus glycoprotein. The tools of recombinant DNA technology allow facile cloning of the glycoprotein gene into suitable expression vectors which mediate efficient in vivo expression of glycoprotein.
2) Nanogram amounts of a plasmid-encoded protein in transfected cells can generate high-affinity antibodies [6]. In situ production of the protein within transfected cells facilitates native post-translational modifications and obviates the issues associated with protein purification in vitro.
3) DNA vaccines provide unique approaches for expanding the spectrum of immune responses, such as by employing multiple or modified antigens and targeted delivery to specific cell types or locations.
4) Remarkable thermal stability of plasmids (over 50˚C) may be of particular advantage in tropical climates.
5) Generic nature of the production and purification processes of plasmid vaccines reduces cost and facilitates easier product development, compared to conventional vaccines.
A DNA rabies vaccine, in its simplest embodiment, is a eukaryotic expression vector containing the rabies virus glycoprotein gene, under a suitable promoter. Several choices exist for expression vectors, and the most common ones employed in the vaccine studies so far are listed in Table 1. The glycoprotein sequences of Pasteur Virus (PV), Challenge Virus Standard (CVS), EvelynRokitnicki-Abelseth (ERA), or street virus isolates have been used by different investigators who evaluated DNA rabies vaccines. Also, significant progress has been made in the design of vectors suited for gene delivery in the recent years [7,8].
Earlier Studies
In the pioneering study, Xiang et al. (1994) reported production of rabies virus neutralizing antibody in female C3H/He mice immunized with pSG5rab.gp vector encoding the full-length glycoprotein gene of ERA strain [9]. Mice immunized thrice with 150 µg of the vector on days 0, 21 and 35 developed low but detectable levels of antibodies after the first dose, which further increased with the boosters. Cytotoxic and helper T-cell responses and complete protection of the immunized mice against a lethal rabies virus challenge confirmed the feasibility of the approach.
Recognizing that promoters differ in tissue specificity and in transcription efficiency, the authors later evaluated the immunogenicity of the vector encoding the glycolprotein placed under CMV promoter [10]. The modified vector induced comparable immune responses to the unmodified one. The vaccine induced long-term protective immune responses and no anti-DNA antibodies were
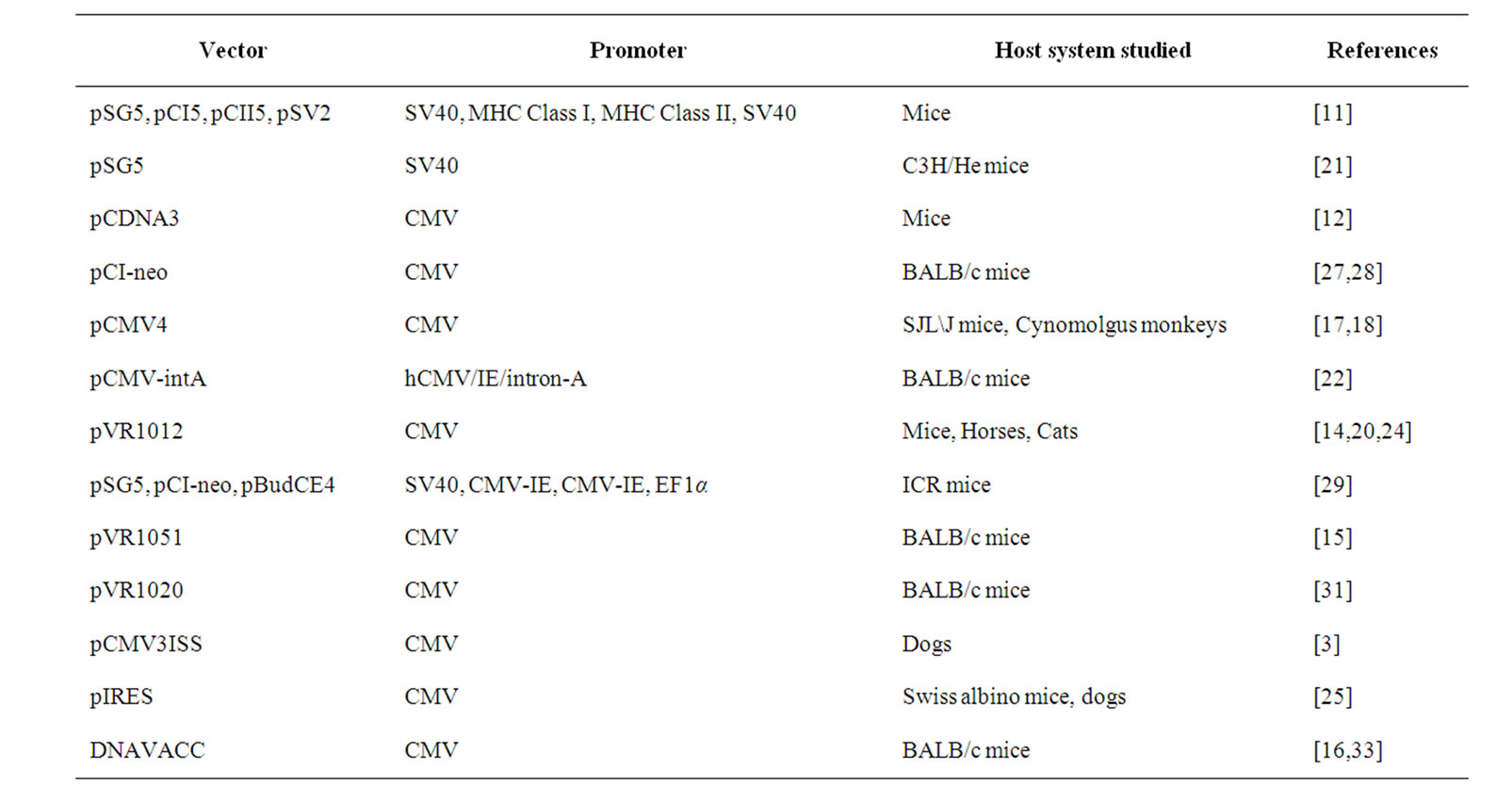
Table 1. Common vectors used in rabies DNA vaccines.
observed in the host.
The need to enhance the immune responses to genetic vaccination was recognized soon after the initial successes in mouse models. A subsequent study investigated the effects of co-administration of vectors encoding mouse Granulocyte Macrophage Colony Stimulating Factor (GMCSF) or interferon-γ (IFN-γ), on the immune responses to a plasmid vector encoding full-length glycoprotein gene, placed under SV40 promoter [11]. The vector produced a slowly increasing antibody response that peaked about 10 - 12 weeks after a single inoculation and persisted for a period of 11 months. Co-inoculation of GM-CSF produced a transient but substantially higher antibody response that eventually declined to low levels. IFN-γ encoding plasmid produced a slight but consistent decrease in the antibody and T-helper cell responses. The immune responses to vectors with the glycoprotein gene under MHC class I or class II promoters, separately, and with or without co-administration of an IFN-γ plasmid, were also studied. Antibody responses comparable to those induced by the SV-40 promoter-controlled glycoprotein construct were observed in mice immunized with the vector having the G gene under MHC class I promoter. Co-immunization of the IFN-γ plasmid reduced the antibody and T-helper cell responses. The immune responses were significantly weaker in the groups immunized with constructs having G gene under MHC class II promoter (thought to have limited the protein expression to cell types such as macrophages, dendritic cells and B-cells). It seems likely that the MHC class II promoter produces a basal immune response that can be augmented by antigen release from other transfected cell types. Co-immunization with the IFN-γ encoding plasmid did not inhibit the low-level immune response from this vector, indicating cell-type specificity in immune downregulation mediated by this cytokine.
Comparison of intramuscular and intradermal routes of vaccination was made in a study that employed a plasmid construct encoding the full-length glycoprotein gene of CVS strain of rabies virus, in BALB/cByJ mice [12]. Two-to-three fold increase of virus neutralizing antibody titers was seen following intramuscular than intradermal delivery. The study also provided the first evidence for enhancement of immunogenicity of DNA rabies vaccines using chemical adjuvants. Pre-treatment of the muscle with snake cardiotoxin 48 hours before immunization produced a 5-fold increase in the antibody responses, compared to no pre-treatment. Seroconversion was observed in 100% of immunized mice, and all survived a subsequent virus challenge. Cardiotoxin-mediated enhancement of antibody responses was seen even at a low DNA dosage of 10 µg. Significant enhancement of neutralizing antibody titres was also seen upon intradermal delivery alongwith monophosphoryl lipid A (MPL), but not following intramuscular immunization. The antisera generated following intradermal immunization with 1 µg of the plasmid constructs exhibited 100% neutralization of a global spectrum of rabies virus variants and CVS and ERA strains.
3. Use of Chemical Adjuvants to Enhance Vaccine Efficacy
The adjuvanting potential of MPL on rabies vaccination via intramuscular and intradermal routes and gene-gun delivery was evaluated in a study [13]. Primary vaccination with DNA by intradermal route was found to confer 100% serocoversion, whereas the intramuscular route required a booster to achieve this. Higher and sustained virus neutralizing antibody titres were observed upon initial intramuscular and intradermal immunization with MPL-adjuvanted DNA, than with DNA alone. However, antibody titres decreased following booster doses. Gene gun-delivery of MPL-adjuvanted DNA was found to produce slightly higher antibody responses, which improved after booster doses of adjuvanted or plain DNA.
Co-administration of a glycoprotein-encoding plasmid with aluminum phosphate [Adju-Phos® (Superphos) at 1 mg/mL] or with the cationic lipid DMRIE-DOPE [N-(1- (2,3-dimyristoyloxypropyl)-N,N-dimethyl-(2-hydroxyethyl) ammoniumbromide/dioleoyl phosphatidylethanolamine] was evaluated in a study performed on equines [14]. A seroconversion rate of 75% was observed following a single dose, and an early onset and higher mean titres of rabies virus neutralizing antibody were seen upon immunization alongwith Adju-Phos. Immunization alongwith DMRIE-DOPE produced 100% seroconvertsion, as early as 14th day after first dose. A stronger and sustained antibody response was seen upon adjuvanting with DMRIE-DOPE. A relatively low dose of 200 µg of plasmid produced protective immunity.
Another study evaluated the immune responses of a DNA rabies vaccine formulated with two different cationic lipid preparations, viz., DMRIE-DOPE and VaxfectinTM [an equimolar mixture of VC1052 ((±N-(3- aminopropyl)-N,N-dimethyl-2,3-bis(myristyloleyloxy)-1propaminium bromide) and DPyPE (Diphytanoylphosphatidyl-ethanolamine) [15]. A greater antibody response was observed with the use of DMRIE-DOPE at a molar ratio of 4:1 than 2.5:1. Mice immunized with the pDNA: VaxfectinTM formulation produced greater virus neutralizing antibody titres than with pDNA:DMRIE-DOPE at either ratios, and achieved 100% seroconversion even with a pDNA dose of 2 µg.
A recent study evaluated Emulsigen® as an adjuvant for a rabies DNA vaccine in a mouse model [16] (discussed later).
4. Delivery via Gene Gun
Gene gun-particle mediated delivery of plasmid constructs for rabies vaccination was reported in 1998 [17]. Gene gun delivery of 2 µg of the plasmid coated onto 2.6 µm gold beads produced 10-fold higher titres of virus neutralizing antibody in SJL\J mice, compared to those elicited by 0.95 µm beads, at 60 and 90 days after the first injection. No seroconversion was seen with intramuscular delivery of the same amount of plasmid. Thirty and 10-fold increases in antibody titres were observed in the respective groups upon an identical booster dose on day 90. Sustained levels of antibody titres > 0.5 IU/mL were observed as late as 300 days in the group immunized with 2.6 µm beads, and all animals in this group survived a lethal viral challenge at day 315. The study suggested that delivery using 2.6 µm beads facilitated closer positioning of the plasmid to the cells, enhancing in vivo expression, antigenic presentation and persistence.
The utility of a single-dose intramuscular or gene-gun delivery was evaluated in a study employing Macaca fascicularis (Cynomolgus) monkeys [18]. Animals were immunized intramuscularly (with a single dose of 100, 500 or 1000 µg of DNA), or via gene gun above axillary and inguinal lymph nodes (with a total of 8, 40 or 60 µg of DNA) using 2.1 µm gold beads. Four out of 6 animals seroconverted by day 7 in the gene gun group, and all by day 60. Only 2 animals in the intramuscular group developed antibodies. Antibody titers were higher in the gene gun group than in the intramuscular group. DNA doses of 500 or 1000 µg induced seroconversion in about 75% animals upon intramuscular vaccination, whereas no dose-dependency was seen with gene gun immunization. Five out of 6 animals survived a viral challenge on day 375 post-vaccination in the intramuscular group, and only 3 out of 5 in the gene gun group. Findings from the study clearly show the requirement of superior DNA vaccination regimens to be effective in rabies prophylaxis of larger animals.
A later study compared the immune responses to DNA vaccination via gene gun delivery, and needle-based intradermal delivery into ear pinnae, against those produced by intramuscular delivery of HDCV, in Macaca fascicularis (Cynomolgus) monkeys [19]. Immunizations were done with either a single dose of 0.5 mL of HDCV, 50 µg of DNA vaccine needle-delivered into ear pinnae, 20 µg of DNA delivered into ear pinnae via gene gun, or 40 µg of DNA delivered above axillary and inguinal lymph nodes. Seroconversion was noticed by day 7 in all animals vaccinated with HDCV, and one animal immunized via gene gun above lymph nodes. The antibody titers increased by day 14, but were undetectable in the animals immunized intradermally via needle, until day 21. Gene gun vaccination above lymph nodes and into ear pinnae induced neutralizing antibodies atleast 14 days earlier compared to needle delivery into ear pinnae. High titres of antibodies were observable in the HDCVand gene gun-vaccinated animals even after 420 - 700 days. Following booster doses, 100% seroconversion was seen in all groups by day 7. Antibody levels increased following each booster, and were maintained upto 308 - 588 days. Multi-site delivery with the involvement of local antigen-presenting cells such as macrophages, dendritic cells and keratinocytes could have enhanced the antibody responses.
5. Combination Rabies Vaccine—Achieving Prime and Boost Together
A novel cost-effective vaccination strategy involving coinoculation of DNA rabies vaccine (DRV) and inactivated rabies virus vaccine has also been reported [20]. A Combination Rabies Vaccine (CRV) prepared by mixing 100 µg of a plasmid construct with different dilutions of PVRV (an inactivated rabies virus vaccine) was evaluated against PVRV, or the DNA, given alone. A formulation consisting of DRV with PVRV625 produced higher levels of rabies virus neutralizing antibodies than that induced by separate immunizations with either. CRV afforded 100% protection to mice against peripheral and intracerebral rabies virus challenge, whereas the corresponding cell culture vaccine dilution and DRV provided only partial protection. A higher anamnestic antibody response was observed with CRV than DRV. Five doses of vaccine on days 0, 3, 7, 14 and 28 produced a further enhancement in anamnestic antibody response than a 2- dose schedule. The experiments were also performed in cattle. The potency of vetCRV100x was observed to be 2- fold higher than that of undiluted veterinary rabies vaccine. However, optimal immune responses required incorporation of protein-based vaccine, which was a drawback.
6. Co-Delivery of Cytokine Genes to Enhance Immune Responses to DNA Rabies Vaccines
Plasmids encoding cytokines (known to recruit immune cells such as dendritic cells) and TRANCE [Tumor Necrosis Factor (TNF)-related activation induced cytokine, a member of the cytokine family] were evaluated as adjuvants for rabies DNA vaccines [21]. Immune responses were studied in C3H/He mice following intramuscular administration of 10 µg of a plasmid expressing the fulllength G gene, or a truncated, secreted form of it, alongwith the plasmids encoding each of the chemokines or TRANCE. None of the genetic adjuvants enhanced the G-specific antibody responses to the full-length glycolprotein. MIP-1α, TRANCE, RANTES and MCP-1 caused a slight decrease in the humoral responses. A marginal increase was observed in the antibody responses to the plasmid encoding the truncated, secreted form of G, upon co-inoculation with vectors encoding MIP-1β, TRANCE, MCP-1 or RANTES. Co-administration of MCP-1, TRANCE and MIP-1β also enhanced the production of IL-2, a Th0 cytokine, while IP-10 and MIP-1α did not. An increase in IgG1/IgG2a ratio of antibodies was observed upon co-administration of the plasmid encoding fulllength G with those encoding IP-10 or MIP-1α, whereas a decrease was seen with co-delivery of RANTES and MIP-1β. Also, the chemokine adjuvants except MCP-1 caused a slight increase in IgG1/IgG2a ratio of antibodies to the plasmid encoding the secreted form of G. It was concluded that chemokines did not have a significant influence on DNA-evoked immune responses. Findings fom the study suggested that genetic adjuvants may not be able to influence the early events of dendritic cell maturation. Activation of naϊve lymphocytes could still be effected by the vector-encoded antigen, by crosspresentation of antigen produced by the other transfected cell types.
7. Rabies DNA Vaccination of Companion Animals (Dogs, Cats, Cattle and Horses)
In a study evaluating the utility of DNA rabies vaccines in companion animals, Beagle dogs and cats were immunized intramuscularly or intradermally with a glycoprotein-encoding plasmid (100 µg/dose). All the dogs immunized intramuscularly showed seroconversion after the first dose, and showed titres > 1:1400 after a booster on day 55. Intradermal scarification produced successful seroconversion in only 50% of animals and lower titres of neutralizing antibodies. The high initial titres in the intramuscular group were maintained even after 289 days, whereas the titres dropped to non-protective levels in the intradermal groups. In contrast, intramuscular immunization with the same amount of DNA resulted in lesser seroconversion frequency and lower mean titres in cats. Efficacy was found to increase when the DNA dose was increased to 300 µg. A four-fold increase in the antibody titres was observed in the intradermally immunized cats, over a period of 7 months following a booster dose. The study suggested that intradermal route is superior in eliciting better immune responses in cats.
It is important that DNA rabies vaccines should be able to induce optimal immune responses in animals under field conditions. Addressing this concern, a study evaluated the immunogenicity and efficacy of a singledose DNA vaccine versus one or two injections of a cell culture vaccine [3]. Under experimental conditions, intramuscular administration of 800 µg or 200 µg of DNA produced weaker and short-lived antibody responses in dogs (0.8 IU/mL by day 35; 0.1 - 0.4 IU/mL by day 90). Intradermal administration using a jet injector produced an early, high-titred and long-lasting antibody response (19 IU/mL at day 35; 6 - 10 IU/mL for upto 4 years) after a single dose. In contrast, the cell culture vaccine induced initial high titres, which declined progressively to nonprotective levels (0.6 IU/mL at day 90; 0.1 IU/mL at 4 years). Full protection against a peripheral viral challenge was observed in dogs immunized with a single intradermal injection of 200 µg of the plasmid vaccine and even when the intradermal vaccine dose was reduced to 45 µg. Following vaccination, the mean titres in the groups receiving 100 or 200 µg DNA vaccine on days 60 were 5 and 3.8 IU/mL, as against 4 IU/mL in dogs immunized with cell culture vaccine. The corresponding titres at day 180 were 2.7, 3.1 and 1.7 IU/mL for the three groups. One year after the vaccination, the titres were 2.2, 1.86 and 0.86 IU/mL, respectively. There was a gradual decline of the titres to 1.2 IU/mL in the DNA vaccine groups by day 600, whereas that in the cell culture vaccine group remained stable at 0.8 IU/mL.
A recent study evaluated the immune responses in cats to intramuscular, intradermal and intranasal delivery of a plasmid vector encoding glycoprotein gene of a Mexican rabies virus isolate [23]. Creole cats were immunized with 100 µg of the plasmid, and a similar booster was given on day 30. Intradermal route was found to be the most effective, achieving seroconversion rate of 75% by day 15. An antibody titre of > 3 IU/mL was noted on day 30, which increased following the booster dose and was maintained at high levels for 180 days. Intranasal immunization produced acceptable levels of neutralizing antibodies (>0.5 IU/mL). In contrast, the intramuscularly immunized cats showed only minimal levels of neutralizing antibodies, even after the booster dose. Upon challenge with CVS strain on day 200, 100% survival was noted in the intradermally immunized cats, as against 50% and 0% in the intranasal and intramuscular groups, respectively.
Biswas et al. evaluated the immunogenicity of a Combination Rabies Vaccine (discussed earlier) in cattle [20]. The vaccine preparations evaluated include 100-, 300- or 600-fold dilutions of a cell culture veterinary rabies vaccine, combination of each of these dilutions with 100 µg of a DNA rabies vaccine, and the DNA vaccine alone. Two doses of the formulations were given on days 0 and 14. The combination rabies vaccine induced higher levels of neutralizing antibodies, than the DNA rabies vaccine. The potency of the combination rabies vaccine was found to be directly proportional to the quantity of the cell culture vaccine contained in it.
The efficacy of plasmid preparations containing various proportions of supercoiled and open circular isoforms was evaluated in a study [24]. Groups of SPF cats were immunized intramuscularly with 50 µg of plasmid preparations containing 81%, 70%, 48% and 20% supercoiled isoform. Significant titres of neutralizing antibodies were detected by day 14 in all groups. No correlation was observed between the level of supercoiled isoforms in the immunogen and the antibody titres at days 14 and 21 post-immunization. The plasmid preparations were found to offer 100% protection against a virulent virus challenge, provided the level of supercoiled isoforms was atleast 48%. Significant Th-cell responses were demonstrated in groups receiving plasmid preparation containing 81% and 70% supercoiled isoforms, at 3 weeks postvaccination. The study suggested that relaxed isoforms of the plasmid are less efficient than the supercoiled forms in inducing protective immune responses, and that atleast 70% of the plasmid isoforms be in the form of supercoiled molecules for optimal immune responses.
A distinct advantage of DNA vaccination is the potential for delivery of multiple antigens in the same vector construct. Such vaccines hold the potential for reduced number of immunizations and a wider spectrum of protection against several pathogens. In this context, a study investigated the immunogenicity of a bicistronic expression vector (pIRES) encoding rabies virus glycoprotein alone, or in combination with VP2 gene of canine parvovirus [25]. Virus neutralizing antibodies to both the pathogens were detected in the immunized animals following a single intramuscular dose of 100 µg. Successful seroconversion was seen in dogs immunized with the constructs, against both the encoded antigens, as early as 14- days post-vaccination, and was maintained till 42 days.
8. Rabies DNA Vaccines in Post-Exposure Prophylaxis
Only a few studies so far have evaluated the utility of DNA vaccines in post-exposure prophylaxis of rabies, probably reflecting the challenges involved. The ability of DNA vaccination to afford protection when performed 6 hours after an intramuscular rabies virus challenge was evaluated in a murine model [26]. One, 3 or 5 doses of the DNA vaccine was given at 24-hour intervals, alongwith a single initial injection of a non-protective dose of immune serum. The vaccine doses were given intradermally in the ear pinna, via gene gun above axillary and inguinal lymph nodes or in a combination of these sites. Seroconversion was observed (a titre of 1:10) on the fifth day in 4 out of 11 animals which received five doses of DNA vaccines, and 100% seroconversion was observed by day 7. A total of 87% of animals in this group survived the challenge, as against 75% in the groups immunized with HDCV.
The utility of this approach in post-exposure rabies prophylaxis of Cynomolgus (Macaca fascicularis) monkeys was evaluated in another study [18]. Six hours after challenge with a coyote rabies virus variant, groups of monkeys received rabies DNA vaccine, control DNA vaccine, or HDCV, alongwith a single-dose HRIG. DNA vaccinations were done via gene gun delivery into ear pinnae and areas above axillary and inguinal lymph nodes, with a total of 60 µg of DNA. HDCV was administered intramuscularly. Booster doses with the same type of vaccine were given on days 3, 7, 14 and 28. Seroconversion was observed by day 7 in all HDCV-vaccinated animals. Two of the DNA vaccinated animals and two control animals showed lower antibody titres at this time. By day 14, 75% (3/4) of negative controls, 50% (2/4) of DNA vaccinated monkeys and 25% (1/4) of HDCV vaccinated monkeys succumbed to rabies. The remaining animals in each group remained rabies-free at the end of 6 months. Conclusive information could not be obtained from the study due to the survival of one unvaccinated control monkey and death of an HDCV-vaccinated monkey.
Intranasal DNA vaccination was also shown to be effective in rabies post-exposure prophylaxis, in mice and rabbit models [27]. An intramuscular challenge was done with CVS strain of rabies virus and 16 hours later, the rabbits received 100 µg of plasmid vaccine intranasally, 0.4 mL of Rabipur or plain PBS. Booster doses with the same immunogen were administered on days 3, 7 and 14. Groups of mice received three doses (on days 3, 7 and 14) of either 50 µg of the plasmid intranasally, 0.25 mL of Rabipur vaccine intramuscularly or 50 µL of PBS intramuscularly. In rabbits, neutralizing antibody titres > 0.5 IU/mL were detectable by day 30 in the DNA-immunized group, and by day 15 in Rabipur-immunized animals. The survival rates in the intranasal DNA-immunized and HDCV-immunized rabbits were 100 and 87%, respectively. Neutralizing antibodies > 0.5 IU/mL were observed in the immunized mice on day 30 after immunization. The survival rates in the intranasal DNA and intramuscular PCEC vaccination groups were 100, and 80%, respectively. Significantly, the plasmid DNA and the encoded mRNA were detected in cerebral cortex, cerebellum and hippocampus, mainly in glial cells and endothelial cells of microvessels, 72 hours after administration. Findings indicate the probable involvement of macrophages, lymphocytes and/or dendritic cells in the transmucosal transport of the plasmid and crossing of blood-brain barrier. It may also be possible that the immune mechanisms triggetred by Th1 or Th2 type of cells in the CNS may afford efficient protection without high levels of antibodies.
9. DNA Rabies Vaccines against Rabies-Related Viruses
Currently available cell culture vaccines do not afford complete protection against the genotypes 2, 3 and the newer genotypes. With recent reporting of newer and phylogenetically distinct genotypes such as Shimoni bat virus, there is an increasing need for expanding the spectrum of protection offered by rabies biologics. DNAbased vaccination may be a suitable strategy for achieving this, because of the possibility of simultaneous delivery of multiple antigens, native or modified. Earlier studies with rabies virus nucleoprotein, the most conserved viral protein, did not seem to be promising (Dietzschold et al., 1990; Perrin et al., unpublished results). An earlier study evaluated immune responses induced by pCI-neo vectors encoding glycoprotein sequences of genotype 1 (PV strain), genotype 3 (Mokola virus) and a vector encoding a chimeric glycoprotein composed of amino-terminal segment of Mokola virus and carboxy-terminal sequence from PV strain [28]. A single dose of pGPV (encoding PV glycoprotein) and pGMok (encoding G of MOKV) into cardiotoxin-pretreated muscles induced 100% seroconversion in the immunized mice, by day 30 post-immunization for pGPV and by day 14 in pGMok groups. Following a single administration of 50 µg of pGPV, IgM antibodies appeared by day 3, peaked at day 7 and declined later. The percentage of seroconversion was 75 and 100, after 7 and 14 days, respectively. Predominance and increasing levels of IgG2a were observed at the time points. IgG2a/IgG1 levels showed a decline by day 39 and thereafter increased. An early and long-lasting Th cell response was also observed to be induced by pGPV. pGPV induced significant neutralizing antibody levels against genotype 1, 4, 5 and 6, but not against genotypes 2 and 3. Significant titres were produced by pGMok against LBV and MOKV, with low titres being produced against DUVV and undetectable levels against PV, EBLV-1 and EBLV- 2. The homologous antibody titres induced by pGPV increased from day 14 to day 160, whereas the antibody titres against other genotypes showed an initial decline from day 14 to day 39, and thereafter increased upto 160 days. Compared to pGPV and pGMok, the chimeric plasmid pGMokPV induced lower but significant neutralizing antibody titres against genotypes 1, 2, 3 and 6, and lower levels at genotypes 4 and 5. It was also found to produce an earlier and stronger cell mediated immune response than by the other two constructs. pGPV and pGMok protected 100% of the immunized mice against an intracerebral challenge with the homologous viruses, whereas partial protection (50% against CVS by pGMok and 17% against MOKV by pGPV) was induced against heterologous challenges.
Another study compared the immunogenicity and protective efficacy of several DNA vaccine constructs against Mokola virus [29]. Two intramuscular doses of pCl-neo and pSG5-based constructs expressing Mokola virus glycoprotein provided 100% protection against an intracranial viral challenge in ICR mice. Virus neutralizing antibodies appeared by three weeks post-immunization and increased after a booster dose. The authors also evaluated immunizations with pBudCE4 (a dual promoter vector) expressing Mokola virus glycoprotein alone (pBudCE4-mokG), or nucleoprotein also (pBudCE4- mokG + N). Comparatively lower levels of Mokola VNA were observed in mice immunized with pBudCE4-mokG + N even after two doses, than in the group immunized with the construct encoding glycoprotein alone. Addition of N gene in the construct did not enhance the immune responses or protection. A survival rate of 40% was observed in the group immunized with pBudCE4-mokG. The findings suggest that the inclusion of N gene in a Gencoding Mokola DNA vaccine may be advantageous under specific conditions. A heterologous prime-boost strategy was also evaluated in the study, employing a vaccinia rabies glycoprotein (V-RG) vaccine boost following immunization with a Mokola DNA vaccine, revealing no significant cross-protective immunity against a heterologous viral challenge.
10. DNA Rabies Vaccines: Recent Trends
10.1. Glycoprotein Gene Modifications
A recent study explored the utility of plasmids encoding complete glycoprotein versus those encoding the secreted form, and observed enhanced antibody responses but no increase in protection rates [30]. Findings from the study emphasized the importance of inclusion of the transmembrane domain in eliciting protective antibody responses.
The relevance of the signal sequence (SS) and transmembrane domain on the immunogenicity of rabies DNA vaccine has also been examined [31]. Four constructs were developed with the vector VR1020, encoding the glycoprotein ectodomain and (1) without SS and TD (rGVR) (2) without SS but with TD (rGVRt) (3) with SS but without TD (rGVRs) and (4) with the SS and TD (rGVRst). Three doses of the constructs were given, in saline, or using gene gun (2 µg/shot) on days 0, 21 and 35. On day 45, the animals were given a booster with E. coli-derived or Baculovirus-expressed glycoprotein. Separate groups of mice were also immunized with each type of expressed glycoprotein (50 µg/mouse) and boosted after 30 and 60 days intraperitoneally with the same amount of protein, alongwith incomplete Freund’s adjuvant. The highest titres of neutralizing antibodies were seen in mice immunized with rGVRt construct, either intramuscularly or delivered via gene gun and boosted with the glycoprotein. In comparison, the antibody titres were lesser in the group immunized with rGVRst. The remaining two groups also had lower antibody titres. Boosting with E. coli-expressed as well as baculovirusexpressed glycoprotein increased the antibody titres. It was shown that vectors incorporating the ectodomain plus transmembrane domain of the native glycoprotein would be ideal immunogens for DNA vaccination.
Another study evaluated plasmid constructs bearing unmodified glycoprotein gene (pERAG) of ERA strain and modified glycoprotein gene with an arginine-toglutamine mutation at position 333 (pCDAG3) in ICR mice, following several routes of administrations [32]. Following a single intramuscular administration of 100 µg of pCDAG3, 80% of the immunized mice developed adequate titres of neutralizing antibodies by day 30, and survived a viral challenge on day 30. Subcutaneous administration of three doses of pCDAG3 on days 0, 21 and 42 produced seroconversion in 60% of the mice but only 20% survived a viral challenge, and pERAG did not produce seroconversion when administered subcutaneously. Seroconversion or protection was not observed upon oral immunization. Intradermal immunization using 10 µg of pCDAG3 was found to protect 75% of challenged mice. Findings from the study indicate that mutated glycoprotein might enhance apoptosis, thereby increasing the immune responses.
10.2. Specific Subcellular Targeting
The possibility of enhancement of immune responses to rabies DNA vaccine constructs by specific targeting to subcellular compartments was evaluated in a recent study [33]. Vectors encoding G gene fused to Tissue Plasminogen Activator [facilitating expression and secretion, and enhanced uptake by APC], Lysosomal-Associated Membrane Protein-1 (LAMP-1) [signal sequence for targeting MHC class II pathway] or Ubiquitin A-76 (UQ) [MHC Class I-targeting signal] were evaluated in mice and dogs. BALB/c mice were immunized intramuscularly with three doses of the constructs at 3-weekly intervals. Successful seroconversion was observed in all mice after the initial dose, and maximum antibody titers were found after the 2nd booster, for both modified and unmodified constructs. Highest antibody response was observed in mice immunized with the construct bearing the LAMP-1 tag, followed by the one with the TPA and LAMP-1 tags. No influence of the signal tags was observed on the antibody isotypes induced. The IgG1/ IgG2a ratio was consistently above 1, indicating a Th2 bias. survival rate of 60% was observed in mice immunized with LAMP-1 bearing glycoprotein construct, upon lethal viral challenge.
In a subsequent study, the authors attempted further improvement of immune responses to the LAMP-1 bearing construct, by employing various doses, delivery using intramuscular, gene gun, or oral route, and adjuvanting with Emulsigen® or Emulsigen-D [16]. Intramuscular immunization of 100 µg of the DNA vaccine supplemented with Emulsigen® D was observed to produce the highest level of neutralizing antibodies, a predominantly IgG1/ IgG2a subclass distribution and effective cellular immune responses. Three doses of this formulation were found to protect 100% of mice against a later challenge with a lethal dose of CVS virus. Seroconversion was not observed in mice following oral immunization. Five doses of the formulation protected all immunized mice following an initial intramuscular administration of 50 LD50 of CVS. Immunogenicity of the DNA vaccine construct (without adjuvant) was evaluated in 2-3 month old dogs, wherein neutralizing antibodies were observed following three intramuscular doses of 100 µg of the plasmid.
11. Conclusion and Future Prospects
Despite the demonstration of proof of concept about 16 years ago, plasmid DNA-based rabies vaccination is yet to witness significant commercial success or application in routine rabies prophylaxis. Drawbacks relating to poor immunogenicity and requirement of higher DNA doses in larger animals remain unsolved issues in rabies DNA vaccination. However, current progresses in the field of vector design hold considerable promise for rabies DNA vaccine development. Insights into the roles played by the antigen presenting cells in shaping up adaptive immune responses and the benefits of targeted antigen delivery to these cells hold great potential in devising better strategies for rabies DNA vaccination. Co-delivery with molecularly defined adjuvants is another approach that needs further evaluation in DNA rabies vaccination. Advances in the field of chemical biology also hold importance in the development of delivery agents and adjuvants for rabies DNA vaccines. It is felt that DNA rabies vaccination will sustain research interest pertaining to rabies control atleast in the animal hosts, provided its current drawbacks are addressed effectively.
12. Acknowledgements
PTU thanks the Indian Council of Medical Research for a research fellowship.
REFERENCES
- World Health Organisation, “WHO Expert Consultation on Rabies,” Technical Report Series, Vol. 931, 2005, pp. 1-88.
- C. E. Rupprecht, C. A. Hanlon and T. Hemachuda, “Rabies Re-Examined,” Lancet Infectious Diseases, Vol. 2, No. 6, 2002, pp. 327-343. doi:10.1016/S1473-3099(02)00287-6
- C. Bahloul, D. Taieb, M. F. Diouani, S. B. Ahmed, Y. B. Chtourou and B. I. B’chir, “Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions,” Vaccine, Vol. 24, No. 8, 2006, pp. 1063-1072. doi:10.1016/j.vaccine.2005.09.016
- M. A. Liu, “DNA Vaccines: An Historical Perspective and View to the Future,” Immunological Reviews, Vol. 239, No. 1, 2011, pp. 62-84. doi:10.1111/j.1600-065X.2010.00980.x
- D. C. Tang, M. DeVit and S. A. Johnston, “Genetic Immunization is a Simple Method for Eliciting an Immune Response,” Nature, Vol. 356, No. 6365, 1992, pp. 152-154. doi:10.1038/356152a0
- H. L. Robinson, “Nucleic Acid Vaccines: An Overview,” Vaccine, Vol. 15, No. 8, 1997, pp. 785-787.
- J. A. Williams, A. E. Carnes and C. P. Hodgson, “Plasmid DNA vaccine Vector Design: Impact on Efficacy, Safety and Upstream Production,” Biotechnology Advances, Vol. 27, No. 4, 2009, pp. 353-370. doi:10.1016/j.biotechadv.2009.02.003
- F. Faurez, D. Dory, V. Le Moigne, R. Gravier and A. Jestin, “ Biosafety of DNA Vaccines: New Generation of DNA Vectors and Current Knowledge on the Fate of Plasmids after Injection,” Vaccine, Vol. 28, No. 23, 2010, pp. 3888-3895. doi:10.1016/j.vaccine.2010.03.040
- Z. Q. Xiang, S. L. Spitalnik, M. Tran, W. H. Wunner, J. Cheng and H. C. Ertl, “Vaccination with a Plasmid Vector Carrying Rabies Virus Glycoprotein Gene Induces Protective Immunity against Rabies Virus,” Virology, Vol. 199, No. 1, 1994, pp. 132-140. doi:10.1006/viro.1994.1105
- Z. Q. Xiang, S. L. Spitalnik, J. Cheng, J. Erikson, B. Wojczyk and H. C. Ertl, ”Immune Responses to Nucleic Acid Vaccines to Rabies Virus,” Virology, Vol. 209, No. 2, 1995, pp. 569-579. doi:10.1006/viro.1995.1289
- Z. Q. Xiang, Z. He, Y. Wang and H. C. Ertl, “The Effect of interferon-γ on genetic immunization,” Vaccine, Vol. 15, No. 8, 1997, pp. 896-898.
- N. B. Ray, L. C. Ewalt and D. L. Lodmell, “Nanogram Quantities of Plasmid DNA encoding the rabies virus glycoprotein protect mice against Lethal Rabies Virus Infection,” Vaccine, Vol. 15, No. 8, 1997, pp. 892-895. doi:10.1016/S0264-410X(96)00281-2
- D. L. Lodmell, N. B. Ray, J. T. Ulrich and L. C. Ewalt, “DNA Vaccination of Mice against Rabies Virus: Effects of the Route of vaccination and the Adjuvant Monophosphoryl Lipid A (MPL),” Vaccine, Vol. 18, No. 11-12, 2000, pp. 1059-1066. doi:10.1016/S0264-410X(99)00352-7
- L. Fischer, J. Minke, N. Dufay, P. Baudu and J. C. Audonnet, “Rabies DNA Vaccine in the Horse: Strategies to Improve Serological Responses,” Vaccine, Vol. 21, No. 31, 2003, pp. 4593-4596. doi:10.1016/S0264-410X(03)00504-8
- M. Margalith and A. Vilalta, “Sustained Protective Rabies Neutralizing Antibody Titers after Administration of Cationic Lipid-Formulated pDNA Vaccine,” Genetic Vaccines and Therapy, Vol. 4, No. 2, 2006, pp. 1-6.
- M. Kaur, A. Saxena, A. Rai and R. Bhatnagar, “Rabies DNA Vaccine Encoding Lysosome-Targeted Glycoprotein Supplemented with Emulsigen-D Confers Complete Protection in Preexposure and Postexposure Studies in BALB/c Mice,” FASEB Journal, Vol. 24, No. 1, 2010, pp. 173-183. doi:10.1096/fj.09-138644
- D. L.Lodmell, N. B. Ray and L. C. Ewalt, ” Gene Gun Particle-Mediated Vaccination with plasmid DNA Confers Protective Immunity against Rabies Virus Infection,” Vaccine, Vol. 16, No. 2-3, 1998, pp. 115-118. doi:10.1016/S0264-410X(97)88325-9
- D. L. Lodmell, M. J. Parnell, J. R. Bailey, L. C. Ewalt and C. A. Hanlon, “Rabies DNA Vaccination of Non-Human Primates: Post-Exposure Studies Using Gene Gun Methodology That Accelerates Induction of Neutralizing Antibody and Enhances Neutralizing Antibody Titers,” Vaccine, Vol. 20, No. 17-18, 2002, pp. 2221-2228. doi:10.1016/S0264-410X(02)00143-3
- D. L. Lodmell, M. J. Parnell, J. T. Weyrich, D. L. Lodmell, M. J. Parnell, J. R. Bailey, L. C. Ewalt and C. A. Hanlon, “One-Time Gene Gun or Intramuscular Rabies DNA Vaccination of Non-Human Primates: Comparison of Neutralizing Antibody Responses and Protection against Rabies Virus 1 Year after Vaccination,” Vaccine, Vol. 20, No. 5-6, 2001, pp. 838-844. doi:10.1016/S0264-410X(01)00392-9
- S. Biswas, G. S. Reddy, V. A. Srinivasan and P. N. Rangarajan, “Preexposure Efficacy of a Novel Combination DNA and Inactivated Rabies Virus Vaccine,” Human Gene Therapy, Vol. 12, No. 15, 2001, pp. 1917-1922. doi:10.1089/104303401753153965
- A. R. Pinto, A. Reyes-Sandoval and H. C. J. Ertl, “Chemokines and TRANCE as Genetic Adjuvants for a DNA Vaccine to Rabies Virus,” Cellular Immunology, Vol. 224, No. 2, 2003, pp. 106-113. doi:10.1016/j.cellimm.2003.08.006
- J. E. Osorio, C. C. Tomlinson, R. S. Frank, E. J. Haanes, K. Rushlow and J. R. Haynes, “Immunization of Dogs and Cats with a DNA vaccine against rabies virus,” Vaccine, Vol. 17, No. 9-10, 1999, pp. 1109-1116. doi:10.1016/S0264-410X(98)00328-4
- E. Tesoro-Cruz, R. Calderon-Rodriguez, R. HernandezGonzalez, F. Blanco-Favela and A. Aguilar-Setien, “Intradermal DNA Vaccination in Ear Pinnae is an Efficient Route to Protect Cats against Rabies Virus,” Veterinary Research, Vol. 39, No. 2, 2008, p. 16. doi:10.1051/vetres:2007054
- L. Cupillard, V. Juillard, S. Latour, G. Colombet, N. Cachet and S. Richard, “Impact of Plasmid Supercoiling on the Efficacy of a Rabies DNA Vaccine to Protect Cats,” Vaccine, Vol. 23, No. 16, 2005, pp. 1910-1916. doi:10.1016/j.vaccine.2004.10.018
- S. Patial, V. K. Chaturvedi, A. Rai, M. Saini, R. Chandra, Y. Saini and P. K. Gupta, “Virus Neutralizing Antibody Response in Mice and Dogs with a Bicistronic DNA Vaccine Encoding Rabies Virus Glycoprotein and Canine Parvovirus VP2,” Vaccine, Vol. 25, No. 20, 2007, pp. 4020-4028. doi:10.1016/j.vaccine.2007.02.051
- D. L. Lodmell and L. C. Ewalt, “Post-Exposure DNA Vaccination Protects Mice against Rabies Virus,” Vaccine, Vol. 19, No. 17-19, 2001, pp. 2468-2473. doi:10.1016/S0264-410X(00)00475-8
- E. Tesoro-Cruz, I. A. Feria Romero, J. G. Lopez Mendoza, S. Orozco Suarez, R. Hernandez Gonzalez and F. B. Favela, “Efficient Post-Exposure Prophylaxis against Rabies by Applying a Four-Dose DNA Vaccine Intranasally,” Vaccine, Vol. 26, No. 52, 2008, pp. 6936-6944. doi:10.1016/j.vaccine.2008.09.083
- C. Bahloul, Y. Jacob, N. Tordo and P. Perrin, “DNA-Based Immunization for Exploring the Enlargement of Immunological Cross-Reactivity against the Lyssaviruses,” Vaccine, Vol. 16, No. 4, 1998, pp. 417-425. doi:10.1016/S0264-410X(97)00204-1
- L. H. Nel, M. Niezgoda, C. A. Hanlon, P. A. Morril, P. A. Yager and C. E. Rupprecht, “A comparison of DNA Vaccines for the Rabies-Related Virus, Mokola,” Vaccine, Vol. 21, No. 19-20, 2003, pp. 2598-2606. doi:10.1016/S0264-410X(03)00036-7
- P. K. Gupta, S. Sharma, S. S. Walunj, A. A. Patil, A. Rai A and M. Saini, “A DNA Vaccine That Encodes Rabies Virus Glycoprotein Lacking Transmembrane Domain Enhances Antibody Response but Not Protection,” Acta Virologica, Vol. 50, No. 2, 2006, pp. 87-92.
- A. Rath, S. Choudhury, D. Batra, S. V. Kapre, C. E. Rupprecht and S. K. Gupta, “DNA vaccine for rabies: Relevance of the trans-membrane domain of the glycoprotein in generating an antibody response,” Virus Research, Vol. 13, No. 2, 2005, pp. 143-152. doi:10.1016/j.virusres.2005.05.002
- M. O. Osinubi, X. Wu, R. Franka, M. Niezgoda, A. J. Nok, A. B. Ogunkoya and C. E. Rupprecht, “Enhancing Comparative Rabies DNA Vaccine Effectiveness through Glycoprotein Gene Modifications,” Vaccine, Vol. 27, No. 51, 2009, pp. 7214-7218. doi:10.1016/j.vaccine.2009.09.031
- M. Kaur, A. Rai and R. Bhatnagar, “Rabies DNA vaccine: No impact of MHC Class I and Class II targeting sequences on immune response and protection against lethal challenge,” Vaccine, Vol. 27, No. 15, 2009, pp. 2128- 2137. doi:10.1016/j.vaccine.2009.01.128
NOTES
*Corresponding author.

