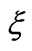Soft Nanoscience Letters
Vol.1 No.3(2011), Article ID:6164,5 pages DOI:10.4236/snl.2011.13014
Light Scattering Study of Mixture of Polyethylene Glycol with C12E5 Microemulsion
![]()
1Department of Physics, University of Sistan and Baluchestan, Zahedan, Iran; 2Institut für Experimentelle Physik, Universität Ulm, Albert-Einstein-Allee, Ulm, Germany.
Email: soheil.sharifi@gmail.com, masoud.amirkhani@uni-ulm.de
Received March 20th, 2011; Revised June 3rd, 2011; Accepted July 5th, 2011.
Keywords: Nano-Droplet, Microemulsion, PEG, Light Scattering, SAXS
ABSTRACT
The mixture of C12E5 microemulsion with PEG have been studied by small-angle X-ray scattering and dynamic light scattering in order to determine structure and dynamic of the system. Light scattering experiment shown an exponential relaxation for pure C12E5 microemulsion that with increasing of PEG concentration in the C12E5 microemulsion, relaxation becomes non-exponential, which demonstrates that increase of cooperativity. The study structure of the system with SAXS experiment, shown with increasing of PEG concentration, the size of the droplet doesn’t change but interaction between droplets increases that have agreement with light scattering data’s.
1. Introduction
One of the interesting topic in the soft matter physics is mixture of microemulsion with polymer and study depletion interaction between droplets of microemulsion that induced by polymer. The depletion phenomena play an important role in many industrial and biological applications. The study of the depletion interaction is important because of the some phenomena that it can produce with this effect. It is well known that depletion interaction can make phase separation in the colloidal dispersions [1], protein crystallization and the helical conformation of long molecular chains [2-4]. In the mixture of non-adsorbing polymer with microemulsion, near the surfaces of droplets, there exists a depletion volume usually characterized by a depletion layer thickness [5-8]. Ternary microemulsions, consisting of water, decane, and nonionic surfactant, pentaethylene glycol dodecyl ether (C12E5), have been well characterized by many researchers [9-11]. These systems form an oil-inwater droplet microemulsion, a lamellar structure, a bicontinuous microemulsion, or a hexagonal structure depending on the concentration and temperature. The thermodynamic properties of the nonionic microemulsion with the C12E5 at a constant surfactant-to-oil weight ratio of 1.08 had been studied extensively, and the system is well characterized. The C12E5 microemulsion at this weight ratio have between 24 C to 30 C shown a L1 phase and phase tradition change with add polymer to the microemulsion. Dynamic and structure study of this system showed a single relaxation and well-defined spherical oil droplets with hydrocarbon radius 75 Å and low polydispersity [12]. In this work, the effects of increasing of nonadsorbing polymer, polyethylene glycol (PEG), on the structure and relaxation of droplet of nanionic microemulsions (C12E5 microemulsions) is studied with SAXS and light scattering. The viscosity and light scattering of the mixture of PEG with C12E5 microemulsion was studied and analyzed within polymer depletion theory [13]. In that studied, dilution viscometry and dynamic light scattering were used to confirm that these microemulsions behave as hardsphere dispersions. However, the system is different to ours with respect to the molecular weight of the polyethylene glycol.
2. Experiment
Materials and Istruments
The Pentaethylene glycol monododecyl ether (C12E5), ndecane and polyethylene glycol (PEG) (Mn = 2200) were obtained from Sigma-Aldrich. Chemicals were used as received and MilliQ water was used in preparing all samples. The bottles of C12E5, which are hermetically sealed, have always been stored in a refrigerator well below the melting point (23˚C). After a container was opened, the surfactant was stored in a nitrogen environment; it was still found that the surfactant did not remain stable for more than a month. The samples were prepared by mixing the components directly in glass a ampoule in order to minimize the number of transfers and the glass ampoules were tightly sealed with a gas flame. The microemulsions were prepared by weight, in terms of surfactant-oil mass ratio of 1.08 and the mass fraction of droplets (mf, drop = (mDec + mC12E5)/(mTotal)), which varies by the respective mass of n-decane (mDec), C12E5 (mC12E5) and total sample mass (mTota). The microemulsions are thermodynamically stable dispersions in oil-in-water (O/W) droplets surrounded by a surfactant film: For these O/W microemulsions, it has been shown that the microemulsion phase (L1) is well-modeled as a dispersion of hard-sphere particles. In all experiments, the mass ratio of surfactant to the oil is constant and with a hydrocarbon core radius of 75 Å over a wide range of droplet concentrations, when the system is near the limit of maximum oil solubilization. The samples of microemulsions with polymer were prepared by weight in the terms of the mass fraction of triblock polymer (mf, poly = mpoly/(mTotal)) that mpoly is the mass of polymer in the sample. The mixed samples were prepared at a constant mass fraction of droplets (mf, drop = 0.1) and a surfactant-oil mass ratio of 1.08 with the different mass fractions of polymer. The samples were thoroughly shaken to ensure homogenization and then kept at the temperature 26˚C in a water bath for several days before the experiment. We observed that all samples were transparent at 26˚C.
Small-angle X-ray scattering (SAXS) measurements were performed using the pinhole SAXS instrument at the University of Aarhus. The instrument consists of an X-ray camera (NanoSTAR, Bruker AXS) with a rotating anode X-ray (Cu Ka radiation) source, crosscoupled Gobel mirrors, collimation using three pinholes and an evacuated beam path, and a 2D position-sensitive gas detector (HiSTAR). The experiments were done at a fixed wavelength of 1.54 Å and two different sample-detector distances. In the current experiments small pinholes were used, giving a range of scattering vectors as 0.001 < Q (1/Å) < 0.2, where q is the wave vector. Samples were held in 1 mm quartz capillaries and measurements were made at room temperature. The scattering from capillaries was measured as background and was subtracted to yield the excess scattering as a function of Q for microemulsion samples. DLS measurements were performed using an ALV single-detector version compact goniometer system, from ALV-GmbH, Langen, Germany. The light source is a He-Ne laserOperating at a wavelength of 632.8 nm, with vertically polarized light. The beam was focused on the sample cell through a temperature-controlled cylindrical quartz container (with two plane-parallel windows), which is filled with a refractive index matching liquid (toluene). All the correlation functions in this work were fitted by a single stretched exponential function.
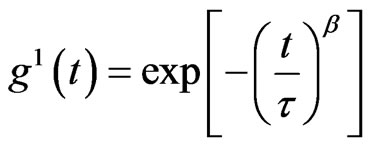 (1)
(1)
The stretched exponential function describes the decay processes that have a distribution of relaxation times ( ). The parameter
). The parameter 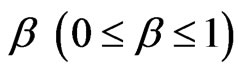 measures the width of the distribution function. A smaller β value corresponds to a broader distribution of relaxation times or more cooperative movement [14-16]. The mean value of the relaxation time is given by
measures the width of the distribution function. A smaller β value corresponds to a broader distribution of relaxation times or more cooperative movement [14-16]. The mean value of the relaxation time is given by
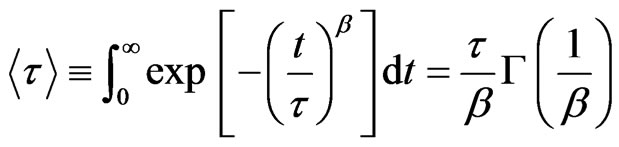 (2)
(2)
where 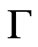 is the gamma function and the collective diffusion constant [17-21] was calculated through
is the gamma function and the collective diffusion constant [17-21] was calculated through 
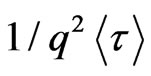 .
.
3. Results and Discussion
Figure 1 shows time correlation function data (at a scattering angle of 90˚) for polymer mass fraction of 0.027. The decay of the time correlation function shows a single relaxation that can be well described by Equation (1) for all the samples at 26˚C. The fittings of the correlation functions for the pure microemulsion show that the size distribution of the particles is not changing by changing the droplet mass fraction and also has rather narrow (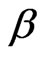 = 0.98), Figure 2. The value of β, for mixed polymer and microemulsion is slightly lower than pure microemulsion, which demonstrates that either the polydispersity or cooperativity [16] of the mixed system is practically increases by the addition of polymer (Figure 2). In addition Figure 2, illustrates that is increasing by decreasing the mass fraction of droplet and for the lowest droplet mass fraction the value of virtually is not affected the addition of polymer. The size of microemulsion in the presence of non-adsorbing polymer is expected to be constant [13,19] so decreasing of
= 0.98), Figure 2. The value of β, for mixed polymer and microemulsion is slightly lower than pure microemulsion, which demonstrates that either the polydispersity or cooperativity [16] of the mixed system is practically increases by the addition of polymer (Figure 2). In addition Figure 2, illustrates that is increasing by decreasing the mass fraction of droplet and for the lowest droplet mass fraction the value of virtually is not affected the addition of polymer. The size of microemulsion in the presence of non-adsorbing polymer is expected to be constant [13,19] so decreasing of 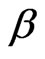 value can be the contribution of cooperative movement due to the depletion effect. At the lower droplet mass fraction, with the increase of polymer amount the
value can be the contribution of cooperative movement due to the depletion effect. At the lower droplet mass fraction, with the increase of polymer amount the 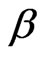 decrease less significantly compare the pure microemulsion. So, decreasing
decrease less significantly compare the pure microemulsion. So, decreasing 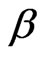 value in lower droplet mass fraction can be rationalized by decreasing depletion effect. It is obvious from Figure 3 that the diffusion decreases monotonously with increasing PEG concentration for all systems, and is lower for the higher mass fraction of the polymer. More-
value in lower droplet mass fraction can be rationalized by decreasing depletion effect. It is obvious from Figure 3 that the diffusion decreases monotonously with increasing PEG concentration for all systems, and is lower for the higher mass fraction of the polymer. More-
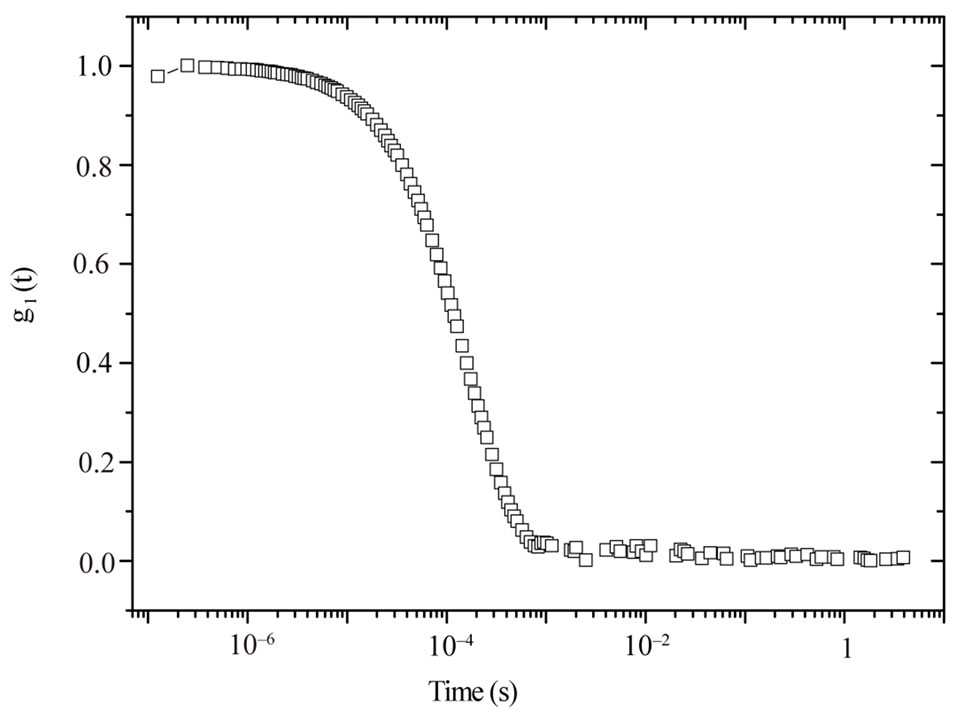
Figure 1. The first-order field correlation function versus time for C12E5 microemulsion at a droplet mass fraction of 0.098 and polymer mass fraction of 0.027 at the temperature 26˚C.
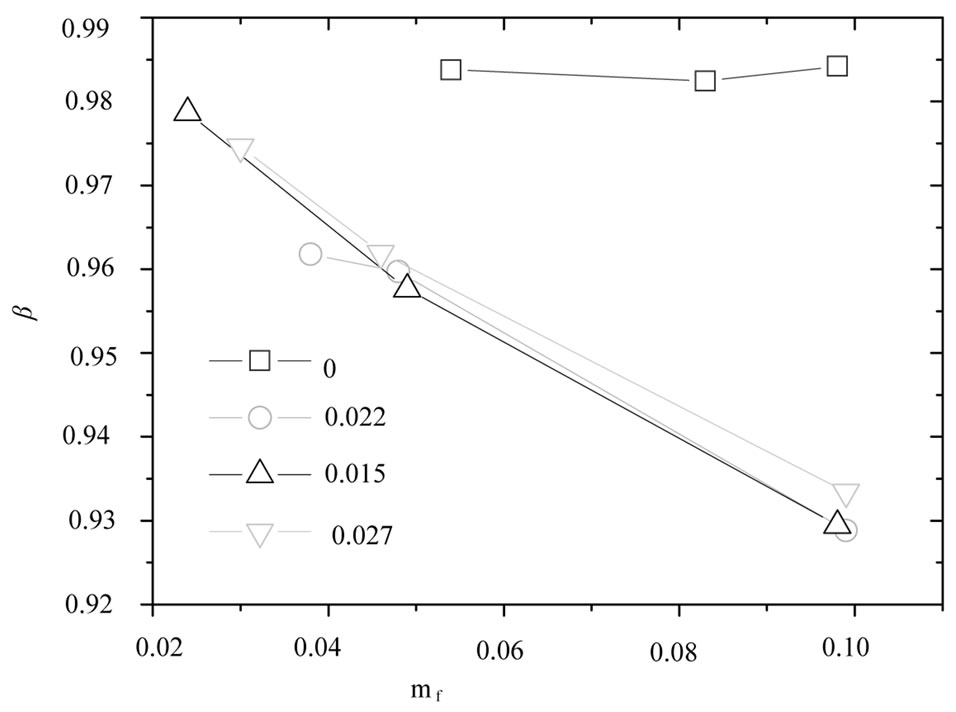
Figure 2. The 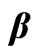 value of the mixtures of C12E5 microemulsion for different polymer mass fractions (mf,poly = 0¢, 0.015▲, 0.022˜, 0.027▼), versus the mass fraction of droplets at temperature 26˚C.
value of the mixtures of C12E5 microemulsion for different polymer mass fractions (mf,poly = 0¢, 0.015▲, 0.022˜, 0.027▼), versus the mass fraction of droplets at temperature 26˚C.
over the general trend depicted in Figure 3 is that the adding increase amount of PEG the slop of collective diffusion verse droplet mass fraction and the intercept (the dilute-limiting value of diffusion) decreases. This trend is attributed to the change of interaction between microemulsions from repulsion to the attractive interaction [20]. This is a typical behavior that has been reported elsewhere for the mixture of the C12E5 microemulsion and PEG [13].
The structure of the C12E5 microemulsions at the different concentrations of droplets (oil and surfactant) has previously been studied by SAXS and small-angle neutron scattering in the L1 phase of the microemulsion, [14-16]. A studied has shown hard-sphere behaviors’ that
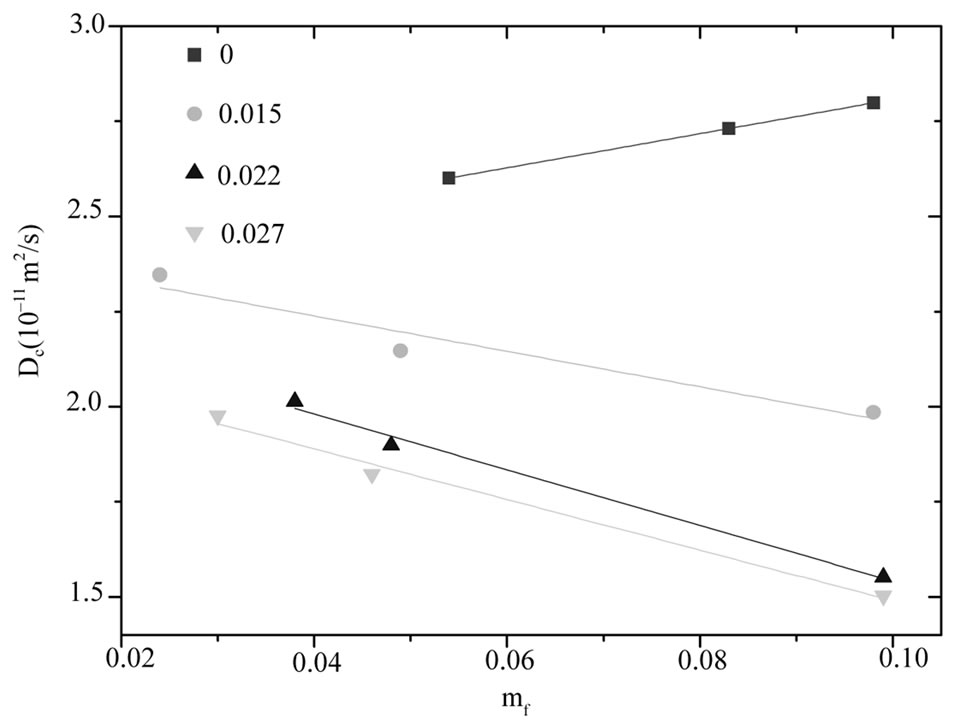
Figure 3. The diffusion coefficient of the mixtures of C12E5 microemulsion with different polymer mass fractions (mf,poly = 0¢, 0.015˜, 0.022▲, 0.027▼), versus the mass fraction of droplets at temperature 26˚C.
it well-modeled as core-shell particles for a surfactant-tooil mass ratio of 1.08 at a temperature 25˚C [14]. In the present work, the variation of structure of the C12E5 microemulsion under addition of the PEG is investigated by SAXS. The generalized indirect Fourier transformation (GIFT) method with a structure factor for a model of monodisperse spheres with an attractive depletion potential is applied to the SAXS data [22-25].
We used GIFT program for analysis SAXS experiments and we present results of this analysis in the Table 1. Our results show that size of the droplet at C12E5 microemulsion is 85 Å that it is in agreement with previous results, [14], and the size ratio of polymer to droplet change from 0.3 to 0.5 with increasing polymer mass fraction. Figure 4, shows the SAXS data for the effect of PEG concentration on C12E5 microemulsion at the constant droplet mass fraction (0.098) and constant mass ratio of oil to surfactant (1.08). The red line is the fit by the depletion model using the GIFT method. The structure factor derived by this approach from the SAXS data using the depletion model shows a peak at q = 0.034 Å. This peak does not change with the PEG concentration, however, the value of the structure factor at q = 0 increases with increasing of PEG concentration, Figure 5.
The pure microemulsion, S(Q) as a function of Q has a peak around Q = 0.036 Å and shows a single broad interference peak. Under addition of the PEG a strong change of the first interference peak together with the development of a second order peak is clearly observed. Moreover, a significant decrease of the full width with increasing PEG is evident, indicating the increase of order with increasing content of polymer. The peak location carries information about the mean intermicellar dis-
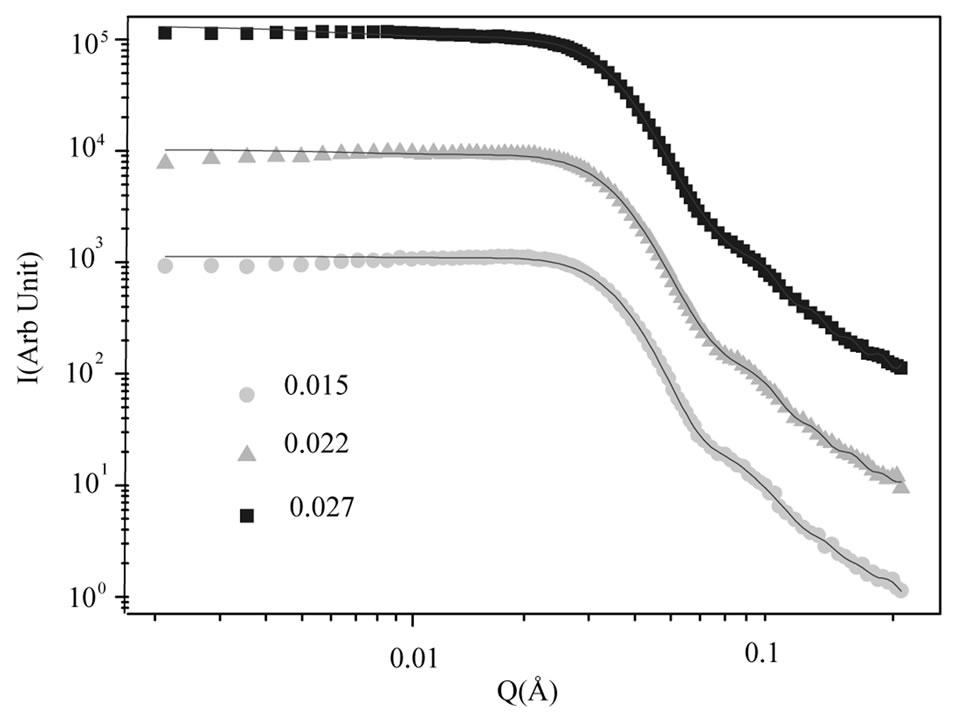
Figure 4. The SAXS experiment of the mixture of C12 E5 microemulsion at a constant droplet mass fraction (0.098) and  = 1.08 with different concentration of PEG (mf,pol = 0.015˜, 0.022▲, 0.027¢). The red line is the fit with the attractive monodisperse spheres with q depletion potential obtained by the GIFT method.
= 1.08 with different concentration of PEG (mf,pol = 0.015˜, 0.022▲, 0.027¢). The red line is the fit with the attractive monodisperse spheres with q depletion potential obtained by the GIFT method.
Figure 5. Structure factor as a function of q for different mass fraction of PEG (mf,pol = 0.015˜, 0.022▲, 0.027¢) mixed with C12E5 microemulsions at constant mass fraction of droplet (0.098) and 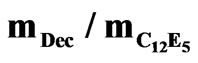 = 1.08. The insert shows the structure factor value at the origin (q = 0) as a function of polymer mass fraction.
= 1.08. The insert shows the structure factor value at the origin (q = 0) as a function of polymer mass fraction.
Table 1. Evaluation parameters for fit data with attractive monodisperse spheres with depletion potential.
| Droplet Mass fraction | Polymer Mass fraction | Droplet Radious (Å) |
|
| 0.098 | 0.015 | 85 | 0.5 |
| 0.098 | 0.022 | 85 | 0.4 |
| 0.098 | 0.027 | 85 | 0.3 |
tance and the sharpness of the peak about the fluctuations around this mean distance, which in turn is dictated by the micellar interaction potential. Hence, the development of a rather sharp peak in S(Q) up on polymer addition shows that the micellar ordering is enhanced and the polydispersity increase with add PEG to the systems.
The SAXS experiment on the mixture of microemulsion with several polymer systems studied before. A study on the mixture of stearate-polyethylene oxidestearate triblock copolymer and C12E5 microemulsion shown that the addition of physically cross-linking triblock copolymer leads to an enhancement of the order between the spherical micelles [11], increase of the network decrease dynamic of the systems. The same results that we can find in the mixture of Hydrophobically EndCapped Poly (ethylene oxide) with C12E5 microemulsion [12]. In the mixture of PEG with C12E5 microemulsion, PEG cannot increase the order in the microemulsion, because of the non-adsorbing origin of it, but it can increase the interaction between the droplets, it is clear from light scattering experiments, Figure 3. So, the best model for the describe the effect of PEG on the C12E5 microemulsion is an attractive sphere model with depletion potential. Å
4. Conclusions
Small-angle X-ray scattering and dynamic light scattering have been used to study the mixture of C12E5 with PEG. Our results show the stretched exponential function decrease in  from 0.98 to 0.92 with increase of mass fraction of the droplet that this behavior is the same for all the PEG concentration in the C12E5 microemulsion which demonstrates increase of the cooperativity between droplets, more ever the study of the C12E5 microemulsion at different droplet mass fraction shows a repulsive behavior that with increase of concentration of PEG on the C12E5 microemulsion, system become attractive. We used an attractive monodisperse sphere model and polydisperse hard sphere model with size polydispersity governed by a Schulz distribution to analyzing the SAXS data of mixture of PEG with C12E5 microemulsion, first model demonstrates a increase of interaction between droplet with increase of the PEG concentration and second model demonstrates a increase of order in the mixture of PEG with C12E5 microemulsion. However, according to the DLS results, the model is more likely to be the right model.
from 0.98 to 0.92 with increase of mass fraction of the droplet that this behavior is the same for all the PEG concentration in the C12E5 microemulsion which demonstrates increase of the cooperativity between droplets, more ever the study of the C12E5 microemulsion at different droplet mass fraction shows a repulsive behavior that with increase of concentration of PEG on the C12E5 microemulsion, system become attractive. We used an attractive monodisperse sphere model and polydisperse hard sphere model with size polydispersity governed by a Schulz distribution to analyzing the SAXS data of mixture of PEG with C12E5 microemulsion, first model demonstrates a increase of interaction between droplet with increase of the PEG concentration and second model demonstrates a increase of order in the mixture of PEG with C12E5 microemulsion. However, according to the DLS results, the model is more likely to be the right model.
5. Acknowledgements
Thanks for the kindly support from Universky of Sistan and Baluchestan.
REFERENCES
- D. H. Napper, “Polymeric Stabilization of Colloidal Dispersions,” Academic Press, New York, 1983.
- R. Tuinier, J. Rieger and C. G. de Kruif, “DepletionInduced Phase Separation in Colloid-Polymer Mixtures,” Advanced Colloid Interface Science, Vol. 103, No. 1, 2003, pp. 1-31. doi:10.1016/S0001-8686(02)00081-7
- W. C. K. Poon, “The Physics of a Model Colloid-Polymer Mixture,” Journal of Physics: Condensed Matter, Vol. 14, No. 33, 2002, pp. R859-R880. doi:10.1088/0953-8984/14/33/201
- V. Snir and R. D. Kamien, “Entropically Driven Helix Formation,” Science, Vol. 307, No. 5712, 2005, p. 1067. doi:10.1126/science.1106243
- H. N. W. Lekkerkerker, “Interactions, Phase Transitions and Metastable States in Concentrated Colloidal Dispersions,” Physica A, Vol. 213, No. 1-2, 1995, pp. 18-29. doi:10.1126/science.1106243
- R. Tuinier, E. ten Grotenhuis, C. Holt, P. A. Timmins, and C. G. de Kruif, “Depletion Interaction of Casein Micelles and an Exocellular Polysaccharide,” Physical Review E, Vol. 60, No. 1, 1999, pp. 848-856. doi:10.1103/PhysRevE.60.848
- J. Janzen and D. E. Brooks, Clinical Hemorheology, Vol. 9, No. 695, 1989.
- A. M. Kulkarni, A. P. Chattarjee, K. S. Schweizer and C. F. Zukoski, “Depletion Interactions in the Protein Limit: Effects of Polymer Density Fluctuations,” Physical Review Letters, Vol. 83, No. 22, 1999, pp. 4554-4557. doi:10.1103/PhysRevLett.83.4554
- E. Feitosa, W. Brown and P. Hansson, “Interactions between the Non-ionic Surfactant C12E5 and Poly (ethylene oxide) Studied Using Dynamic Light Scattering and Fluorescence Quenching,” Macromolecules, Vol. 29, 1996, pp. 2169-2178. doi:10.1021/ma950516g
- M. Nayeri, M. Zackrisson and J. Bergenholtz, “Scattering Functions of Core-Shell-Structured Hard Spheres with Schulz-Distributed Radii,” Journal of Physical Chemistry B, Vol. 113, No. 24, 2009, 8296. doi:10.1021/jp811482w
- M. Schwab and B. Stühn, “Relaxation Phenomena and Development of Structure in a Physically Cross Linked Nonionic Microemulsion Studied by Photon Correlation Spectroscopy and Small Angle X-ray Scattering,” Journal of Chemical Physical, Vol. 112, No. 14, 2000. pp. 6461-6471. doi:10.1063/1.481207
- H. Bagger-Jorgensen, L. Coppola, K. Thuresson, U. Olsson and K. Mortensen, “Phase Behavior, Microstructure, and Dynamics in a Nonionic Microemulsion on Addition of Hydrophobically End-Capped Poly (Ethylene Oxide),” Langmuir, Vol. 13, No. 16, 1997, pp. 4204-4218. doi:10.1021/la962054l
- M. Zackrisson, R. Andersson and J. Bergenholtz, “Depletion Interactions in Model Microemulsions,” Langmuir, Vol. 20, No. 8, 2004, pp. 3080-3089. doi:10.1021/la036132y
- M. Amirkhani, S. Volden, K. Zhu, W. R. Glomm and B. Nystrm, “Adsorption of Cellulose Derivatives on Flat Gold surfaces and on spherical gold particles” Journal of Colloid and Interface Science, Vol. 328, No. 1, 2008, pp. 20-28. doi:10.1021/la036132y
- M. Amirkhani, G. Gorinia and D. Leporinia, “Second Harmonic Generation Studies of Intrinsic and Extrinsic Relaxation Dynamics in Poly (Methy1 Methacrylate),” Journal of Non-Crystalline Solids, Vol. 355, No. 34-36, 2009, pp. 1707-1712. doi:10.1021/la036132y
- E. Y. Sheu, S. Chen, J. S. Huang and J. C. Sung, “Nonexponential Relaxations in Dense Microemulsion near the Glasslike Transition,” Physical Review A, Vol. 39, No. 11, 1989, pp. 5867-5876. doi:10.1103/PhysRevA.39.5867
- J. Appell, G. Porte and E. Buhler “Self-Diffusion and Collective Diffusion of Charged Colloids Studied by Dynamic Light Scattering,” Journal of Physical Chemistry B, Vol. 109, No. 27, 2005, p. 13186. doi:10.1021/jp051016k
- I. Lynch and L. Piculell, “Size, Concentration, and Solvency Effects on the Viscosifying Behavior of PEO-PSPEO Triblock Copolymers in AOT Oil-Continuous Microemulsions,” Journal of Physical Chemistry B, Vol. 108, No. 22, 2004, pp. 7515-7522. doi:10.1021/jp049147t
- C. Van den Broeck, F. Lostak and H. N. W. Lekkerkerker, “The Effect of Direct Interactions on Brownian Diffusion,” Journal of Chemical Physics, Vol. 74, No. 3, 1981, pp. 2006-2010. doi:10.1063/1.441244
- A. Shukla, H. Graener and R. H. H. Neubert, “Observation of Two Diffusive Relaxation Modes in Microemulsions by Dynamic Light Scattering,” Langmuir, Vol. 20, No. 20, 2004, pp. 8526-8530. doi:10.1021/la048883l
- G. Fritz and O. Glatter, “Structure and Interaction in Dense Colloidal Systems: Evaluation of Scattering Data by the Generalized Indirect Fourier Transformation Method,” Journal of Physics: Condensed Matter, Vol. 18, No. 36, 2006, pp. S2403-S2419. doi:10.1088/0953-8984/18/36/S14
- J. Brunner-Popela and O. Glatter, “Small-Angle Scattering of Interacting Particles. I. Basic Principles of a Global Evaluation Technique,” Journal of Applied Crystallography, Vol. 30, No. 4, 1997, pp. 431-442. doi:10.1021/jp811482w
- O. Glatter, “A New Method for the Evaluation of Small- Angle Scattering Data,” Journal of Applied Crystallography, Vol. 10, No. 5, 1977, pp. 415-421. doi:10.1107/S0021889877013879
- O. Glatter, “Comparison of Two Different Methods for Direct Structure Analysis from Small-Angle Scattering Data,” Journal of Applied Crystallography, Vol. 21, No. 6, 1988, pp. 886-890. doi:10.1107/S0021889877013879
- T. Kuhl, Y. Guo, J. L. Alderfer, A. Berman, D. Leckband, J. Israelachvili and S.W. Hui, “Direct Measurement of Polyethylene Glycol Induced Depletion Attraction between Lipid Bilayers,” Langmuir, Vol. 12, No. 12, 1996, pp. 3003-3014. doi:10.1021/la950802l