Journal of Behavioral and Brain Science
Vol.3 No.6(2013), Article ID:38824,10 pages DOI:10.4236/jbbs.2013.36050
Dopamine and GABA Interaction in Basal Ganglia: GABA-A or GABA-B Receptor Stimulation Attenuates L-DOPA-Induced Striatal and Nigral ERK1/2 Signaling in a Rat Model of Parkinson’s Disease
Department of Pharmacology and Toxicology, School of Medicine-Northwest, Indiana University, Gary, USA
Email: lynchsa@iun.edu, *ssivam@iun.edu
Copyright © 2013 Sarah Lynch, Subbiah P. Sivam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 14, 2013; revised September 21, 2013; accepted October 6, 2013
Keywords: Dopamine; ERK1/2; Hemiparkinsonism; GABA; Striatum; Substantia Nigra; Muscimol; Baclofen
ABSTRACT
Parkinson’s disease (PD) is characterized by degeneration of nigrostriatal dopamine (DA) neurons. The primary drug used to treat PD symptoms is L-DOPA, but side effects such as dyskinesias limit its use. Previous findings show that L-DOPA treatment induces extracellular signal-regulated kinase (ERK1/2), a MAP-kinase protein. γ-aminobutyric acid (GABA) is intimately involved in basal ganglia function. Our previous study using a unilaterally lesioned rat model of PD indicated that elevating GABA levels by GABA transaminase inhibitor, aminooxyacetic acid significantly attenuated L-DOPA-induced ERK phosphorylation in the striatum and substantia nigra (SN). The aim of the present study was to assess the role of GABA-A and GABA-B receptor by using a selective agonist, muscimol and baclofen respectively, on L-DOPA-induced ERK phosphorylation in the striatum and SN. Unilaterally 6-OHDA-lesioned rats were prescreened by apomorphine induced rotation test for the extent of DA loss. Lesioned rats were treated with L-DOPA alone or after muscimol or baclofen pretreatment. Appropriate control groups were used. Phospho-ERK levels, tyrosine hydroxylase (to ascertain DA loss) and substance P (an indirect marker for DA loss) levels were assessed by immunohistochemistry using coronal slices at the level of striatum and SN. L-DOPA administration induced a robust increase (>300%) in phospho-ERK1/2 levels in the striatum and SN. Muscimol as well as baclofen pretreatment attenuated the L-DOPA-induced increase in phospho-ERK1/2 levels by >60% in the striatum and SN. Muscimol and baclofen pretreatment also greatly reduced the number of L-DOPA induced phospho-ERK1/2 stained cells in the striatum as well as the contralateral rotational behavior. The present data taken together with our previous study indicate that the L-DOPA induced increase in ERK1/2 is attenuated by GABA via a GABA-A and GABA-B receptor linked mechanism. The study provides further insight into a dopamine-GABA-ERK interaction in the therapeutic and/or side effects of LDOPA in the basal ganglia.
1. Introduction
Dopaminergic neurons in the nigrostriatal pathway of the basal ganglia degenerate in Parkinson’s disease (PD). An imbalance in DA function leads to motor complications associated with the disease [1-3]. D1 receptors receive diminishing amounts of DA compensation by becoming supersensitive to the neurotransmitter. It is well established that L-DOPA and other DA agonists ameliorate certain motor deficits [4-6]. Long-term administration of L-DOPA, however, often leads to side effects. These include dyskinesias, characterized by loss of voluntary motor control as well as the emergence of involuntary motor control [7,8].
Numerous reports have stated that activation of supersensitive D1 receptors in the DA-depleted striatum leads to the phosphorylation of ERK1/2, which belongs to a class of mitogen-activated protein kinases (MAPKs) and is implicated in transcriptional and translational efficiency [5,9,10]. Reports involving both hemiparkinsonian rodents [10-12] and non-human primates [5,8,13]
have attributed this phenomenon to the development of motor side effects. The involvement of supersensitive D1 receptor stimulation and ERK1/2 signaling in the striatum and SN was further supported by more recent studies. D1 agonist, SKF-38393 increased phospho-ERK1/2 levels in the striatum and in the substantia nigra (SN), and both responses were blocked by D1 antagonist SCH- 23390 [14].
GABAergic medium-sized spiny neurons (MSNs) comprise about 95% of the striatum [5], and 99% of the entire basal ganglia [15]. GABA and DA systems in the basal ganglia are cooperatively and interdependently involved. Presynaptic DA modulation of GABA release in the striatum and SN [15] is the major physiological factor in motor control [5,8,16]. Many studies have reported that GABA transmission is indeed influenced by DA imbalance [17-20].
GABA receptors are of two major subtypes: ionotropic (GABA-A) and metabotropic G-protein-coupled (GABAB) receptors [21]. Although altered GABA transmission is well documented in PD, very little is known about the role of GABA receptors in DA-mediated effects as far downstream as ERK1/2. Our recent study [22] showed that elevating GABA levels by aminooxyacetic acid (AOAA) attenuated the L-DOPA induced induction of ERK1/2 in the striatum and SN. The present study employed a pharmacological approach to test whether stimulation of GABA-A or GABA-B receptors, by using selective agonists such as muscimol or baclofen, would modify the L-DOPA-induced rotational response and ERK1/2 phosphorylation in the striatum and SN in a unilaterally lesioned rat model of PD.
2. Materials and Methods
2.1. Animals
Animals were kept as previously described [22]. Female Sprague-Dawley rats (Harlan Laboratories, Inc.) weighing 245 - 320 g were maintained on a 12 h light/12h dark cycle at 22˚C ± 2˚C and 50% ± 10% humidity with ad libitum access to Wayne Lab Box chow and water. Experimental treatments and animal care protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine-Northwest.
2.2. Unilateral Dopaminergic Lesion with 6-Hydroxydopamine (6-OHDA)
Lesions of the nigrostriatal DA pathway were made in the right median forebrain bundle (MFB) as previously described [22] by infusion of 9 µg of free-base 6-OHDA neurotoxin (Research Biochemicals, Inc.) in ascorbic acid (4 μl, 0.1% in saline, Sigma-Aldrich, Inc.) using ketamine HCl/xylazine HCl solution (80 mg/kg, i.p., SigmaAldrich, Inc.) for anesthesia. The stereotaxic coordinates of the MFB relative to the bregma were A: 4.4. L: 1.2, V: 7.8, and the I-bar was set at −2.5 [23]. Noradrenergic neurons were protected from the neurotoxin with desipramine HCl (15 mg/kg, i.p., Sigma-Aldrich, Inc.) administered 60 min prior to neurotoxin infusion [24]. Meloxicam (1 mg/kg, s.c., Sigma-Aldrich, Inc.) was administered for two days post-surgery for pain relief.
2.3. Apomorphine-Induced Rotation Test to Screen Extent of Lesion
The extent of the unilateral 6-OHDA lesion was assessed by apomorphine-induced rotation test as previously described [22]. Two weeks post-lesion, an apomorphineinduced rotation test (apomorphine HCl, 0.1 mg/kg, i.p., Sigma-Aldrich, Inc.) was applied. Animals were observed for contralateral rotation by placing each in an enclosed hemispherical bowl. The number of rotations in 5-minute intervals at 15, 30, and 45 minutes were recorded. Animals with 5 or more average rotations/min were considered to have >90% DA depletion and were used for additional drug treatment.
2.4. L-DOPA, Muscimol, and Baclofen Treatments
The following groups of 6-OHDA-lesioned animals were used: a) vehicle + vehicle (control); b) vehicle + LDOPA; c) muscimol + vehicle; d) muscimol + L-DOPA; e) vehicle + baclofen; f) L-DOPA + baclofen; the numbers of animals in each group were 3, 6, 3, 6, 3,6 respectively. In the present study we administered muscimol or baclofen 30 min prior to L-DOPA and perfused the animals transcardially 30 minutes after L-DOPA administration. RO-4-4602 (50 mg/kg, i.p., Hoffmann-La Roche, Inc.) was given 30 minutes prior to L-DOPA administration (94.5 mg/kg, i.p., Sigma-Aldrich, Inc.). Vehicle + vehicle served as the independent control group. The experimental design allowed the comparison of not only the relative changes from the lesioned versus intact side in the same animal, but also each drug treatment group versus the independent control group. Ten minutes after L-DOPA injection, rats were observed for 5 minutes to observe the rotational response. An overdose of sodium pentobarbital (100 mg/kg, i.p., Sigma-Aldrich, Inc.) was used to perfuse animals.
2.5. Immunohistochemistry
The protocol for immunohistochemistry is as previously described [22]. In brief, animals were transcardially perfused with 10% sucrose and paraformaldehyde solution (Sigma-Aldrich, Inc.). Sections of the striatumand SNcontaining regions were cut coronally. Sections were rinsed in a series of phosphate-buffered saline (PBS) solutions, and incubated in 0.3% H2O2 as well as 10% normal goat serum. Immunoreactivity was detected using affinity-purified monoclonal tyrosine hydroxylase (TH) (Affinity Bioreagents, Inc.), phospho-ERK1/2 (phosphop44/42 MAP kinase (thr202/tyr204); Cell Signaling Technology, Inc.), and substance P (SP). Sections were incubated in 1˚ antibody solutions for 24 hours, followed by PBS rinses and incubated for TH detection in affinitypurified biotinylated anti-mouse IgG and for SP and phospho-ERK1/2 detection in anti-rabbit IgG (Vector Laboratories, Inc.). Ready-to-use Vectastain Elite ABC kit (Vector Laboratories, Inc.) with DAB was used for final antibody staining. Sections were mounted, soaked in a series of ethanol and xylene rinses, and covered using Permount (Fisher Scientific, Inc.).
2.6. Quantification and Statistical Analysis
Quantification of Immunostaining. The immunostaining intensities for TH, SP and phospho-ERK1/2 in the striatum and SN of the intact and lesioned sides were determined as previously described [22] with ImageJ (NIH) and quantified using QuantiScan software (Biosoft, Inc.). The quantified values of TH, SP, and phosphoERK1/2 immunoreactivity of the lesioned side were expressed as percent change from that of the intact side. For analysis of the effect of muscimol or baclofen pretreatment, the percent change in muscimol + L-DOPA or baclofen + L-DOPA treatment group was compared to the percent change in the vehicle + L-DOPA group.
Quantification of Striatal Phospho-ERK1/2 Labeled Cell Counts. Counts of phospho-ERK1/2 immunostained cells were performed as previously described [22] on images of the striatum at 200× magnification using Image-Pro Plus 7.0 (Media Cybernetics, Inc.). Eight sample areas of 0.54 mm2 per striatum were counted, and values are expressed in cells/mm2 [25].
Stastical Analysis. All values are depicted as mean ± SEM. Group means were compared using SigmaStat (Systat Software, Inc.) to apply one-way analysis of variance followed by Newman-Keuls multiple range test. P < 0.05 was considered significant.
3. Results
3.1. Assessment of Rotational Response in Unilaterally 6-OHDA Lesioned Animals
In control, muscimol or baclofen-treated animals, no rotations were observed. In animals treated with vehicle + L-DOPA, an average of 13.3 ± 1.6 rotations per minute was observed. Finally, in animals pretreated with muscimol or baclofen followed by L-DOPA, averages of only 0.9 ± 0.6 and 0.9 ± 0.5 rotations, respectively, were observed, indicating that muscimol or baclofen pretreatment greatly attenuated L-DOPA-induced rotations (Figure 1).
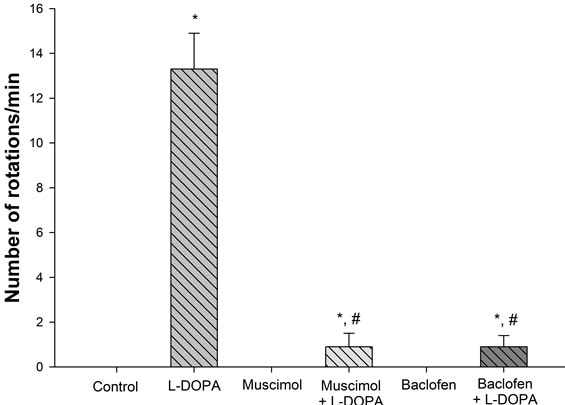
Figure 1. Influence of muscimol or baclofen on L-DOPA-induced rotations. Animals in control and muscimol or baclofen groups did not rotate. Administration of L-DOPA induced a robust rotational response. Pretreatment of muscimol or baclofen greatly reduced L-DOPA-induced rotations. *p < 0.05 as compared to the other groups. #p < 0.05 as compared to L-DOPA group.
3.2. Influence of L-DOPA, Muscimol, and Baclofen on TH, SP, and Phospho-ERK1/2 Levels in the Striatum of Unilaterally 6-OHDA Lesioned Animals
Immunoreactive Intensity of TH, SP, and PhosphoERK1/2 in the Striatum (Figures 2 and 3). A greater than 90% decrease in TH immunoreactivity in the lesioned striatum was observed for control, muscimol, baclofen, L-DOPA, muscimol + L-DOPA and baclofen + L-DOPA groups. The lesioned striatum of animals in all aforementioned groups demonstrated a moderate loss of SP immunoreactivity. Muscimol, baclofen, L-DOPA, musicmol + L-DOPA or baclofen + L-DOPA treatments did not alter the basal loss of TH or SP immunoreactivity in the lesioned striatum. In the unlesioned side (intact striatum), none of the treatment groups showed phosphoERK1/2 immunoreactivity. In control, muscimol or baclofen treated rats, phospho-ERK1/2 immunoreactivity was not apparent in the lesioned or intact striatum. LDOPA treatment resulted in a robust activation of phospho-ERK1/2 in the striatum in the lesioned side as compared to the intact side. Pretreatment of muscimol or baclofen to stimulate GABA-A or GABA-B receptors followed by L-DOPA administration resulted in significantly lower levels of phospho-ERK1/2 in the striatum as compared to phospho-ERK1/2 in the L-DOPA group.
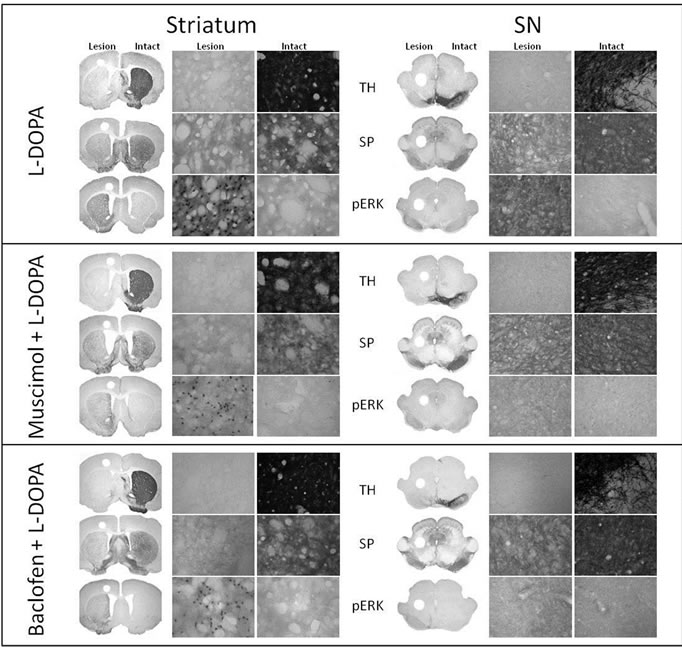
Figure 2. GABA-A and GABA-B receptor agonists attenuate L-DOPA-induced phospho-ERK1/2 signaling. Representative micrographs showing immunohistochemistry analysis of TH, SP, and phospho-ERK1/2 following L-DOPA administration (left), after muscimol + L-DOPA (middle), and after baclofen + L-DOPA (right) in the striatum (upper panel) and SN (bottom panel) of unilaterally 6-OHDA lesioned animals. The loss of TH and SP immunoreactivity was not altered by the treatments. There was a robust increase of phospho-ERK1/2 immunoreactivity in the L-DOPA treatment group. Muscimol and baclofen pretreatment significantly attenuated L-DOPA-induced increase of phospho-ERK1/2 immunoreactivity. The coronal slices represent 1.25× magnification and the higher power images represent 400× magnification. A summary of changes in all of the groups is depicted in Figure 3.
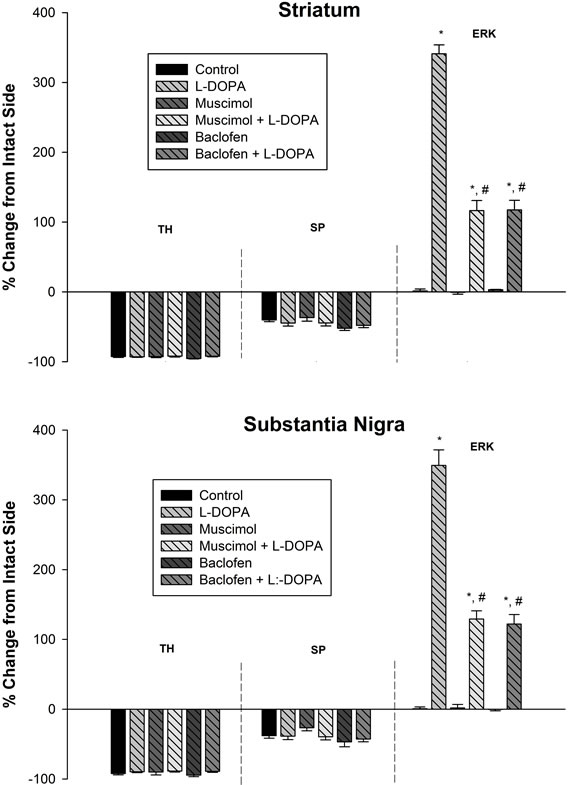
Figure 3. GABA-A and GABA-B receptor agonists attenuate L-DOPA-induced phospho-ERK1/2 signaling: quantitative analysis of TH, SP, and phospho-ERK1/2 immunoreactivity following drug administration in the striatum (upper panel) and SN (lower panel) of unilaterally 6-OHDA lesioned animals. TH immunoreactivity in all groups was severely depleted in the lesioned side as compared to the intact side. SP immunoreactivity in all groups was moderately depleted in the lesioned side as compared to the intact side. In control, muscimol, and baclofen treated animals, phospho-ERK1/2 immunoreactivity was not apparent. In L-DOPA treated animals, phospho-ERK1/2 immunoreactivity increased robustly in the striatum and SN in the lesioned side as compared to the intact side. In muscimol + L-DOPA and baclofen + L-DOPA treated rats, phospho-ERK1/2 immunoreactivity was significantly attenuated in the striatum and SN when compared to the L-DOPA group. Data were expressed as percent change of the lesioned side compared to intact side. *p < 0.05 as compared to the other groups. #p < 0.05 as compared to L-DOPA group.
Cells Labeled with Phospho-ERK1/2 in the Striatum (Figure 4). The number of immunostained cells labeled with phospho-ERK1/2 was counted in the striatum. In control and muscimol or baclofen treated animals, no phospho-ERK1/2 labeled cells were apparent. In LDOPA treated animals, the number of phospho-ERK1/2 labeled cells was 1440 ± 56 cells/mm2. In muscimol + L-DOPA and baclofen + L-DOPA treated animals, the number of phospho-ERK1/2 labeled cells was 1063 ± 61 and 1062 ± 26 cells/mm2 respectively. Thus, both mus-
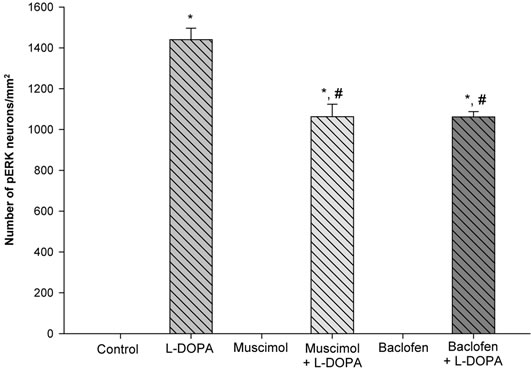
Figure 4. GABA-A and GABA-B receptor agonists attenuate the number of L-DOPA induced phospho-ERK1/2 labeled cells in the striatum of unilaterally 6-OHDA lesioned animals. In control, muscimol, and baclofen treated animals, no cells showing phospho-ERK1/2 immunoreactivity were observed. In L-DOPA treated animals, robust amounts of phospho-ERK1/2 labeled cells were observed. In muscimol + L-DOPA and baclofen + L-DOPA treated animals, significantly fewer phospho-ERK1/2 labeled cells were observed. *p < 0.05 as compared to the other groups. #p < 0.05 as compared to L-DOPA.
cimol + L-DOPA and baclofen + L-DOPA treatments significantly decreased the amount of phospho-ERK1/2 labeled cells as compared to the rats treated with LDOPA alone.
3.3. Influence of L-DOPA, Muscimol, and Baclofen on TH, SP, and Phospho-ERK1/2 Levels in the SN of Unilaterally Lesioned Animals
Results similar to those seen in the striatum were observed within the SN with regard to changes in TH, SP, and phospho-ERK1/2 immunoreactivity. L-DOPA treatment resulted in the robust activation of phospho-ERK1/ 2 in the SN. Administration of muscimol or baclofen to stimulate GABA-A or GABA-B receptors followed by L-DOPA treatment resulted in significantly lower levels of phospho-ERK1/2 in the SN as compared to phospho-ERK1/2 in the L-DOPA group (Figures 2 and 3).
4. Discussion
In the present study, we reported a new finding that pretreatment with GABA-A agonist, muscimol, or GABA-B agonist, baclofen attenuated the L-DOPA-induced rotational response as well as increase in phospho-ERK1/2 levels both in the striatum and SN. These data together with our previous study [22] indicate that there is a DAGABA link in the modulation of L-DOPA-induced increase in ERK1/2.
Basal ganglia activity depends on the direct (striatonigral) and indirect (striatopallidal) pathway, subserved by D1 and D2 receptors, respectively [4]. An imbalance of direct and indirect pathway activity occurs in PD following DA neuron degeneration in the direct pathway [1,26]. DA agonists activate phospho-ERK1/2 via D1 receptors in the DA depleted striatum [14,22,27]. Additionally, alterations in synaptic plasticity [28,29] and gene expression [4,30], such as increased D1 receptor expression at the plasma membrane [8,31] and the switch in the ERK1/ 2 pathway regulation [7], have been attributed to PDassociated D1 supersensitivity. The extent of phosphoERK1/2 activation has been linked to the severity of dyskinesias, a motor side effect of long-term L-DOPA treatment [5,10]. These studies taken together support the conclusion that D1 receptor stimulation is responsible for L-DOPA-induced ERK1/2 phosphorylation in the striatum and SN.
GABAergic medium-sized spiny neurons (MSNs) are morphologically the main neuron type in the striatum [15,16] and the SN pars reticulata (SNr) [15,16]. DA regulates GABA-releasing MSN activity by activating DA receptors [8,32,33]. It has been reported that LDOPA administration induces alterations in GABA transmission in the striatum and SN [17,18,20]. Restored DA activity via D1 receptor activation by L-DOPA administration in unilaterally 6-OHDA lesioned rats results in enhanced GABA transmission in the basal ganglia [34]. Immunofluorescence and western blot [27] and immunohistochemical [10] analyses demonstrated that L-DOPAinduced phospho-ERK1/2 activation occurred selectively on striatal MSNs expressing D1 receptors. We reported recently that pretreatment with GABA level-enhancing agent, AOAA, attenuated the L-DOPA-induced phosphoERK1/2 activation in the striatum and SN [22]. These reports and the present study taken together suggest that GABA modulates MAPK pathways via a DA-dependent mechanism.
The D1 receptor-mediated enhancement of GABA transmission has been linked to GABA-A receptor activation [17,35]. GABA-A receptor activation resulted in increased striatal and nigral DARPP-32 phosphorylation [36]. In dyskinetic mice, sensitized cAMP/cAMP-dependent protein kinase/DARPP-32 signaling leads to the phosphorylation of ERK1/2 following L-DOPA administration [12,37]. It is recently found that L-DOPA-induced ERK1/2 phosphorylation requires DARPP-32 in MSNs that express D1 receptors [38]. These reports suggest that DARPP-32 may also be involved in the mechanism DA, GABA, and ERK1/2 interactions.
Ionotropic GABA-A receptors are generally located on symmetrical synapses, whereas metabotropic GABA-B receptors are found in all regions of the plasma membrane. Because of this localization, it has been concluded that GABA-A receptors in the basal ganglia mediate fast GABA transmission, and GABA-B receptors are involved in subtle and more complex GABAergic effects [39]. Although the two receptor types mediated their effects via different mechanisms, in the present study, stimulation of either GABA-A or GABA-B receptors attenuated L-DOPA-induced ERK1/2 phosphorylation. This finding further extends and supports our recent finding of GABAergic influence on the attenuation of L-DOPAinduced ERK1/2 phosphorylation [22]. It is possible that GABA-A or GABA-B receptors suppress the lesioninduced D1 receptor supersensitivity. This in turn may lead to a reduced induction of ERK1/2 by L-DOPA. However, the exact mechanisms involved in this process have yet to be understood.
Other studies have also suggested that GABA may have a beneficial rather than detrimental effect in treating PD. Local GABA-A receptor activation in the striatum or SNr offers beneficial effects in experimental Parkinsonism. GABA-A receptor agonist muscimol, injected into the SNr of parkinsonian monkeys, has been reported to ameliorate motor symptoms [40]. Implantation of genetically-engineered GABA-releasing cells into the SNr showed similar results in 6-OHDA-lesioned rats [41]. Injection of benzodiazepines, believed to act via GABAA receptors [42], and after transplantation of GABA-rich grafts [43], both into the striatum, resulted in behavioral recovery in 6-OHDA-lesioned rats. In clinical trials, motor symptoms are mildly relieved with GABAergic drugs [16]. These studies taken together further support the concept that enhanced GABAergic transmission may help alleviate motor side effects of L-DOPA.
GABA is also implicated in the effects of adenosine A2A receptor antagonists that appear to be useful in the treatment of PD. Adenosine A2A receptors are densely localized in the basal ganglia, concentrated on GABAergic MSNs [3]. Both animal models and human patients of PD have exhibited prominent therapeutic effects after administration of adenosine A2A receptor antagonists [44, 45] without dyskinetic side effects [46,47] as well as reduced L-DOPA-induced dyskinesias [3,48]. Increased GABA release in the SNr of 6-OHDA lesioned rats has also been demonstrated after administration of adenosine A2A receptor antagonists [3,49]. It has been suggested that facilitated GABA transmission in the striatum reduces striatopallidal neuronal activity, helping to restore the balance of striatal output; such an enhanced GABA transmission could possibly be involved in the amelioration rather than occurrence of L-DOPA-induced motor side effects [3].
In summary, we report for the first time that stimulation of GABA-A or GABA-B receptors attenuates the L-DOPA-induced phospho-ERK1/2 levels in the striatum and SN and also L-DOPA induced rotations. The results of this study further support the importance of GABA systems in L-DOPA induced effects, suggesting the possibility of GABAergic drugs to enhance therapeutic benefits and/or reduce motor side effects of L-DOPA therapy.
5. Acknowledgements
This work was supported in part by a Research Enhancement Award from IUPUI, a summer research internship award from IUSM-NW, and an award from IUN Research Fund. The generous supply of SP antiserum used in this study from Dr. J.-S. Hong, NIEHS, Research Triangle Park is greatly appreciated.
REFERENCES
- C. R. Gerfen, “D1 Dopamine Receptor Supersensitivity in the Dopamine-Depleted Striatum: Animal Model of Parkinson’s Disease,” Neuroscientist, Vol. 9, No. 6, 2003, pp. 455-462. http://dx.doi.org/10.1177/1073858403255839
- J. W. Mink, “The Basal Ganglia and Involuntary Movements: Impaired Inhibition of Competing Motor Patterns,” Archives of Neurology, Vol. 60, No. 10, 2003, pp. 1365-1368. http://dx.doi.org/10.1001/archneur.60.10.1365
- P. Hickey and M. Stacy, “Available and Emerging Treatments for Parkinson’s Disease: A Review,” Drug Design, Development, and Therapy, Vol. 5, 2011, pp. 241-254.
- C. R. Gerfen, “Molecular Effects of Dopamine on Striatal Projection Pathways,” Trends in Neurosciences, Vol. 23, 2000, pp. S64-S70.
- A. Berthet and E. Bezard, “Dopamine Receptors and L- Dopa-Induced Dyskinesia,” Parkinsonism & Related Disorders, Vol. 15, No. 4, 2009, pp. S8-12. http://dx.doi.org/10.1016/S1353-8020(09)70827-2
- A. Nadjar, C. R. Gerfen and E. Bezard, “Priming for lDopa-Induced Dyskinesia in Parkinson’s Disease: A Feature Inherent to the Treatment or the Disease?” Progress in Neurobiology, Vol. 87, No. 1, 2009, pp. 1-9. http://dx.doi.org/10.1016/j.pneurobio.2008.09.013
- C. R. Gerfen, S. Miyachi, R. Paletzki and P. Brown, “D1 Dopamine Receptor Supersensitivity in the Dopamine- Depleted Striatum Results from a Switch in the Regulation of ERK1/2/MAP Kinase,” Journal of Neuroscience, Vol. 22, No. 12, 2002, pp. 5042-5054.
- M. Feyder, A. Bonito-Oliva and G. Fisone, “L-DOPA- Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission,” Frontiers in Behavioral Neuroscience, Vol. 5, 2011, p. 71.
- D. S. Kim, R. D. Palmiter, A. Cummins and C. R. Gerfen, “Reversal of Supersensitive Striatal Dopamine D1 Receptor Signaling and Extracellular Signal-Regulated Kinase Activity in Dopamine-Deficient Mice,” Neuroscience, Vol. 137, No. 4, 2006, pp. 1381-1388. http://dx.doi.org/10.1016/j.neuroscience.2005.10.054
- J. E. Westin, L. Vercammen, E. M. Strome, C. Konradi, and M. A. Cenci, “Spatiotemporal Pattern of Striatal ERK1/2 Phosphorylation in a Rat Model of L-DOPA- Induced Dyskinesia and the Role of Dopamine D1 Receptors,” Biological Psychiatry, Vol. 62, No. 7, 2007, pp. 800-810. http://dx.doi.org/10.1016/j.biopsych.2006.11.032
- N. Pavon, A. B. Martin, A. Mendialdua and R. Moratalla, “ERK Phosphorylation and FosB Expression Are Associated with L-DOPA-Induced Dyskinesia in Hemiparkinsonian Mice,” Biological Psychiatry, Vol. 59, No. 1, 2006, pp. 64-74. http://dx.doi.org/10.1016/j.biopsych.2005.05.044
- E. Santini, E. Valjent, A. Usiello, M. Carta, A. Borgkvist, J. A. Girault, D. Herve, P. Greengard and G. Fisone, “Critical Involvement of cAMP/DARPP-32 and Extracellular Signal-Regulated Protein Kinase Signaling in LDOPA-Induced Dyskinesia,” Journal of Neuroscience, Vol. 27, No. 26, 2007, pp. 6995-7005. http://dx.doi.org/10.1523/JNEUROSCI.0852-07.2007
- M. A. Cenci, K. E. Ohlin and D. Rylander, “Plastic Effects of L-DOPA Treatment in the Basal Ganglia and Their Relevance to the Development of Dyskinesia,” Parkinsonism & Related Disorders, Vol. 15, No. 3, 2009, pp. S59-S63. http://dx.doi.org/10.1016/S1353-8020(09)70782-5
- C. Moreno and S. Sivam, “The Time Course of D1 Agonist Induced Striatonigral ERK1/2 Signaling in a Rat Model of Parkinson’s Disease,” Journal of Behavioral and Brain Science, Vol. 2, No. 1, 2012, pp. 1-9. http://dx.doi.org/10.4236/jbbs.2012.21001
- J. M. Tepper, E. D. Abercrombie and J. P. Bolam, “Basal Ganglia Macrocircuits,” Progress in Brain Research, Vol. 160, 2007, pp. 3-7. http://dx.doi.org/10.1016/S0079-6123(06)60001-0
- A. Galvan and T. Wichmann, “GABAergic Circuits in the Basal Ganglia and Movement Disorders,” Progress in Brain Research, Vol. 160, 2007, pp. 287-312. http://dx.doi.org/10.1016/S0079-6123(06)60017-4
- T. Trevitt, B. Carlson, M. Correa, A. Keene, M. Morales and J. D. Salamone, “Interactions between Dopamine D1 Receptors and Gamma-Aminobutyric Acid Mechanisms in Substantia Nigra Pars Reticulata of the Rat: Neurochemical and Behavioral Studies,” Psychopharmacology (Berl), Vol. 159, No. 3, 2002, pp. 229-237. http://dx.doi.org/10.1007/s002130100908
- M. Ochi, S. Shiozaki and H. Kase, “L-DOPA-Induced Modulation of GABA and Glutamate Release in Substantia Nigra Pars Reticulata in a Rodent Model of Parkinson’s Disease,” Synapse, Vol. 52, No. 2, 2004, pp. 163- 165. http://dx.doi.org/10.1002/syn.20006
- H. Wang, J. Katz, P. Dagostino and J. J. Soghomonian, “Unilateral 6-Hydroxydopamine Lesion of Dopamine Neurons and Subchronic L-DOPA Administration in the Adult Rat Alters the Expression of the Vesicular GABA Transporter in Different Subsets of Striatal Neurons and in the Substantia Nigra, Pars Reticulata,” Neuroscience, Vol. 145, No. 2, 2007, pp. 727-737. http://dx.doi.org/10.1016/j.neuroscience.2006.12.001
- C. Rangel-Barajas, I. Silva, L. M. Lopez-Santiago, J. Aceves, D. Erlij and B. Floran, “L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats Is Associated with UpRegulation of Adenylyl Cyclase Type V/VI and Increased GABA Release in the Substantia Nigra Reticulata,” Neurobiology of Disease, Vol. 41, No. 1, 2011, pp. 51-61. http://dx.doi.org/10.1016/j.nbd.2010.08.018
- F. Calon, M. Morissette, A. H. Rajput, O. Hornykiewicz, P. J. Bedard and T. Di Paolo, “Changes of GABA Receptors and Dopamine Turnover in the Postmortem Brains of Parkinsonians with Levodopa-Induced Motor Complications,” Movement Disorders, Vol. 18, No. 3, 2003, pp. 241-253. http://dx.doi.org/10.1002/mds.10343
- S. Lynch and S. P. Subbiah, “GABA Attenuates L- DOPA-Induced Striatal and Nigral ERK1/2 Signaling in a Rat Model of Parkinson’s Disease,” Journal of Behavioral and Brain Science, Vol. 3, No. 3, 2013, pp. 320-330. http://dx.doi.org/10.4236/jbbs.2013.33032
- G. Paxinos and C. Watson, “The Rat Brain in Stereotaxic Coordinates,” 5th Edition, Elsevier Academic Press, New York, 2005.
- G. R. Breese, A. A. Baumeister, T. J. McCown, S. G. Emerick, G. D. Frye, K. Crotty and R. A. Mueller, “Behavior Differences between Neonatal and Adult 6-Hydrocydopamine-Treated Rats to Dopamine Agonists: Relevance to Neurological Symptoms in Clinical Syndromes with Reduced Brain Dopamine,” Journal of Pharmacology and Experimental Therapeutics, Vol. 231, 1984, pp. 343-354.
- M. Andersson, C. Konradi and M. A. Cenci, “cAMP Response Element-Binding Protein Is Required for Dopamine-Dependent Gene Expression in the Intact But Not the Dopamine-Denervated Striatum,” Journal of Neuroscience, Vol. 21, No. 24, 2001, pp. 9930-9943.
- M. Lebel, P. Robinson and M. Cyr, “Canadian Association of Neurosciences Review: The Role of Dopamine Receptor Function in Neurodegenerative Diseases,” Canadian Journal of Neurological Sciences, Vol. 34, No. 1, 2007, pp. 18-29.
- E. Santini, C. Alcacer, S. Cacciatore, M. Heiman, D. Herve, P. Greengard, J. A. Girault, E. Valjent and G. Fisone, “L-DOPA Activates ERK Signaling and Phosphorylates Histone H3 in the Striatonigral Medium Spiny Neurons of Hemiparkinsonian Mice,” Journal of Neurochemistry, Vol. 108, No. 3, 2009, pp. 621-633. http://dx.doi.org/10.1111/j.1471-4159.2008.05831.x
- G. M. Thomas and R. L. Huganir, “MAPK Cascade Signalling and Synaptic Plasticity,” Nature Reviews Neuroscience, Vol. 5, No. 3, 2004, pp. 173-183. http://dx.doi.org/10.1038/nrn1346
- C. R. Gerfen, R. Paletzki and P. Worley, “Differences between Dorsal and Ventral Striatum in Drd1a Dopamine Receptor Coupling of Dopamineand cAMP-Regulated Phosphoprotein-32 to Activation of Extracellular Signal- Regulated Kinase,” Journal of Neuroscience, Vol. 28, No. 28, 2008, pp. 7113-7120. http://dx.doi.org/10.1523/JNEUROSCI.3952-07.2008
- J. D. Berke, R. F. Paletzki, G. J. Aronson, S. E. Hyman and C. R. Gerfen, “A Complex Program of Striatal Gene Expression Induced by Dopaminergic Stimulation,” Journal of Neuroscience, Vol. 18, No. 14, 1998, pp. 5301- 5310.
- A. Berthet, G. Porras, E. Doudnikoff, H. Stark, M. Cador, E. Bezard and B. Bloch, “Pharmacological Analysis Demonstrates Dramatic Alteration of D1 Dopamine Receptor Neuronal Distribution in the Rat Analog of L-DOPAInduced Dyskinesia,” Journal of Neuroscience, Vol. 29, No. 15, 2009, pp. 4829-4835. http://dx.doi.org/10.1523/JNEUROSCI.5884-08.2009
- E. Santini, E. Valjent and G. Fisone, “Parkinson’s Disease: Levodopa-Induced Dyskinesia and Signal Transduction,” FEBS Journal, Vol. 275, No. 7, 2008, pp. 1392- 1399. http://dx.doi.org/10.1111/j.1742-4658.2008.06296.x
- E. Moreno, H. Hoffmann, M. Gonzalez-Sepulveda, G. Navarro, V. Casado, A. Cortes, J. Mallol, M. Vignes, P. J. McCormick, E. I. Canela, C. Lluis, R. Moratalla, S. Ferre, J. Ortiz and R. Franco, “Dopamine D1-Histamine H3 Receptor Heteromers Provide a Selective Link to MAPK Signaling in GABAergic Neurons of the Direct Striatal Pathway,” Journal of Biological Chemistry, Vol. 286, No. 7, 2011, pp. 5846-5854. http://dx.doi.org/10.1074/jbc.M110.161489
- J. Aceves, B. Floran, D. Martinez-Fong, A. Sierra, S. Hernandez and S. Mariscal, “L-DOPA Stimulates the Release of [3H]gamma-Aminobutyric Acid in the Basal Ganglia of 6-Hydroxydopamine Lesioned Rats,” Neuroscience Letters, Vol. 121, 1991, pp. 223-226. http://dx.doi.org/10.1016/0304-3940(91)90690-U
- H. Ikeda, A. Kotani, N. Koshikawa and A. R. Cools, “Differential Role of GABAA and GABAB Receptors in Two Distinct Output Stations of the Rat Striatum: Studies on the Substantia Nigra Pars Reticulata and the Globus Pallidus,” Neuroscience, Vol. 167, No. 1, 2010, pp. 31-39. http://dx.doi.org/10.1016/j.neuroscience.2010.01.054
- G. L. Snyder, G. Fisone and P. Greengard, “Phosphorylation of DARPP-32 Is Regulated by GABA in Rat Striatum and Substantia Nigra,” Journal of Neurochemistry, Vol. 63, No. 5, 1994, pp. 1766-1771. http://dx.doi.org/10.1046/j.1471-4159.1994.63051766.x
- Y. Ding, L. Won, J. P. Britt, S. A. Lim, D. S. McGehee and U. J. Kang, “Enhanced Striatal Cholinergic Neuronal Activity Mediates L-DOPA-Induced Dyskinesia in Parkinsonian Mice,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 108, No. 2, 2011, pp. 840-845. http://dx.doi.org/10.1073/pnas.1006511108
- E. Santini, M. Feyder, G. Gangarossa, H. S. Bateup, P. Greengard and G. Fisone, “Dopamineand cAMP-Regulated Phosphoprotein of 32 KDa (DARPP-32)-Dependent Activation of ERK and Mammalian Target of Rapamycin Complex 1 (TORC1) Signaling in Experimental Parkinsonism,” Journal of Biological Chemistry, Vol. 287, , 2012, pp. 27806-27812. http://dx.doi.org/10.1074/jbc.M112.388413
- J. Boyes, and J. P. Bolam, “Localization of GABA Receptors in the Basal Ganglia,” Progress in Brain Research, Vol. 160, 2007, pp. 229-243. http://dx.doi.org/10.1016/S0079-6123(06)60013-7
- T. Wichmann, M. A. Kliem and M. R. DeLong, “Antiparkinsonian and Behavioral Effects of Inactivation of the Substantia Nigra Pars Reticulata in Hemiparkinsonian Primates,” Experimental Neurology, Vol. 167, No. 2, 2001, pp. 410-424. http://dx.doi.org/10.1006/exnr.2000.7572
- B. B. Carlson, S. Behrstock, A. J. Tobin and J. D. Salamone, “Brain Implantations of Engineered GABA-Releasing Cells Suppress Tremor in an Animal Model of Parkinsonism,” Neuroscience, Vol. 119, No. 4, 2003, pp. 927- 932. http://dx.doi.org/10.1016/S0306-4522(03)00218-5
- C. C. Tenn and L. P. Niles, “Mechanisms Underlying the Antidopaminergic Effect of Clonazepam and Melatonin in Striatum,” Neuropharmacology, Vol. 36, No. 11-12, 1997, pp. 1659-1663. http://dx.doi.org/10.1016/S0028-3908(97)00165-2
- C. Winkler, C. Bentlage, G. Nikkhah, M. Samii and A. Bjorklund, “Intranigral Transplants of GABA-Rich Striatal Tissue Induce Behavioral Recovery in the Rat Parkinson Model and Promote the Effects Obtained by Intrastriatal Dopaminergic Transplants,” Experimental Neurology, Vol. 155, No. 2, 1999, pp. 165-186. http://dx.doi.org/10.1006/exnr.1998.6916
- R. A. Hauser, J. P. Hubble and D. D. Truong, “Randomized Trial of the Adenosine A2A Receptor Antagonist Istradefylline in Advanced PD,” Neurology, Vol. 61, No. 3, 2003, pp. 297-303. http://dx.doi.org/10.1212/01.WNL.0000081227.84197.0B
- H. Kase, S. Aoyama, M. Ichimura, K. Ikeda, A. Ishii, T. Kanda, K. Koga, N. Koike, M. Kurokawa, Y. Kuwana, A. Mori, J. Nakamura, H. Nonaka, M. Ochi, M. Saki, J. Shimada, T. Shindou, S. Shiozaki, F. Suzuki, M. Takeda, K. Yanagawa, P. J. Richardson, P. Jenner, P. Bedard, E. Borrelli, R. A. Hauser and T. N. Chase, “Progress in Pursuit of Therapeutic A2A Antagonists: The Adenosine A2A Receptor Selective Antagonist KW6002: Research and Development toward a Novel Nondopaminergic Therapy for Parkinson’s Disease,” Neurology, Vol. 61, No. 11, Suppl. 6, 2003, pp. S97-S100. http://dx.doi.org/10.1212/01.WNL.0000095219.22086.31
- P. Jenner, “Istradefylline, a Novel Adenosine A2A Receptor Antagonist, for the Treatment of Parkinson’s Disease,” Expert Opinion on Investigational Drugs, Vol. 14, No. 6, 2005, pp. 729-738. http://dx.doi.org/10.1517/13543784.14.6.729
- A. Pinna, J. Wardas, N. Simola and M. Morelli, “New Therapies for the Treatment of Parkinson’s Disease: Adenosine A2A Receptor Antagonists,” Life Sciences, Vol. 77, No. 26, 2005, pp. 3259-3267. http://dx.doi.org/10.1016/j.lfs.2005.04.029
- J. M. Savola, M. Hill, M. Engstrom, H. Merivuori, S. Wurster, S. G. McGuire, S. H. Fox, A. R. Crossman and J. M. Brotchie, “Fipamezole (JP-1730) Is a Potent Alpha2 Adrenergic Receptor Antagonist that Reduces Levodopa- Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease,” Movement Disorders, Vol. 18, No. 8, 2003, pp. 872-883. http://dx.doi.org/10.1002/mds.10464
- M. Ochi, S. Shiozaki and H. Kase, “Adenosine A2A Receptor-Mediated Modulation of GABA and Glutamate Release in the Output Regions of the Basal Ganglia in a Rodent Model of Parkinson’s Disease,” Neuroscience, Vol. 127, No. 1, 2004, pp. 223-231. http://dx.doi.org/10.1016/j.neuroscience.2004.04.050
NOTES
*Corresponding author.

