Agricultural Sciences
Vol.06 No.12(2015), Article ID:62134,9 pages
10.4236/as.2015.612141
Arbuscular Mycorrhizal Fungus Mediate Changes in Mycorrhizosphere Soil Aggregates
Tao Liang1,2,3, Xiaojun Shi1,2*, Tao Guo1,2, Sili Peng1
1College of Resources and Environment, Southwest University, Chongqing, China
2The National Monitoring Base for Purple Soil Fertility and Fertilizer Efficiency, Southwest University, Chongqing, China
3Beibei District Agricultural Committee, Chongqing, China

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 September 2015; accepted 21 December 2015; published 23 December 2015
ABSTRACT
Many studies have shown that arbuscular mycorrhizal (AM) fungus has an important role in soil aggregate formation and stabilization. While most studies about the effects of AM fungus on soil aggregate have experimental set-ups in single pots or containers with two compartments, these studies cannot differentiate the effects of roots, mycorrhizal roots or hyphae. In this study we used containers with four compartments to split the roots and quantitatively compare the change of soil aggregate in the mycorrhizosphere soil, rhizosphere soil, hyphosphere soil and bulk soil. Our results demonstrate a significant positive correlation among hyphal length density, easily extractable glomalin (EEG) and aggregate mean weight diameter (MWD), geometric mean diameter (GMD) and percentage of soil macroaggregate with a diameter larger than 0.25 mm (R0.25). The GMD and MWD of R0.25 in the hyphal compartment were higher than those in the non-inoculated root compartment, but were lower than those in the mycorrhizal compartment. This suggests the mycorrhizal hyphae had a greater effect than the non-inoculated roots, but less of an effect than the mycorrhizal roots on the formation and stabilization of soil aggregate. The results reveal that plant roots, mycorrhizal roots and mycorrhizal hyphae contribute to aggregate stability in individual ways and that their effects are additive, creating a synergistic stabilizing effect.
Keywords:
Hyphae, Hyphosphere, Mycorrhizae, Rhizosphere, Water Stable Aggregate

1. Introduction
Soil structure refers to the aggregate stability and the arrangement of individual soil particles. Soil aggregate stability is a critical soil property affecting a wide range of biogeochemical and physical processes in agricultural and natural environments [1] . Statistics such as geometric mean diameter (GMD), mean weight diameter (MWD) and R0.25 (the percentage of the soil macroaggregate with a diameter more than 0.25 mm) have been historically used [2] [3] to quantitatively describe the stability characteristics and distribution of soil aggregate. Most high plants, including pasture plants and major crop species [4] , can form associations with arbuscular mycorrhizal (AM) fungus [5] and AM are important components of soil and plant systems. Extensive networks of AM fungal hyphae spread from mycorrhizal-infected plant roots into the surroundings and play important roles in physical and chemical processes [6] . Glomalin is a hydrophobic proteinaceous and insoluble substance produced by AM fungus [7] that acts as glue, binding soil particles together [8] and forming soil aggregates. It can be detected or quantified in different ways and Rillig [9] developed a method of soil extraction (121˚C in citrate buffer) that was then evaluated with a Bradford assay as glomalin-related soil protein (GRSP). Many reports have shown that AM associations are able to affect the formation and maintenance of soil aggregates through large networks of fungal hyphae and their exudates (glomalin, for example) and residues [6] [10] [11] .
However, most of these earlier studies have been based on single container experiments using sterilized soil [12] -[14] , field observations [15] or experimental studies [16] . In these studies the roots and hyphae were studied together and the effects solely attributed to AM hyphae were detected by conceptual models [17] or mathematical analysis (e.g. path analysis) [18] .
In this study, we present a novel experimental device that consists of four compartments used to independently measure the effects of plant roots, mycorrhizal roots, and mycorrhizal hyphae on soil aggregate. These culturing conditions are different from the conditions used in other studies. Our objectives were to determine: 1) the direct effects of mycorrhizae and its root and fungal components on soil aggregate; and 2) the relationship among hyphae, hyphal exudates (GRSP) and the stability and distribution of soil aggregate in the light of MWD, GMD and R0.25.
2. Materials and Methods
2.1. Mycorrhizae, Soil and Plants
The AM fungi used were Glomus intraradices, G. mosseae and G. etunicatum provided by the College of Resources and Environmental Sciences, Southwest University. The mycorrhiazal inoculum consisted of soil containing spores and colonized clover-root fragments. The host plant species used was clover (Trifolium repens L.). Soil was collected from the National Monitoring Base of Purple Soil Fertility and Fertilizer Efficiency, Southwest University, Beibei District, Chongqing, China. The soil was comprised of a purple soil (termed regosol in the taxonomy of the Food and Agriculture Organization of the United Nations (FAO), and entisolsin U.S. taxonomy) and a sandy loam (clay 24.1%, silt 46.6%, sand 29.3%), with pH (soil: water ratio of 1:2.5) of 7.1, organic matter of 10.79 g∙kg−1, total N of 0.78 g∙kg−1, total P of 0.82 g∙kg−1, total K of 20.54 g∙kg−1 and available P(Olson-P) of 10.28 mg∙kg−1. The soil material was sieved (10 mm) to remove stones and roots. Soil was irradiated with gamma-rays to remove native microbes from the soil while leaving the soil structure intact.
2.2. Experimental Unit and Design
Plants were grown in containers made of acrylic material with thickness of 3 mm [19] . The containers consisted of four compartments (L × W × H: 5 × 15 × 20 cm for each) with two compartments on either side of a solid barrier, and each two adjacent compartments were separated by screens (thickness, 37.5 μm; L × W: 21 × 17 cm).
Plant roots were split and trained to grow into the soils of the two (inside) compartments on either side of the central solid barrier. The soil on one side was inoculated with an AM fungus strain; the soil on the other side was not (non-AM). The screens were placed between the two outer and inner compartments to prevent penetration by plant roots into the outside compartments, but to permit the growth of hyphae and allow for an exchange of soil solution between compartments. By use of this arrangement, four different soils with different mycorrhizal influences were obtained, which were respectively labeled as plain soil (S: no roots, no AM hyphae), rhizosphere soil (R: plants roots without AM-infection), mycorrhizosphere soil (M: AM roots, AM hyphae) and hyphosphere soil (H: AM hyphae only). These four treatments (S, R, M and H) in this study were conducted in triplicate. Clover seeds were disinfected and rinsed with sterile deionized water. Then they were placed in culture pots filled with a 1:1 mixture of sand and soil (steam-sterilized at 121˚C for 60 min). The uniform seedlings were chosen when the roots were approximately 5 cm long and ten plants were transferred to the growth compartments, where an equal number of roots were trained to separate over the central solid barrier and grow into the soils of the R and M treatments.
2.3. Growth Conditions
During the cultivation period from Dec. 2012 to Mar. 2013, cultivated plants were placed in a culturing room (located in Southwest University, Chongqing) for 14 hours every day with a constant temperature of about 30˚C, relative humidity of 50% - 70% and an average photosynthetic photon flux density of 230 - 280 μ∙mol∙m−2∙s−1 which was provided by reflector sunlight dysprosium lamps (DDF400, Nanjing, China). In order to keep soils in all four compartments at a similar moisture, the soils in the inside (R and M) and outside (S and H) compartments were watered daily and twice a week with tap water, respectively. Sponge was employed to cover the soil surface to minimize vaporization and avoid direct irradiation by sunlight.
2.4. Lab Analysis
After three months’ growth, plants were harvested and analyzed. Roots subsamples (0.5 g fresh weight) were cut into segments with length of 1 cm for determining the percentage of root length colonized by the mycorrhizal fungi, as described by Giovannetti and Mosse [20] . The shoot and the remaining root were dried in an oven at 70˚C and then weighed to measure the dry matter yield. The soil samples (with particle size < 5 cm) collected from around the growing system were carefully broken manually into smaller aggregates (with size of about 1 cm), and then air-dried at ambient temperature. Wet sieving method described by Kemper and Rosenau [21] was employed to determine the composition of soil aggregates. Hyphae length was determined as described by Abbott [22] . Soil organic matter (OM) was detected by the potassium dichromate-volumetric method (digested by K2Cr2O7-H2SO4; Lu, 2000). In our study, EEG and TG (Total Glomalin) were determined using the procedures described by Wright and Upadhyaya [8] . Briefly, 0.25 mm sieved soil was added into a centrifuge tube with citrate solution, followed by being autoclaved and centrifuged at speed of 4000 r for 5 min to separate the liquid and solid phases. The supernatant was transferred into another tube and stored at 4˚C before analysis. EEG was extracted with 8 ml of 200 mM citrate solution (pH = 7.0), by autoclaving at 121˚C for 30 min. TG was extracted repeatedly using with 50 ml of 200 mM citrate (pH 8.0) by autoclaving at 121˚C for 60 min. This extraction processes continued until the glomalin supernatant concentrations were below detection limit. Extracts from each cycle were merged and then centrifuged at 10,000 r for 10 min to remove residual soil particles. Protein content was measured by Bradford assay with bovine serum albumin as the standard.
Soil structure stability was evaluated by mean weight diameter (MWD) and geometric mean diameter (GMD) taking micro-aggregates with diameter of 1 - 2 mm as a standard, due to its sensitivity to short-term treatments of soil [8] .
2.5. Data Analysis
Mean weight diameter (MWD) and geometric mean diameter (GMD) were used to evaluate the stability of soil aggregates and calculated with the following formula [21] :
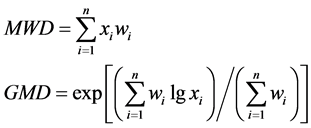
where xi is the sieve opening size (mm); wi is the proportion of the total sample mass occurring in the i size fraction; n is the number of particle fractions.
The proportion of soil macroaggregate of a diameter larger than 0.25 mm was calculated by:
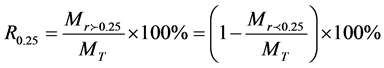
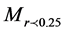 is the weight of macroaggregates with a diameter larger than 0.25 mm, MT is the total sample weight,
is the weight of macroaggregates with a diameter larger than 0.25 mm, MT is the total sample weight,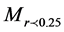 is the weight of microaggregates with a diameter less than 0.25 mm.
is the weight of microaggregates with a diameter less than 0.25 mm.
SAS statistical software package (vers. 6.12; SAS Institute, Cary, NC) were used to analyze the date by two- way or one-way analysis of variance.
3. Results
3.1. Plant Growth
The barrier treatment failed to show any significant impact on Plant growth (Table 1, Table 2), but plants in the M compartment tended to higher more root biomass than in the R compartment. The P status of roots was significantly affected by mycorrhizal inoculation so that roots inoculated with G. etunicatum and G. intraradices had significant higher P uptake than corresponding roots in the R compartment.
The mycorrhizal inoculation rate was not significantly different among the three fungi (Table 2). Roots of inoculated plants were extensively mycorrhizal, with the mean percentage of root length colonized ranging from 56.2% - 61.3%. The roots in R compartments were not inoculated, as expected.
Our results demonstrate that hyphae can penetrate a nylon barrier between compartments M and H. Hyphae length extracted from the soil ranged from 71 cm∙g−1 to 87 cm∙g−1 and showed a trend of M > H. G. etunicatum had higher hyphal densities in comparison to G. intraradices and G. mosseae.
Table 1. Shoot dry weight (g), shoot nitrogen concentration (g∙kg−1) and shoot phosphorus content uptake (mg) of clover plants cultivated in the special containers consisting of four compartments.
Notes: The values shown are the mean of four replicates. Letters in the table within each cell relate to significant differences between the mycorrhizal inoculants at the 5% level based on analysis of variance. a = we cannot reject the null hypothesis that these values are the same.
Table 2. Colonization rate (proportion of root length colonized by AM, %), mean dry root weight (g), root P concentration (g∙kg−1) and root P uptake (mg) of clover in the mycorrhizosphere and rhizosphere compartments.
Notes: Values in the table are the means of xx replicates. Letters in the table within each cell relate to significant differences between the mycorrhizal inoculants at the 5% level based on analysis of variance. NS = not significant; * = P < 0.05; ** = P < 0.01; *** = P < 0.001; ND = not detected.
3.2. Soil Variables
The barrier treatment significantly affected the TG concentration in the soil (Table 3). TG declined in the following pattern: M > H > R > S. The highest value of TG was 4.96 g∙kg−1 measured in the M compartments inoculated with G. intraradices. Due to the different extraction procedures used, the value for TG (4.56 - 4.96 g∙kg−1) was larger than the values for EEG (0.89 - 1.05 g∙kg−1). The differences of TG or EEG values between the AM fungus isolates were not significant. Neither AM inoculation nor the barrier treatment significantly affected the contents of EEG or OM in the soil.
In this experiment, the values for soil MWD, GMD and R0.25 in M compartment were significantly higher than in the other compartments. There was a pattern of soil aggregate size of M > H > R > S from largest to smallest (Table 4).
3.3. Relationships between Fungal and Soil Variables
MWD, GMD and R0.25 were significantly related to each other, their correlation coefficients were 0.958, 0.863 and 0.791, respectively (P < 0.001, Table 5). Additionally, MWD, GMD and R0.25 were positively related to EEG. Although the correlation coefficients were small, correlation between the length of hyphae and the three indices of soil aggregate size was significant and positive, and the correlations of latter were higher than the former.
Additionally, hyphae length was positively related to TG, EEG and colonization rate. Out of these variables, colonization rate had the highest correlation with hyphae length (r = 0.603, P < 0.05).
Table 3. Hyphal density (cm∙g−1), concentration of organic matter (g∙kg−1), total glomalin (TG, g∙kg−1) and easily-extractable glomalin (EEG, g∙kg−1) content of soils in the four compartments.
Notes: Values in the table are the means of xx replicates. Letters in the table within each cell relate to significant differences between the mycorrhizal inoculants at the 5% level based on analysis of variance. NS = not significant; * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
Table 4. Mean weight diameter (MWD), geometric mean diameter (GMD) and the percentage of soil macroaggregates with a diameter larger than 0.25 mm (R0.25, %).
Notes: Values in the table are the means of xx replicates. Letters in the table within each cell relate to significant differences between the mycorrhizal inoculants at the 5% level based on analysis of variance. NS = not significant; * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
Table 5. Correlation coefficients and significance levels among the soil aggregate characteristics (wet-sieving, MWD = mean weight diameter; GMD = geometric mean diameter; R0.25 = the percentage of soil macroaggregates with a diameter larger than 0.25 mm, %), easily extractable glomalin (EEG, g∙kg−1), total glomalin (TG, g∙kg−1), organic matter (OM, g∙kg−1), colonisation rate (proportion of the root length colonised by AM, %) and length of hyphae (cm∙g−1). Sample size = 48.
Significant at * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
4. Discussion
4.1. Changes in Soil Aggregates
Numerous studies have shown that roots, mycelia and GRSP all affect soil macroaggregate formation and stability [6] , but differing experimental conditions have prevented the comparison of the magnitude of the effects of these three factors. Furthermore, the interaction effects of these three variables have been understudied.
The higher GMD, MWD, and R0.25 in the M and H compartments (Table 5) demonstrates that even within the short time of this experiment, the positive mycorrhizae effects on the soil aggregate were measurable. The mycorrhizal inoculation contributed to aggregate stability in a direct way that was able to be separated from the effects of the plant roots alone. The combined effects of the plant roots and the mycorrhizae were additive as has been described by Andrade [19] . The stabilization of soil aggregates is probably influenced by the individual action and the interaction of the plant and fungal systems.
4.2. Extraradical Mycelium and Soil Aggregates
The significant and positive correlation between the hyphae length and MWD and GMD (r = 0.609, 0.541 P < 0.01, Table 4) demonstrated the importance of the mycelial network for the formation and stability of soil aggregations. This is thought to occur by hyphal enveloping of soil particles [18] and such a relationship has been directly observed [12] [23] [24] , but included the potentially confounding effects of the plant roots. Our results show that these effects are independent of the effects of plant roots. Rillig et al. [24] and Siddiky et al. [25] also considered the influence of plant roots and used a nylon screen compartment and an in vitro bioreactor system to detect the unique effect of the hyphae. However, these studies prepared the soil by steaming it and eliminating the resident AM communities. We found that the relative abundance of hyphae of G. etunicatum produced a corresponding higher MWD and GMD in soil aggregates and confirms a former study about the effects of various AM taxa on soil aggregate stabilization [26] . Given how many species of ACM potentially infect plant roots, the differences in their effects on soil aggregates deserves further study.
4.3. GRSP and Soil Aggregates
The special role of GRSP in soil aggregate formation and stability has increasingly been the subject of a number of research projects [7] [8] [27] . Approximately 80% of GRSP (by weight) produced by fungi is contained in hyphae and spores in comparison to the amount of GRSP released into the culture medium by the fungi [28] , strongly suggesting that the impact of GRSP on soil aggregates is primarily derived from the hyphae. While TG levels had a positive correlation with macroaggregate abundance under each metric, none of these relations were significant. TG high-temperature extraction from soil can inadvertently be contaminated with proteins and polyphenols [29] , and therefore EEG may be a better measure of glomalin abundance.
A clear result from our study is that roots, mycelia, and GRSP have independent and synergistic impacts on macroaggregate formation and stability that have not been detectable with the experimental conditions used in previous research. This is the first study to compare the magnitude of effects between these three factors and our results suggest that their interactions deserve further study. AM fungus-mediated contributions to soil aggregates can be particularly important in managed ecosystems and natural. Although experimental results obtained under specified conditions are not necessarily representative of field conditions [26] , the method used in this research may help us better understand the relationships between mycorrhizal symbiosis, plant productivity, and soil quality.
5. Conclusion
We found that even within the short time of this experiment, there were direct, positive effects of AM fungus inoculation on WSA stability that were measurable. Plant non-inoculated roots, mycorrhizal roots, and mycorrhizae hyphae contributed to soil aggregate stability in individual ways and their effects were additive when they acted in concert.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 40701085) and the Fundamental Research Funds for the Central Universities (Grant NO.XDJK2010B012).
Cite this paper
TaoLiang,XiaojunShi,TaoGuo,SiliPeng, (2015) Arbuscular Mycorrhizal Fungus Mediate Changes in Mycorrhizosphere Soil Aggregates. Agricultural Sciences,06,1455-1463. doi: 10.4236/as.2015.612141
References
- 1. Amezketa, E. (1999) Soil Aggregate Stability: A Review. Journal of Sustainable Agriculture, 14, 83-151.
http://dx.doi.org/10.1300/J064v14n02_08 - 2. van Bavel, C.H.M. (1949) Mean Weight-Diameter of Soil Aggregates as a Statistical Index of Aggregation. Soil Science Society of America Journal, 14, 20-23.
http://dx.doi.org/10.2136/sssaj1950.036159950014000C0005x - 3. Gardner, W.R. (1956) Representation of Soil Aggregate-Size Distribution by a Logarithmic-Normal Distribution. Soil Science Society of America Journal, 20, 151-153.
http://dx.doi.org/10.2136/sssaj1956.03615995002000020003x - 4. Koide, R.T. and Mosse, B. (2004) A History of Research on Arbuscular Mycorrhiza. Mycorrhiza, 14, 145-163.
http://dx.doi.org/10.1007/s00572-004-0307-4 - 5. Smith, S.E. and Read, D.J. (2008) Mycorrhizal Symbiosis. Academic Press, Elsevier, New York.
- 6. Rillig, M.C. and Mummey, D.L. (2006) Mycorrhizas and Soil Structure. New Phytologist, 171, 41-53.
http://dx.doi.org/10.1111/j.1469-8137.2006.01750.x - 7. Rillig, M.C., Wright, S.F and Eviner, V.T. (2002) The Role of Arbuscular Mycorrhizal Fungi and Glomalin in Soil Aggregation: Comparing Effects of Five Plant Species. Plant and Soil, 238, 325-333.
http://dx.doi.org/10.1023/A:1014483303813 - 8. Wright, S. and Upadhyaya, A. (1998) A Survey of Soils for Aggregate Stability and Glomalin, a Glycoprotein Produced by Hyphae of Arbuscular Mycorrhizal Fungi. Plant and Soil, 198, 97-107.
http://dx.doi.org/10.1023/A:1004347701584 - 9. Rillig, M.C. (2004) Arbuscular Mycorrhizae, Glomalin, and Soil Aggregation. Canadian Journal of Soil Science, 84, 355-363.
http://dx.doi.org/10.4141/S04-003 - 10. Tisdall, J.M. and Oades, J. (1982) Organic Matter and Water-Stable Aggregates in Soils. Journal of Soil Science, 33, 141-163.
http://dx.doi.org/10.1111/j.1365-2389.1982.tb01755.x - 11. Miller, R. and Jastrow, J. (1990) Hierarchy of Root and Mycorrhizal Fungal Interactions with Soil Aggregation. Soil Biology and Biochemistry, 22, 579-584.
http://dx.doi.org/10.1016/0038-0717(90)90001-G - 12. Piotrowski, J.S., Denich, T., Klironomos, J.N., Graham, J.M. and Rillig, M.C. (2004) The Effects of Arbuscular Mycorrhizas on Soil Aggregation Depend on the Interaction between Plant and Fungal Species. New Phytologist, 164, 365-373.
http://dx.doi.org/10.1111/j.1469-8137.2004.01181.x - 13. Bedini, S., Avio, L., Argese, E. and Giovannetti, M. (2007) Effects of Long-Term Land Use on Arbuscular Mycorrhizal Fungi and Glomalin-Related Soil Protein. Agriculture Ecosystems & Environment, 120, 463-466.
http://dx.doi.org/10.1016/j.agee.2006.09.010 - 14. Siddiky, M.R.K., Kohler, J., Cosme, M. and Rillig, M.C. (2012) Soil Biota Effects on Soil Structure: Interactions between Arbuscular Mycorrhizal Fungal Mycelium and Collembola. Soil Biology & Biochemistry, 50, 33-39.
http://dx.doi.org/10.1016/j.soilbio.2012.03.001 - 15. Hontoria, C., Velasquez, R., Benito, M., Almorox, J. and Moliner, A. (2009) Bradford-Reactive Soil Proteins and Aggregate Stability under Abandoned versus Tilled Olive Groves in a Semi-Arid Calcisol. Soil Biology & Biochemistry, 41, 1583-1585.
http://dx.doi.org/10.1016/j.soilbio.2009.04.025 - 16. Wilson, G.W.T., Rice, C.W., Rillig, M.C., Springer, A. and Hartnett, D.C. (2009) Soil Aggregation and Carbon Sequestration Are Tightly Correlated with the Abundance of Arbuscular Mycorrhizal Fungi: Results from Long-Term Field Experiments. Ecology Letters, 12, 452-461.
http://dx.doi.org/10.1111/j.1461-0248.2009.01303.x - 17. Caruso, T. and Rillig, M.C. (2011) Direct, Positive Feedbacks Produce Instability in Models of Interrelationships among Soil Structure, Plants and Arbuscular Mycorrhizal Fungi. Soil Biology & Biochemistry, 43, 1198-1206.
http://dx.doi.org/10.1016/j.soilbio.2011.02.009 - 18. Miller, R. and Jastrow, J. (2000) Mycorrhizal Fungi Influence Soil Structure. In: Kapulnik, Y. and Douds Jr., D.D., Eds., Arbuscular Mycorrhizas: Physiology and Function, Kluwer Academic, Dordrecht, 3-18.
http://dx.doi.org/10.1007/978-94-017-0776-3_1 - 19. Andrade, G., Mihara, K.L., Linderman, R.G. and Bethlenfalvay, G.J. (1998) Soil Aggregation Status and Rhizobacteria in the Mycorrhizosphere. Plant and Soil, 202, 89-96.
http://dx.doi.org/10.1023/A:1004301423150 - 20. Giovannetti, M. and Mosse, B. (1980) An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytologist, 84, 489-500.
http://dx.doi.org/10.1111/j.1469-8137.1980.tb04556.x - 21. Kemper, W. and Rosenau, R. (1986) Aggregate Stability and Size Distribution. In: Klute, A., Ed., Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, Agronomy Monograph, American Society of Agronomy and Soil Science Society of America, Madison, 425-442.
- 22. Abbott, L.K., Robson, A.D. and Deboer, G. (1984) The Effect of Phosphorus on the Formation of Hyphae in Soil by the Vesicular Arbuscular Mycorrhizal Fungus, Glomus fasciculatum. New Phytologist, 97, 437-446.
http://dx.doi.org/10.1111/j.1469-8137.1984.tb03609.x - 23. Bearden, B.N. and Petersen, L. (2000) Influence of Arbuscular Mycorrhizal Fungi on Soil Structure and Aggregate Stability of a Vertisol. Plant and Soil, 218, 173-183.
http://dx.doi.org/10.1023/A:1014923911324 - 24. Rillig, M.C., Mardatin, N.F., Leifheit, E.F. and Antunes, P.M. (2010) Mycelium of Arbuscular Mycorrhizal Fungi Increases Soil Water Repellency and Is Sufficient to Maintain Water-Stable Soil Aggregates. Soil Biology & Biochemistry, 42, 1189-1191.
http://dx.doi.org/10.1016/j.soilbio.2010.03.027 - 25. Siddiky, M.R.K., Schaller, J., Caruso, T. and Rillig, M.C. (2012) Arbuscular Mycorrhizal Fungi and Collembola Non-Additively Increase Soil Aggregation. Soil Biology & Biochemistry, 47, 93-99.
http://dx.doi.org/10.1016/j.soilbio.2011.12.022 - 26. Stefano, B., Elisa, P., Luciano, A., Sergio, P., Paolo, B., Emanuele, A. and Manuela, G. (2009) Changes in Soil Aggregation and Glomalin-Related Soil Protein Content as Affected by the Arbuscular Mycorrhizal Fungal Species Glomus mosseae and Glomus intraradices. Soil Biology & Biochemistry, 41, 1491-1496.
http://dx.doi.org/10.1016/j.soilbio.2009.04.005 - 27. Fokom, R., Adamou, S., Teugwa, M.C., Boyogueno, A.D.B., Nana, W.L., Ngonkeu, M.E.L., Tchameni, N.S., Nwaga, D., Ndzomo, G.T. and Zollo, P.H.A. (2012) Glomalin Related Soil Protein, Carbon, Nitrogen and Soil Aggregate Stability as Affected by Land Use Variation in the Humid Forest Zone of South Cameroon. Soil & Tillage Research, 120, 69-75.
http://dx.doi.org/10.1016/j.still.2011.11.004 - 28. Driver, J.D., Holben, W.E. and Rillig, M.C. (2005) Characterization of Glomalin as a Hyphal Wall Component of Arbuscular Mycorrhizal Fungi. Soil Biology & Biochemistry, 37, 101-106.
http://dx.doi.org/10.1016/j.soilbio.2004.06.011 - 29. David, P.J., Sara, G. and Bray, B. (2008) Glomalin Extraction and Measurement. Soil Biology and Biochemistry, 240, 728-739.
NOTES

*Corresponding author.


