Agricultural Sciences
Vol.4 No.12(2013), Article ID:40714,5 pages DOI:10.4236/as.2013.412087
Response of cranberry and kidney beans to linuron
![]()
University of Guelph Ridgetown Campus, Ridgetown, Canada; *Corresponding Author: soltanin@uoguelph.ca
Copyright © 2013 Nader Soltani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 24 June 2013; revised 15 November 2013; accepted 28 November 2013
Keywords: Cranberry Bean; Height; Injury; Seed Moisture; Kidney Bean; Yield
ABSTRACT
Field studies were conducted in 2009 and 2010 at the Huron Research Station, Exeter, Ontario and the University of Guelph Ridgetown Campus, Ridgetown, Ontario to determine the tolerance of four cultivars of cranberry bean (“Etna”, “Hooter”, “SVM Taylor”, and “Capri”) and four cultivars of kidney bean (“Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty”) to linuron applied preemergence at 1125 and 2250 g∙ai∙ha−1. One week after emergence (WAE), linuron applied PRE caused 0.4% to 1.2% injury in “Etna”, “Hooter”, “SVM Tayler”, and “Capri” cranberry bean and 3.1% to 3.6% injury in “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” kidney bean. At 2 and 4 WAE, there was no difference in injury among the dry bean cultivars. Contrast comparing injury due to linuron in cranberry vs kidney bean cultivars indicated 2.3%, 1.7%, and 1.2% greater injury in kidney bean compared to cranberry bean at 1, 2, and 4 WAE, respectively. Linuron PRE caused slightly greater injury in kidney bean compared to cranberry bean but crop injury was minimal with no adverse effect on plant height, shoot dry weight, seed moisture content, and yield under the environments evaluated. Based on this research, linuron applied PRE at the proposed rate of 1125 g∙ai∙ha−1 can be safely used in cranberry and kidney beans in Ontario.
1. INTRODUCTION
Canada, a major producer of dry bean, produces nearly 10% of the world’s dry bean valued at $226 million dollars annually [1]. Ontario, one of the leading provinces in Canada in dry bean production, produces 129,000 MT of dry bean with a farm-gate value of approximately $90 million [2]. Major market classes of dry bean grown in Ontario include black, cranberry, kidney, and white (navy) bean.
Dry bean is a short season crop with short physical stature and therefore is very sensitive to weed interference [3-7]. Dry bean seed yield has been reduced as much as 70% due to weed interference [8]. Presence of weeds at the harvest time can also cause seed staining and interfere with harvesting efficiency in dry bean [9- 12].
There are few herbicides registered for broadleaf weed control in dry bean in Ontario. Imazethapyr is the only soil applied herbicide for annual broadleaf weed control. Imazethapyr can cause significant dry bean injury under some environmental conditions and provides marginal control of common ragweed (Ambrosiaartemisiifolia L.) and common lambsquarters (Chenopodium album L.). More research is needed to identify new herbicide options that provide consistent broadleaf weed control and have adequate margin of crop safety in various cultivars within different market classes of dry bean.
Linuron is a substituted urea herbicide registered for use in a number of crops including corn and soybean [13]. Linuron is readily absorbed through roots following a soil application [14]. Linuron applied pre-emergence (PRE) controls many broadleaf weeds such as velvetleaf (Abutilon theophrasti Medicus), redwood pigweed (Amaranthus retroflexus L.), common lambsquarters (Chenopodium album L.), common ragweed (Ambrosiaartemisiifolia L.), common chickweed [Stellaria media (L.) Cyrillo], field pennycress (Thlaspi arvense L.), prostrate knotweed (Polygonum arenastrum L.), purslane (Portulaca oleracea L.), shepherd’s purse [Capsella bursa-pastoris (L.) Medic.], smartweed (Polygonum spp.), annual sowthistle (Sonchus oleraceus L.), wild buckwheat (Polygonum convovulus L.) and wormseed mustard (Erysimum cheiranthoides L.), including acetolactate synthaseand triazine-resistant biotypes [13-15]. Linuron is registered at a rate of 1125 to 2250 g∙ai∙ha−1 in soybean in Ontario with rate applied dependent on soil texture and organic matter. Linuron has the potential to provide broad spectrum broadleaf weed control in some market classes of dry bean [16].
Tolerance of dry beans to various soil applied herbicides is influenced by herbicide rate, market class, cultivar and environmental conditions [4,7,15,17]. There is little published data on the sensitivity of “Etna”, “Hooter”, “SVM Taylor”, and “Capri” cranberry bean cultivars and “Red Hawk”, “Pink Panther”, “Calmont”, and Majesty kidney bean cultivars to linuron.
The objective of this study was to evaluate the tolerance “Etna”, “Hooter”, “SVM Taylor”, and “Capri” cranberry bean and “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” kidney bean to linuron applied pre-emergence at 1125 and 2250 g∙ai∙ha−1, representing the 1X and 2X manufacturer’s recommended rate in soybean under Ontario environmental conditions.
2. MATERIALS AND METHODS
Field studies were conducted in 2009 and 2010 at the Huron Research Station, Exeter, Ontario and the University of Guelph, Ridgetown Campus, Ridgetown, Ontario. The soil at Exeter was a Brookston clay loam (Orthic Humic Gleysol, mixed, mesic, and poorly drained) with 38% sand, 41% silt, 21% clay, 3.7% organic matter and pH of 7.8 in 2009, and 36% sand, 39% silt, 25% clay, 3.6% organic matter and pH of 7.8 in 2010. The soil at Ridgetown was a Wattford (Grey-Brown Brunisolic, mixed, mesic, sandy, and imperfectly drained)-Brady (Gleyed Brunisolic Grey-Brown Luvisol, mixed, mesic, sandy, and imperfectly drained) with 54% sand, 27% silt, 19% clay, 5.6% organic matter and pH of 6.4 in 2009, and 40% sand, 35% silt, 25% clay, 7.1% organic matter and pH of 6.6 in 2010. Seedbed preparation at all sites consisted of fall moldboard plowing followed by two passes with a field cultivator in the spring.
The experiments were established as a 2-way factorial design arranged in a completely randomized block with four replications. Factor 1 was cultivar (variety) of dry bean (Cranberry: “Etna”, “Hooter”, “SVM Taylor”, and “Capri”; Kidney: “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty”) and Factor 2 was rate of linuron (0, 1125 and 2250 g∙ai∙ha−1), applied preemergence. Plots were 6 m wide (8 rows spaced 0.75 m apart) and 10 m long at Exeter and 8 m long at Ridgetown. Within each plot there was one row of “Etna”, “Hooter”, “SVM Taylor”, “Capri”, “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” beans. Beans were planted in late May to early June of each year.
Herbicide applications were made with a CO2-pressurized backpack sprayer calibrated to deliver 200 L∙ha−1 of spray solution at a pressure of 240 kPa using ultra low drift nozzles (ULD120-02, Hypro, New Brighton, MN). Treatments were applied at one day after seeding and were left undisturbed on the surface of soil. All plots were maintained weed-free during the season with hand hoeing and cultivation as required.
Dry bean injury was visually estimated on a scale of 0 (no injury) to 100% (complete plant death) at 1, 2 and 4 weeks after crop emergence (WAE). Bean shoot dry weight was evaluated 4 WAE by cutting plants at the soil surface from 1m of row per plot. Plants were dried at 60 C to a constant moisture and then weighed. Dry bean height was measured for 10 plants in each plot 6 WAE and the average height was recorded. Dry bean was considered mature when 90% of the pods in the weed-free check had turned from green to a golden colour. Beans were harvested from each plot with a small plot combine, weight and moisture were recorded, and yields were adjusted to 18% moisture.
Data were analyzed as a 2-way factorial using PROC MIXED in SAS 9.2. The two treatment factors, dry bean cultivar and herbicide rate, as well as their interaction were considered fixed effects, while environment (yearlocation combinations), interactions between environments and the fixed effects, and replicate nested within environment were considered random effects. Significance of fixed effects was tested using F-tests and random effects were tested using a Z-test of the variance estimate. Environments were combined for a given variable if the environment by cultivar by rate interaction was not significant. The UNIVARIATE procedure was used to test data for normality and homogeneity of variance. For all injury ratings, the untreated check (assigned a value of zero) was excluded from the analysis. However, all values were compared independently to zero to evaluate treatment differences with the untreated check. To satisfy the assumptions of the variance analyses, injury and moisture were log transformed. Treatment comparisons were made using Fisher’s Protected LSD at a level of P < 0.05. Additionally, a contrast was performed for each variable comparing dry bean type (four cranberry vs four kidney bean cultivars) to determine if bean market class influenced responses. Data compared on the transformed scale were converted back to the original scale for presentation of results.
3. RESULTS AND DISCUSSION
Environment by Cultivar by Rate interaction was not significant for injury 2 and 4 WAE, shoot dry weight, height, moisture and yield and all four datasets were analyzed together. Environment by Cultivar by Rate interaction was significant for injury 1 WAE: Exeter 2009 and Ridgetown 2010 were all zero (not shown in Table 1) and were separated from Exeter 2010 and Ridgetown 2009.
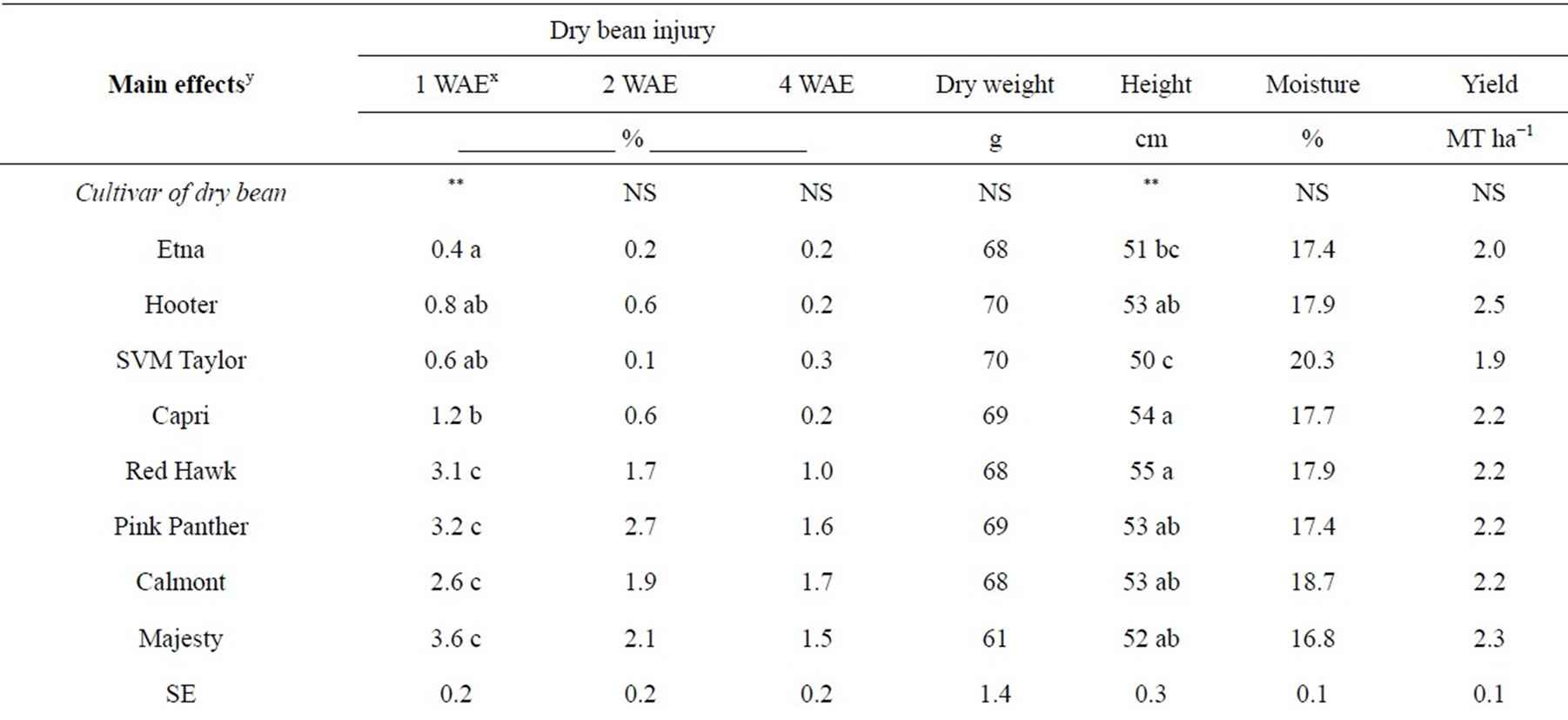
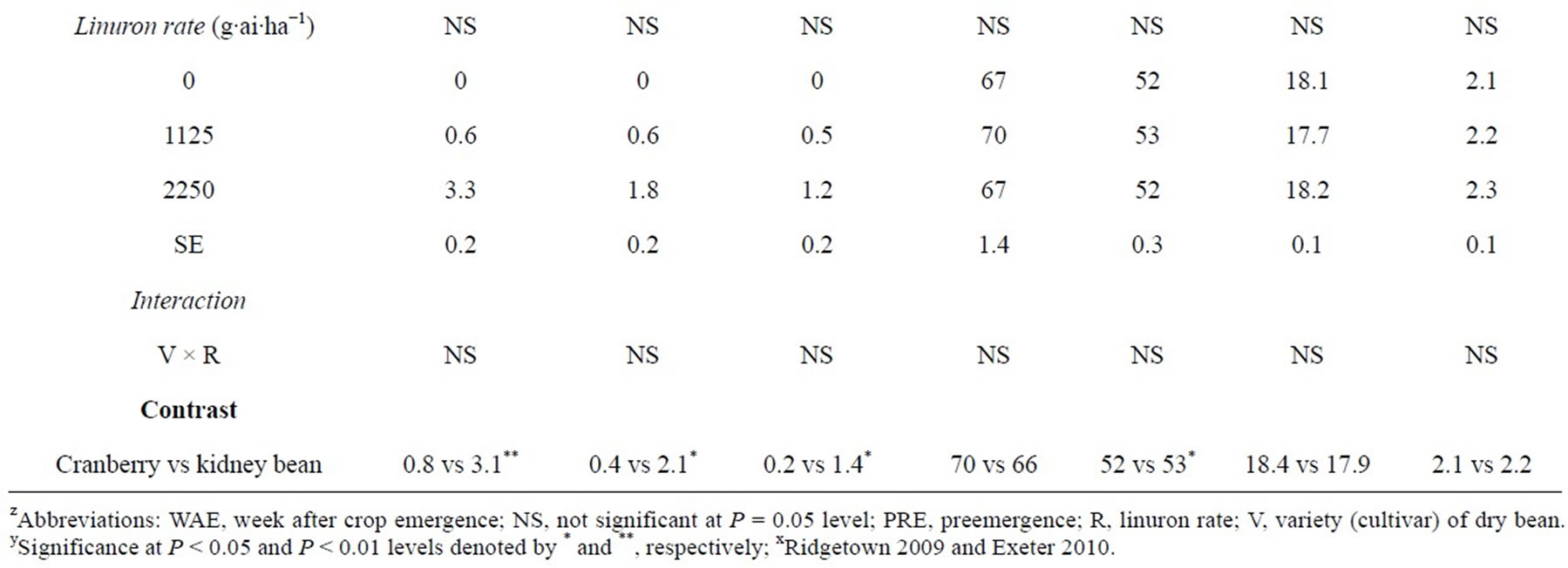
Table 1. Significance of main effects and interactions for percent visual injury, height, shoot dry weight, moisture and seed yield of dry bean when linuron was applied PRE. Means followed by the same letter within a column are not significantly different according to Fisher’s Protected LSD at P < 0.05. Means for a main effect were separated only if there was no significant interaction involving that main effectz.
3.1. Crop Injury
At 1 WAE, linuron caused lower injury in cranberry bean compared to kidney bean (Table 1). Linuron applied PRE caused 0.4% to 1.2% injury in “Etna”, “Hooter”, “SVM Tayler”, and “Capri” cranberry bean and 2.6 to 3.6% injury in “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” kidney bean (Table 1). At 2 and 4 WAE, there was no significant difference in injury among dry bean cultivars. Contrasts comparing cranberry verses kidney bean cultivars indicated 2.3%, 1.7%, and 1.2% greater injury in kidney bean compared to cranberry bean due to linuron PRE at 1, 2, and 4 WAE, respectively.
In other studies, linuron applied PRE caused as much as 12% injury in cranberry and kidney beans [16]. The minimal injury observed in this study is similar to studies with other soil-applied herbicides in dry bean [3,6,17-20]. Also, the variation seen in visible crop injury among cultivars or market classes of dry bean is consistent with other studies that have shown differential sensitivity with soil applied herbicides in dry bean [7,4,21]. Market classes of dry beans have different geographic origins and consequently have different gene pool which affects their tolerance to herbicides [22-24].
3.2. Plant Height and Shoot Dry Weight
Among dry bean cultivars evaluated “SVM Taylor” and “Etna” had the lowest height, however, there was no significant difference in height of “Hooter”, “Capri”, “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” dry beans (Table 1). The kidney bean cultivars were on average 1 cm taller than cranberry bean cultivars evaluated in this study (Table 1).
Linuron applied PRE did not have any adverse effect on shoot dry weight of “Etna”, “Hooter”, “SVM Taylor”, “Capri”, “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” beans (Table 1). There was no difference in shoot dry weight of the cranberry and kidney bean cultivars included in this study (Table 1).
In other studies linuron applied PRE at 500 to 2500 g∙ai∙ha−1 did not affect plant height of cranberry and kidney bean but plant height decreased as much 15% in black bean at 2500 g∙ai∙ha−1 and 10%, 13% and 23% in white bean with linuron applied PRE at 1500, 2000 and 2500 g∙ai∙ha−1, respectively [16]. Studies with other soil applied studies have shown no adverse effect on cranberry and kidney bean height [18-20].
3.3. Seed Moisture Content and Yield
Linuron applied PRE at 1125 and 2250 g∙ai∙ha−1 did not have any adverse effect on the seed moisture content and yield of “Etna”, “Hooter”, “SVM Taylor”, “Capri”, “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” beans (Table 1). In addition, there were no significant differences in seed moisture content and yield of cranberry and kidney bean. In other studies, linuron applied PRE at various doses did not cause any adverse affect on the yield of cranberry, kidney, and white beans but yield of black bean was reduced 16% compared to the nontreated control at 2500 g∙ha−1 [16]. These results are similar to other studies in which flumioxazin, pyroxasulfone, and alachlor applied preemergence which caused no losses in cranberry and kidney bean seed yield [6, 25,26].
4. CONCLUSIONS
Based on this research, linuron applied PRE at the proposed rate of 1125 g∙ai∙ha−1 has an adequate margin of crop safety in “Etna”, “Hooter”, “SVM Taylor”, “Capri”, “Red Hawk”, “Pink Panther”, “Calmont”, and “Majesty” dry beans. There is slight differential sensitivity between dry bean cultivars and market classes to linuron. Linuron PRE caused slightly greater injury in kidney bean compared to cranberry bean but crop injury was minimal with no adverse effect on plant height, shoot dry weight, seed moisture content, and yield under the environmental condition in this study. Availability of linuron for weed management would provide dry bean growers with a new herbicide option for the control of troublesome weeds such as common lambsquarters, common pigweed, common ragweed, and other annual broadleaf weeds.
5. ACKNOWLEDGEMENTS
The authors would like to acknowledge Todd Cowan for his expertise and technical assistance in these studies. Funding for this project was provided by the Ontario White Bean Producers, Ontario Coloured Bean Growers Association, and the Can Advance program of Agricultural Adaptation Council.
REFERENCES
- Agriculture and Agri-Food Canada (2007) Canada: Dry bean imports and exports. http://www4.agr.gc.ca/AAFC-AAC/display-afficher.do?id=1174506503179&lang=eng
- Kulasekera, K. (2013) Estimated area, yield, production and farm value of specified field crops, Ontario, 2001- 2012, (Metric Units). http://www.omafra.gov.on.ca/english/stats/crops/estimate_metric.htm
- Arnold, N.R., Murray, W.M., Gregory, J.E. and Smeal, D. (1993) Weed control in pinto beans (Phaseolus vulgaris) with imazethapyr combinations. Weed Technology, 7, 361- 364.
- Bauer, T.A., Renner, K.A., Penner, D. and Kelly J.D. (1995) Pinto bean (Phaseolus vulgaris) varietal tolerance to imazethapyr. Weed Science, 43, 417-424.
- Blackshaw, R.E. and Esau, R. (1991) Control of annual broadleaved weeds in pinto beans (Phaseolus vulgaris). Weed Technology, 5, 532-538.
- Urwin, C.P., Wilson, R.G. and Mortensen, D.A. (1996) Responses of dry edible bean (Phaseolus vulgaris) cultivars to four herbicides. Weed Technology, 10, 512-518.
- Wilson, R.G. and Miller, S.D. (1991). Dry edible bean (Phaseolus vulgaris) responses to imazethapyr. Weed Technology, 5, 22-26.
- Malik, V.S., Swanton, C.J. and Michaels, T.E. (1993) Interaction of white bean (Phaseolus vulgaris) cultivars, row spacing, and seeding density with annual weeds. Weed Science, 41, 62-68.
- Wilson, R.G. (1993) Wild proso millet (Panicum miliaceum) interference in dry bean (Phaseolus vulgaris). Weed Science, 41, 607-610.
- Zimdahl, R.L. (1980) Weed-crop competition. International Plant Protection Center, Oregon State University, Corvallis.
- Bassett, I.J. and Munro, D.B. (1985) The biology of Canadian weeds. 67. Solanum ptycanthum Dun., S. nigrum L., and S. sarrachoides Sendt. Canadian Journal of Plant Science, 65, 401-414. http://dx.doi.org/10.4141/cjps85-055
- Ogg, A.G. and Rogers, B.S. (1989) Taxonomy, distribution, biology, and control of black nightshade (Solanum nigrum) and related species in the United States and Canada. Weed Science, 4, 25-58.
- Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA) (2011) Guide to weed control. Ontario Ministry of Agriculture, Food, and Rural Affairs, Toronto.
- Senseman, S.A. (2007) Herbicide handbook. 9th Edition, Weed Science Society of America, Champaign.
- Van Gessel, J.M., Monks, W.D. and Quintin, R.J. (2000) Herbicides for potential use in lima bean (Phaseolus lunatus) production. Weed Technology, 14, 279-286. http://dx.doi.org/10.1614/0890-037X(2000)014[0279:HFPUIL]2.0.CO;2
- Sikkema, P.H., Hekmat, S., Shropshire, C. and Soltani, N. (2009) Response of black, cranberry, kidney, and white bean to linuron. Weed Biology and Management, 9, 173- 178. http://dx.doi.org/10.1111/j.1445-6664.2009.00336.x
- Renner, K.A. and Powell, G.E. (1992) Responses of navy bean (Phaseolus vulgaris) and wheat (Triticum aestivum) grown in rotation to clomazone, imazethapyr, bentazon, and acifluorfen. Weed Science, 40, 127-133.
- Sikkema, P., Soltani, N., Shropshire, C. and Cowan, T. (2004) Sensitivity of kidney beans (Phaseolus vulgaris) to soil applications of S-metolachlor and imazethapyr. Canadian Journal of Plant Science, 84, 405-407. http://dx.doi.org/10.4141/P03-069
- Soltani, N., Shropshire, C., Cowan, T. and Sikkema, P. (2003) Tolerance of cranberry beans (Phaseolus vulgaris) to soil applications of S-metolachlor and imazethapyr. Canadian Journal of Plant Science, 83, 645-648. http://dx.doi.org/10.4141/P03-006
- Soltani, N., Shropshire, C., Cowan, T. and Sikkema, P. (2004) Tolerance of black beans (Phaseolus vulgaris) to soil applications of S-metolachlor and imazethapyr. Weed Technology, 18, 166-173. http://dx.doi.org/10.1614/WT-03-044R
- Poling, K. (1999) Dry edible bean responses to dimethenamid and metolachlor. M.S. Thesis, Michigan State University, East Lansing.
- Singh, S.P., Gepts, P. and Debouck, D.G. (1991) Races of common bean (Phaseolus vulgaris), fabaceae. Economic Botany, 45, 379-396. http://dx.doi.org/10.1007/BF02887079
- Singh, S.P., Gutierrez, J.A., Molina, A., Urrea, C. and Gepts, P. (1991) Genetic diversity in cultivated common bean: II. Marker-based analysis of morphological and agronomic traits. Crop Science, 31, 23-29. http://dx.doi.org/10.2135/cropsci1991.0011183X003100010005x
- Singh, S.P., Nodari, R. and Gepts, P. (1991) Genetic diversity in cultivated common bean: I. Allozymes. Crop Science, 31, 19-23. http://dx.doi.org/10.2135/cropsci1991.0011183X003100010004x
- Soltani, N., Bowley, S. and Sikkema, P.H. (2005) Responses of dry beans (Phaseolus vulgaris) to flumioxazin. Weed Technology, 19, 351-358. http://dx.doi.org/10.1614/WT-04-146R1
- Sikkema, P., Shropshire, C. and Soltani, N. (2007) Dry bean response to preemergence-applied KIH-485. Weed Technology, 21, 230-234. http://dx.doi.org/10.1614/WT-06-050.1

