Journal of Minerals and Materials Characterization and Engineering
Vol.1 No.4(2013), Article ID:34238,8 pages DOI:10.4236/jmmce.2013.14025
Physico-Mechanical Responses of Polypropylene-CaCO3 Composite
1Department of Metallurgical and Materials Engineering, University of Lagos, Lagos, Nigeria
2Department of Chemical Engineering, University of Lagos, Lagos, Nigeria
Email: samsonoluropo@yahoo.com, *mawwal04@yahoo.com, ramadanwasiu@yahoo.com, mbodude@unilag.edu.ng
Copyright © 2013 Samson Oluropo Adeosun et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 3, 2013; revised April 25, 2013; accepted May 13, 2013
Keywords: Polypropylene; Calcium Carbonate; Water Absorption Capacity; Density; Ultimate Tensile Strength; Impact Resistance
ABSTRACT
This study examines some physical and mechanical characteristics of polypropylene (PP) and calcium trioxocarbonate IV (CaCO3) composites prepared by melt blending. The mechanical (tensile strength and impact energy resistance) and physical (density and water absorption capacity) properties of the composites were evaluated using 0% - 40% of the filler. The results showed that 20%-CaCO3 addition improved the ultimate tensile stress by 58%, and the UTS is 84% better when 25%-CaCO3 addition is made while the impact resistance decline by 8 and 12% respectively at these two compositions. In addition, the density only differ from that of pure PP at 25% CaCO3 addition by 18% increment, however, water absorption increased by 400% at 10%-CaCO3 addition.
1. Introduction
Polypropylene (PP) is an amorphous thermoplastic polymer which has wide engineering application because it possesses useful properties such as transparency, dimensional stability, flame resistance, high heat distortion temperature, and high impact strength [1]. PP filled with particulate fillers has continued to attract research attention due to its versatility of application and low cost [2-6].
Incorporation of fillers in thermoplastics has been a common practice in the plastics industry for the dual purposes of reducing production costs of moulded products as well as improving the mechanical properties of the thermoplastics such as strength, rigidity, durability and hardness [7]. The fillers that have been widely studied includes CaCO3 [8-10], talc [11-13], rice husk ash [14], kaolin and mica [15-18], and organic fillers [19,20]. Talc, mica and kaolin are used to enhance the stiffness and strength of filled plastics; whereas CaCO3 filled grades are reported to have higher impact strength. The properties of particulate-filled polymers are determined by several factors such as the component properties (matrix and filler), composition, and structure. In addition to the component properties, the mechanical characteristics of these materials are significantly influenced by the interfacial interactions, which depend on the size of the interface and the strength of the interaction [10]. This explains the incorporation of additives such as coupling agents and compatibilizer to further enhance properties [21-24].
CaCO3 is one of the most commonly used inorganic fillers in thermoplastics such as polyvinyl chloride (PVC) and PP [25]. The particle size of commercial grade CaCO3 is in the range of micrometer. Minimal strength enhancement observed in micro-sized CaCO3 filled composites was largely attributed to lack of surface interaction between the filler and the matrix. Attempt have been to improve the surface interactions of CaCO3/PP composite through use of nano-size particles [23,26]. However, homogeneous dispersion of nanoparticles in the thermoplastic matrix is a difficult process [26]. There is also the tendency for the production of craze associated with nanoparticles. These together with the extra cost of producing nanoparticles have made industrial utilization of nanoparticles relatively unattractive.
Consequently, there is the need to explore means of significantly improving on the mechanical properties of polymer composites with little or no increase in production cost in order to have wide application prospect. This study is therefore aimed at reinforcing PP with comercial grade CaCO3 using relatively cheap technology (melt blending) with the view to determining the optimum filler load for the enhancement of both mechanical and physical properties of CaCO3/PP composite.
2. Experimental Methodology
2.1. Materials
The calcium carbonate was obtained from Kesco chemicals located at Bariga area of Lagos, while the polypropylene was obtained from Gee Plast industry, Lagos, Nigeria. The instrument used includes sieve machine, furnace, digital weighing balance, Hounsfield Tensometer tensile machine, and scanning electron microscopy.
2.2. Method
Two (2) kg of calcium carbonate sample was measured on a weighing device and sieved into 100BSS using a sieve machine with an electrical vibrator operating at 3 hertz and vibrating for 30 mins. The measured polypropylene (PP) sample was charged into a preheated crucible pot using a camp gas as the furnace to remove all moisture and later melt at a temperature around 176˚C. The calcium carbonate was then added to the melt. The mixture was then poured into a metal mould and allowed to solidify (see Figure 1). The filler loading of CaCO3 ranges from 0 wt - 40 wt percent while PP make up for the rest to add up to 100 wt percent of the mix samples. The pouring was done from one side of the mould at a fast rate and the container was held close to the mould during pouring. The cast composite samples on cooling have dimension 137 × 137 × 10 mm3.
The densities of the cast samples were obtained after fettling by weighing each sample on the digital weighing scale to obtain the mass (m). The volume of water used was measured as V1 then the sample was placed into the water and the new volume was measured as V2, hence the final volume (v) of water used was determined and densities (ρ) were calculated using Equation (1)
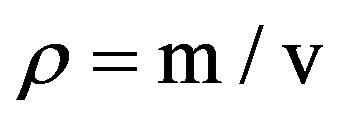 (1)
(1)
Water absorption samples were cut into sizes (see Figure 2) suitable for cylinder used for the water absorption process, weighed dried, W1 at first instance. Subsequently, samples were then weighed after every 24 hrs for a total of 168 hrs to obtain the new sample weights W2. The percentage weight gained was calculated and recorded for each sample using the Equation (2).
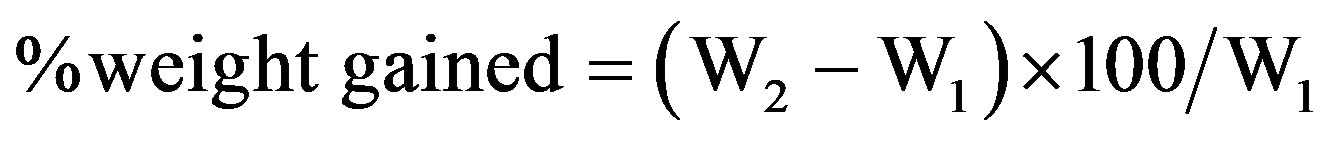 (2)
(2)
 (a)
(a)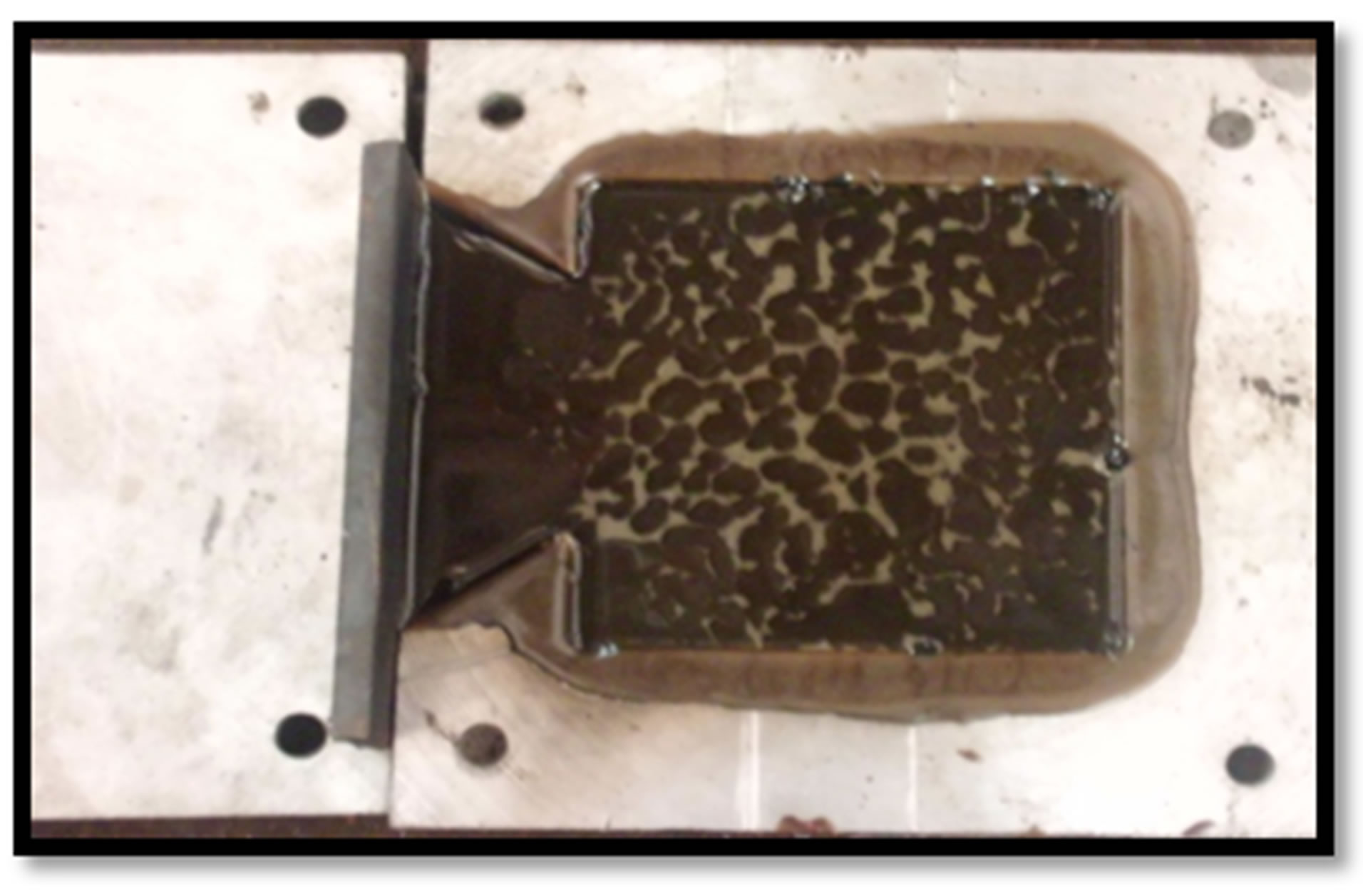 (b)
(b) (c)
(c)
Figure 1. Shows (a) metal mould (b) molten PP/CaCO3 composite solidifying in mould cavity (c) solid PP/CaCO3 composite samples.

Figure 2. Shows water absorption test of solid PP/CaCO3 composite samples.
The tensile test specimens were machined (see Figure 3) and test carry out using Hounsfield Tensometer tensile test machine. The test specimen was secured firmly between the gaps of the tensometer which was loaded me chanically until it was stressed to fracture. The test was carried out at a strain rate of 4.7 × 10−2 S−1.
Impact resistance test was performed in accordance to ASTM D4812 standard. The sample was also machined to the required specimen size; length of 100 mm and height of 8 mm, 6 mm thick and a V-notch at the centre of the specimen with depth of 2 mm.
Scanning electron microscopy of the samples was carried out. The samples were cut into small bits required by the SEM machine; 1 cm by 1 cm. Variable current (VP) was used because the samples are not electrically conducting. The variable current makes the image clearer and works between pressures of 10 Pa - 200 Pa. High vacuum could have been used, but it is restricted to only electrically conducting materials and works at pressures much higher than the VP. The pressure target was set to 50 Pa.
3. Results and Discussion
3.1. Physical Properties
The density of the polymer composite decline from about 0.00675 g/cm3 for pure polypropylene to 0.004 g/cm3 for composite containing 10% CaCO3 addition (see Figure 4). Between 10% and 25% CaCO3 addition there was an increase in composite density to 0.008 g/cm3 before its density again declined to 0.004 g/cm3 at addition of 40% CaCO3. The density of PP −25% CaCO3 composite does not significantly differ from that of pure polypropylene with about 18 percent increase. At higher density water of hydration could be present at the surfaces of inorganic fillers giving fillers with high tendency to agglomerate. The agglomeration of particles could cause air pockets to be trapped between the particles leading to increase in density of composite. At lower density there is the tendency of enhanced filler-matrix compatibility as the polymer matrix displaced the trapped air pockets and this resulted in deagglomeration of the particles with improved filler dispersion [21].
In Figure 5, the water absorption tendency of PPCaCO3 composite is shown. Generally, as soak time increases, the material become more susceptible to water absorption. Composite with 10 wt% CaCO3 displayed highest water absorption capacity (0.1%) at 168 hours. Between 145 - 168 hours soak-time, composite with 25% CaCO3 addition showed the lowest water absorption capacity of 0.04 wt% after 180 hours. The hydrophobic behaviour of PP −25% CaCO3 over convectional PP began above 100 hours soak time. Thus, PP −25% CaCO3 composite is seen to have superior water absorption resistance over pure PP. The amount of water absorb varies differently with various plastics and the absorbed water may affect the plastics in different ways. The electrical property change most noticeable with water absorption. Plastics which absorb water tend to change dimension in the process and when water stability is required in products made of such plastics, grades with less tendency to absorb water are chosen. Mechanical properties may also be affected negatively with water absorption [1].
3.2. Mechanical Properties
The ultimate tensile strengths (UTS) of the PP-CaCO3 composites improves steadily as CaCO3 is added and reached peak of 7.5 MPa at 25% CaCO3 (see Figure 6) which is superior to the conventional polypropylene (4.75 MPa) by about 58%. However with further increase in CaCO3 addition the UTS decreases in almost similar fashion as it increases from 0% - 25% CaCO3. The initial increase in strength of the composite is due to good filler-matrix interactions, which also enable more stress to be transferred from the matrix to the fillers during external loading [27].
The elongation responses of the PP-CaCO3 composite increases from 5.75% for conventional pure polypropylene to 9.5% for polypropylene −25% CaCO3 content (see Table 1). Further increment in CaCO3 content does not improve its elongation as it declines in a similar manner to its increment. The reverse increment in elongation of the composite above 20% filler content can be attributed to the formation of filler aggregates which are made up of particles held together by strong forces and not as agglomerates while are made up of particles held together by weak forces. The aggregates debond easily from the matrix and tend to reduce the strength of the composite while increasing its elongation [28]. Matrix-filler debonding occurred and this permits unhindered plastic deformation around the filler particles thereby increasing the ductility of the composite.
Figure 7 shows the Young modulus (E) of the composite at various filler loading. There is a sharp decrease in modulus from the conventional PP (160 MPa) to 20% CaCO3 with about 92 MPa thereafter an upward trend was observed with filler content uptill 40% CaCO3. The rigidity of the conventional PP is therefore superior to that of composites with CaCO3 content below 28%. Thus there is poor transfer of the elastic deformation between filler and matrix materials with interface fracture below
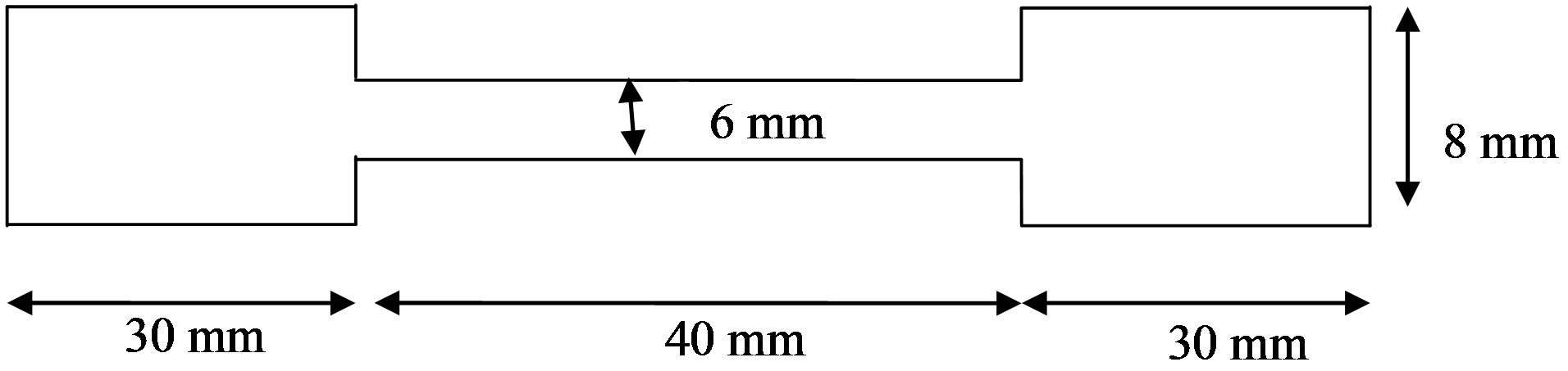
Figure 3. Tensile test specimen for PP/CaCO3 composite.
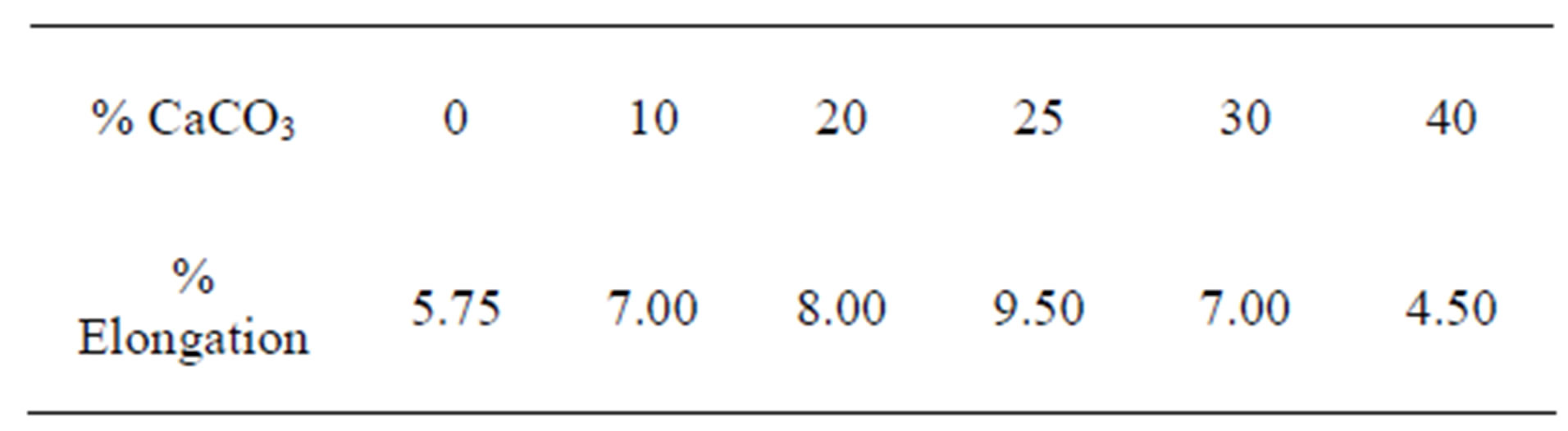
Table 1. Elongation response of PP/CaCO3 composite.
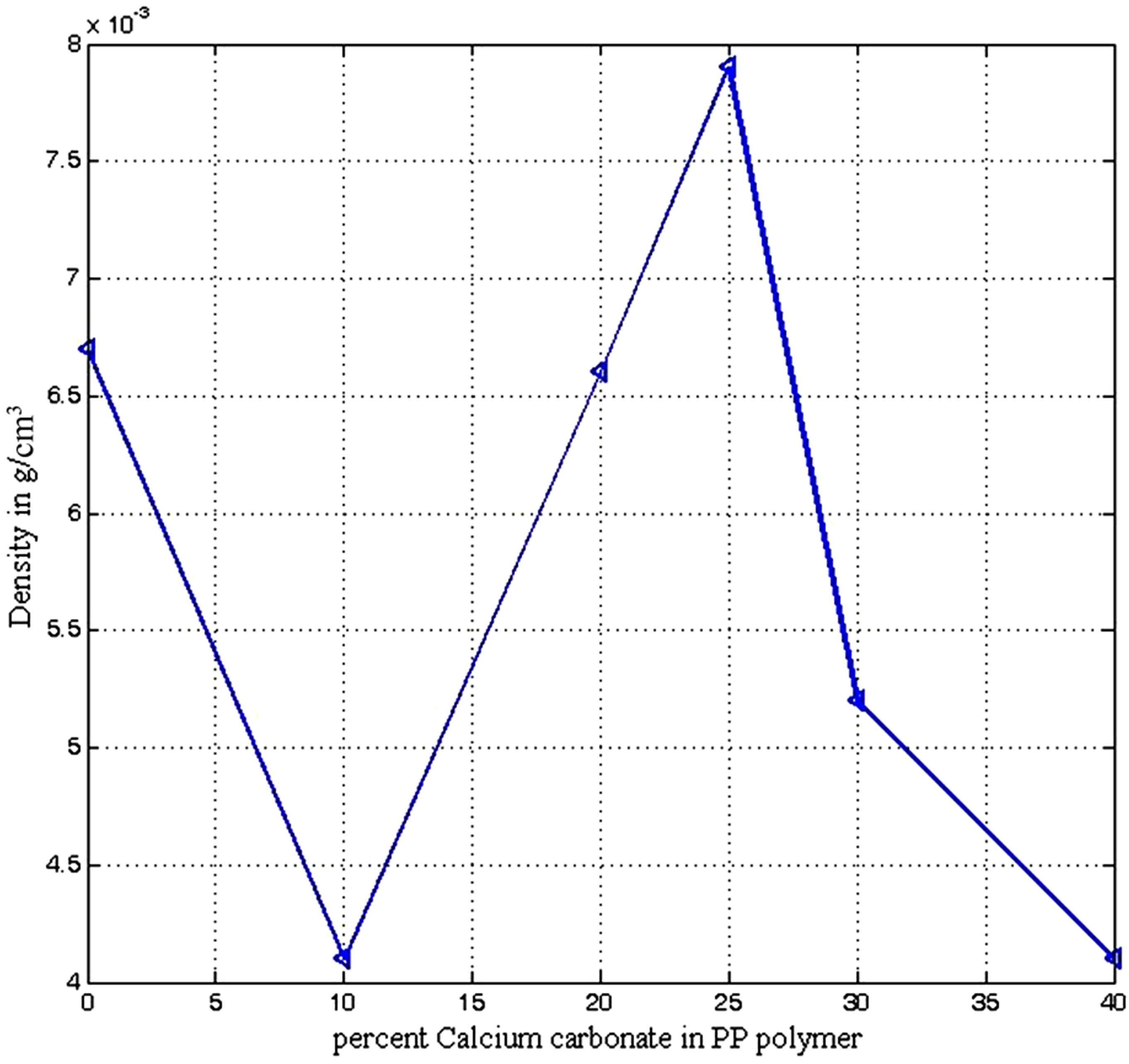
Figure 4. Density of polypropylene calcium carbonate composite.
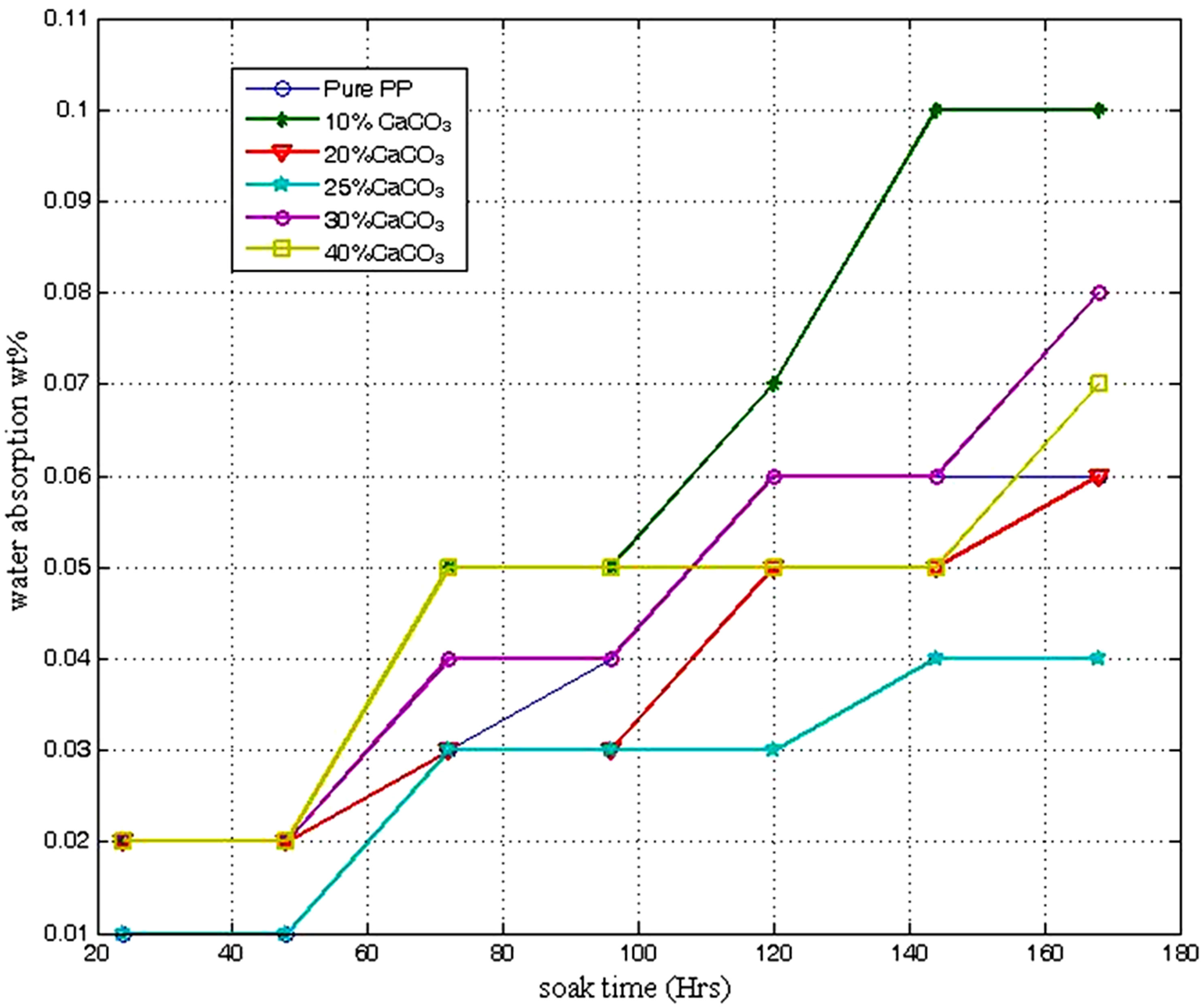
Figure 5. Water absorption responses of polypropylene calcium carbonate composite.
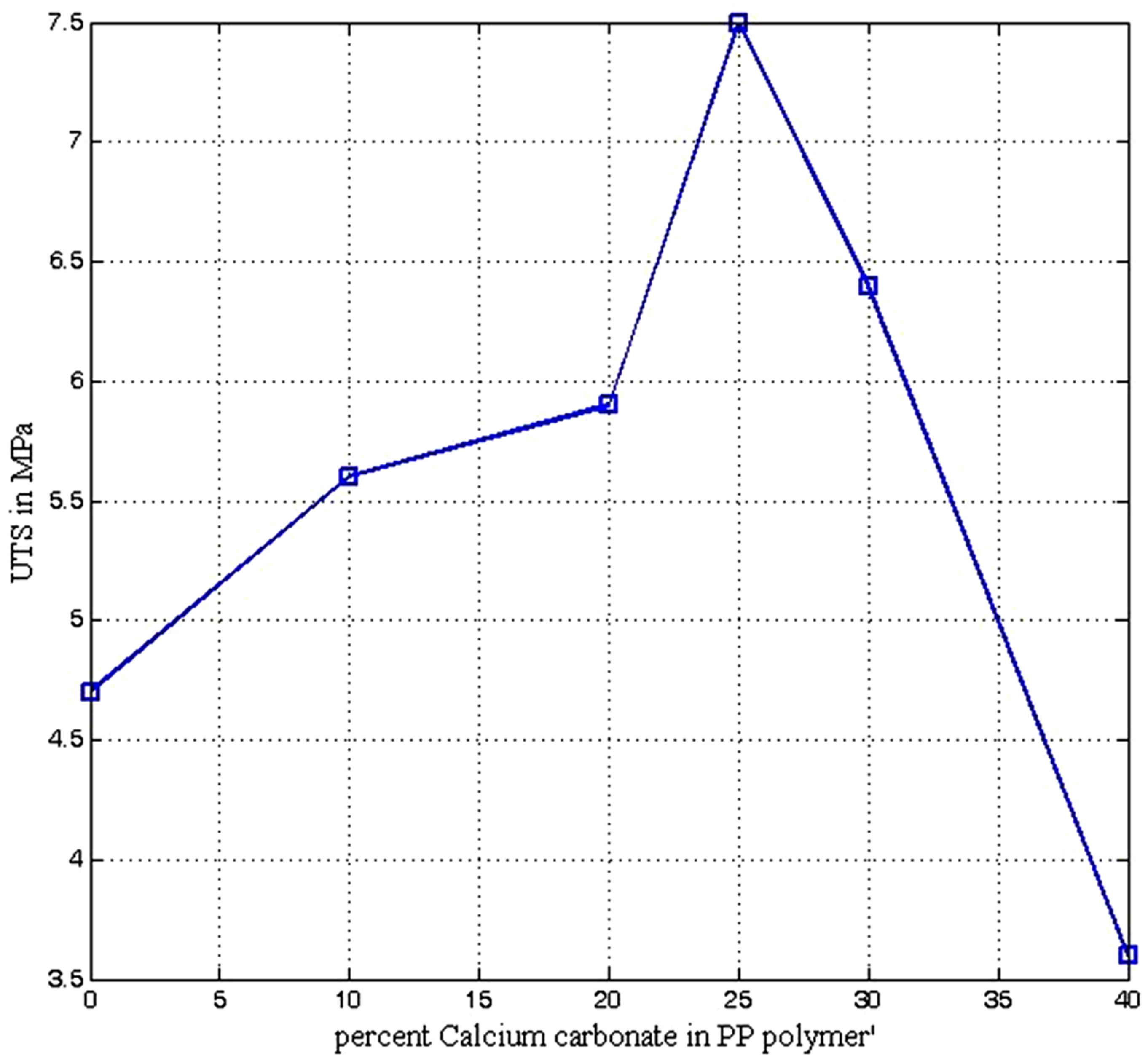
Figure 6. Ultimate tensile strength of polypropylene calcium carbonate composite.
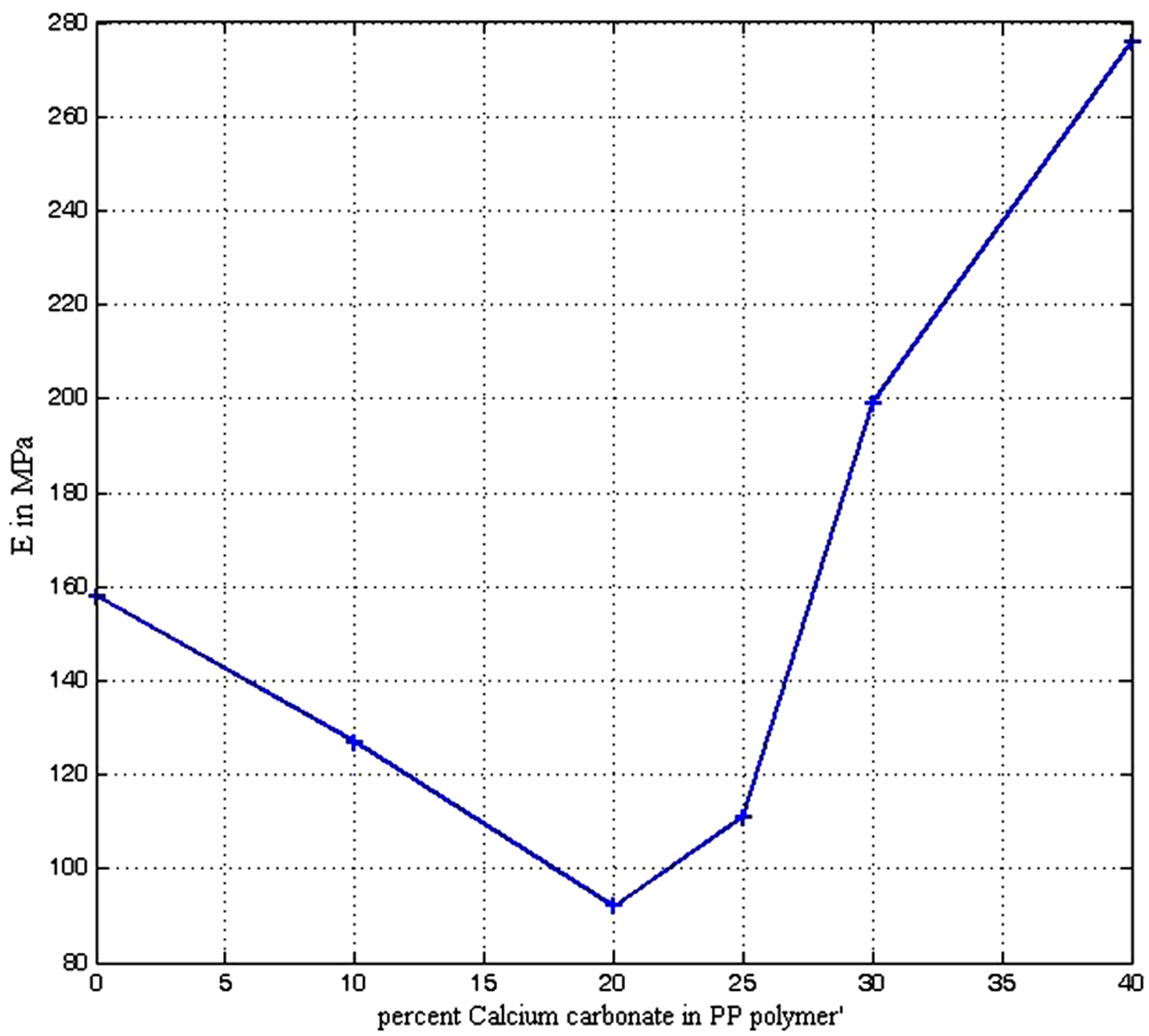
Figure 7. Young modulus (E) of polypropylene calcium carbonate composite.
20% CaCO3. Beyond 20% CaCO3, there is an improved interaction. At 25% CaCO3 the modulus is 116 MPa representing a decrease of 27.5% relative to the conventional PP. Tensile modulus is known to be less sensitive variation of interfacial adhesion than the tensile strength which is strongly associated with interfacial failure behaviour [29]. Increase in tensile modulus of some composite samples is attributed to better distribution of Al dross filler in the matrix for both filler sizes. The higher surface area of filler gives better adhesion in composite, thus forming a stiffer material which is attributed to higher modulus [30].
The impact energy resistance of PP composite decreases with increase in CaCO3 addition from 68 J to 55 J for 0 to 5 percent CaCO3 addition. It should be stated that the impact energy resistance of the composite at 25% CaCO3 content (60 J) is not too serious a decline (12%) from that of conventional PP (68 J) when the higher density of the composite is taken into account as it will compensate for the likely effect of the fall in impact energy resistance (see Figure 8). The decline in impact resistance of the composite material as filler content increases is due to the effect posed by big filler particles which may act as stress concentration points or points of discontinuity in composites thereby promoting crack initiation and propagation [25].
3.3. Microstructural Analysis
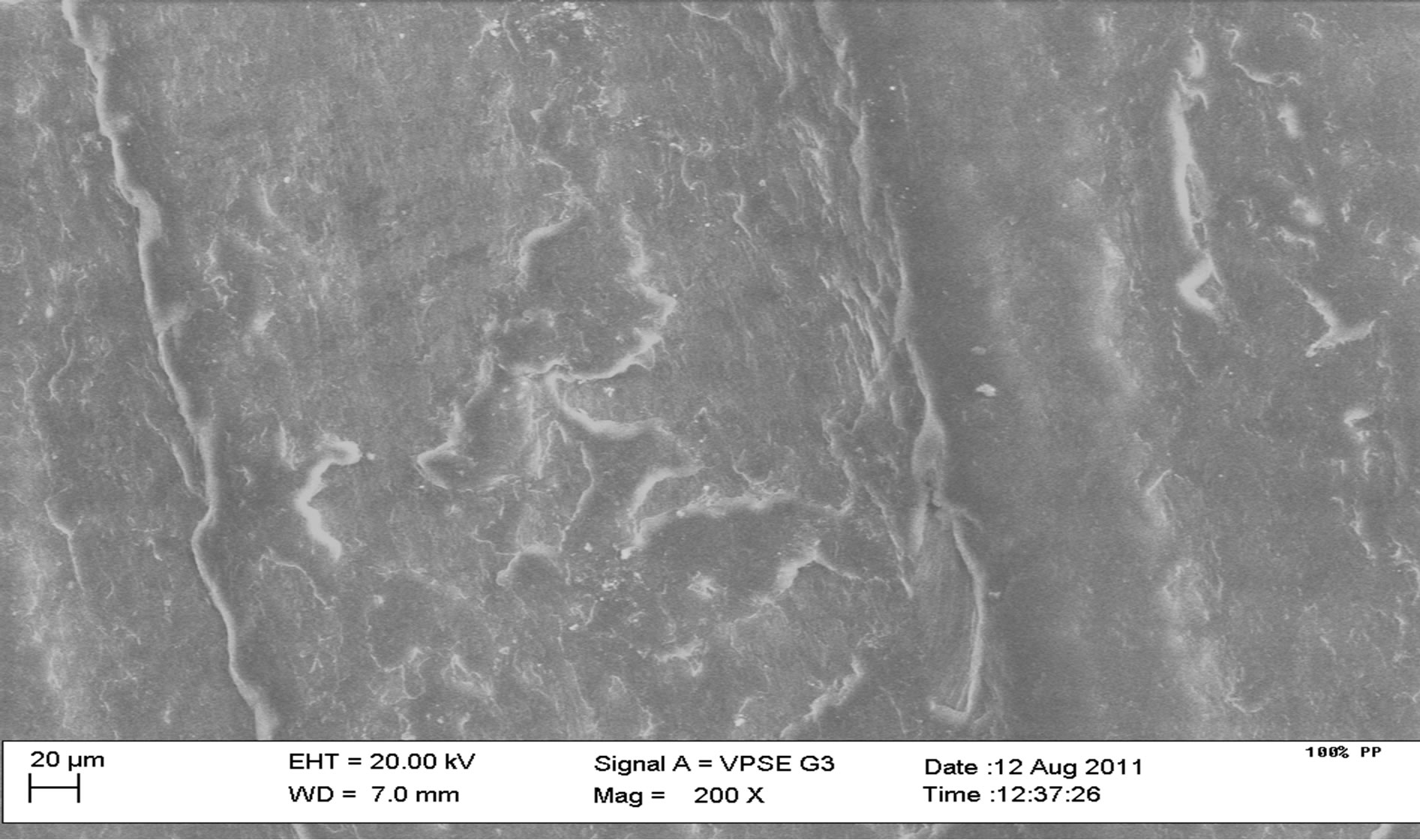
Figure 9(a) shows an uneven distribution of particles for polypropylene samples and this is due to the mode of pouring during casting. Figure 9(b) shows the microstructure of 10% calcium carbonate (CaCO3) addition with some degree of agglomeration of the particles. Figure 9(c) shows the microstructure of the 40% calcium carbonate (CaCO3) addition at 200× magnification with a greater degree of agglomeration of particles than the 10% CaCO3 addition.
4. Conclusion
There is a general enhancement of desirable mechanical properties when the calcium carbonate is added to the polypropylene. The better strengthening effect of the calcium carbonate can be attributed to the formation of aggregates by the filler which can debond easily to promote ductility. However, the impact strength of the composite decline because of poor bonding between the matrix and filler particles. The percentage calcium carbonate addi-
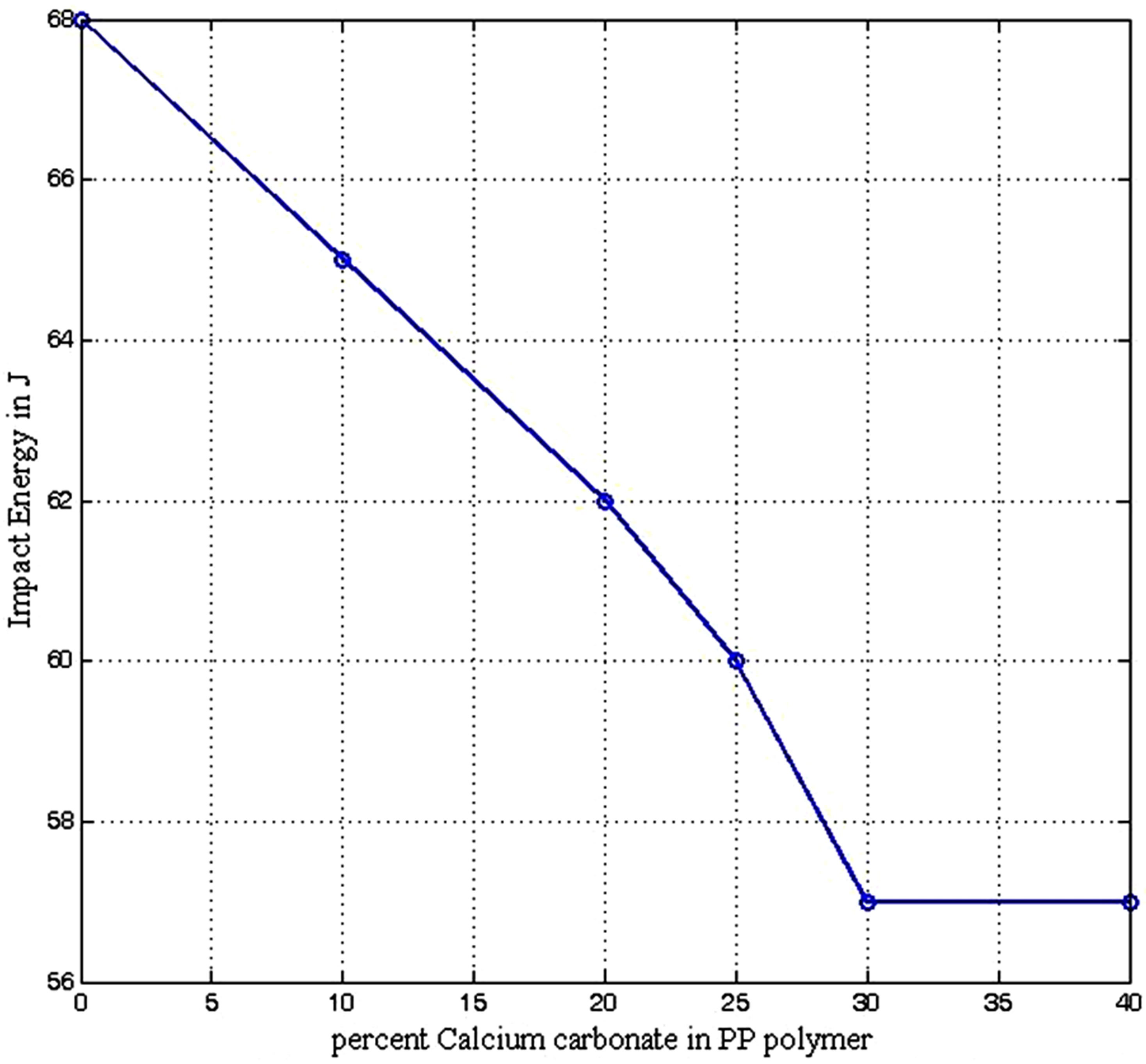
Figure 8. Impact energy resistance of polypropylene calcium carbonate composite.
 (a)
(a)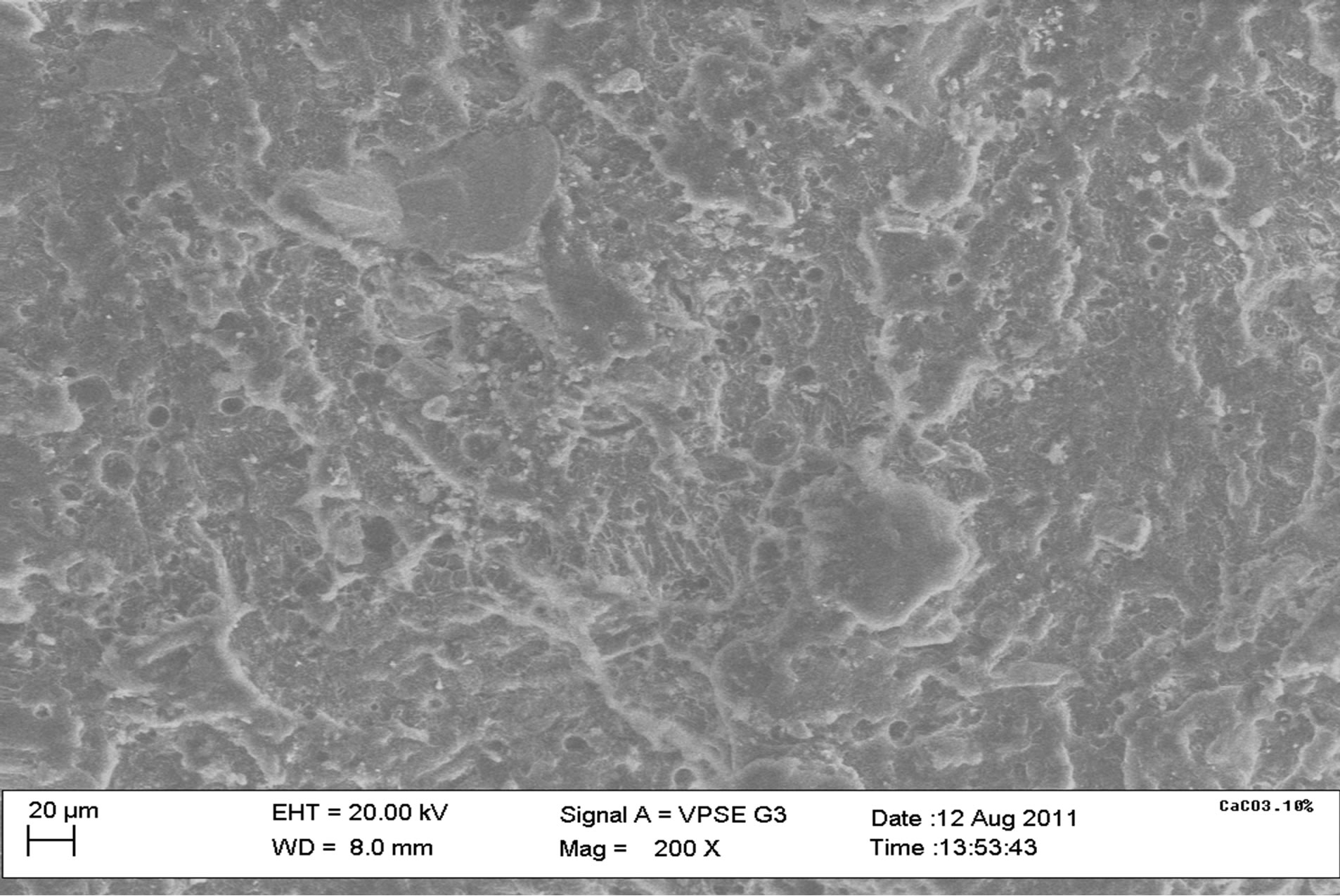 (b)
(b)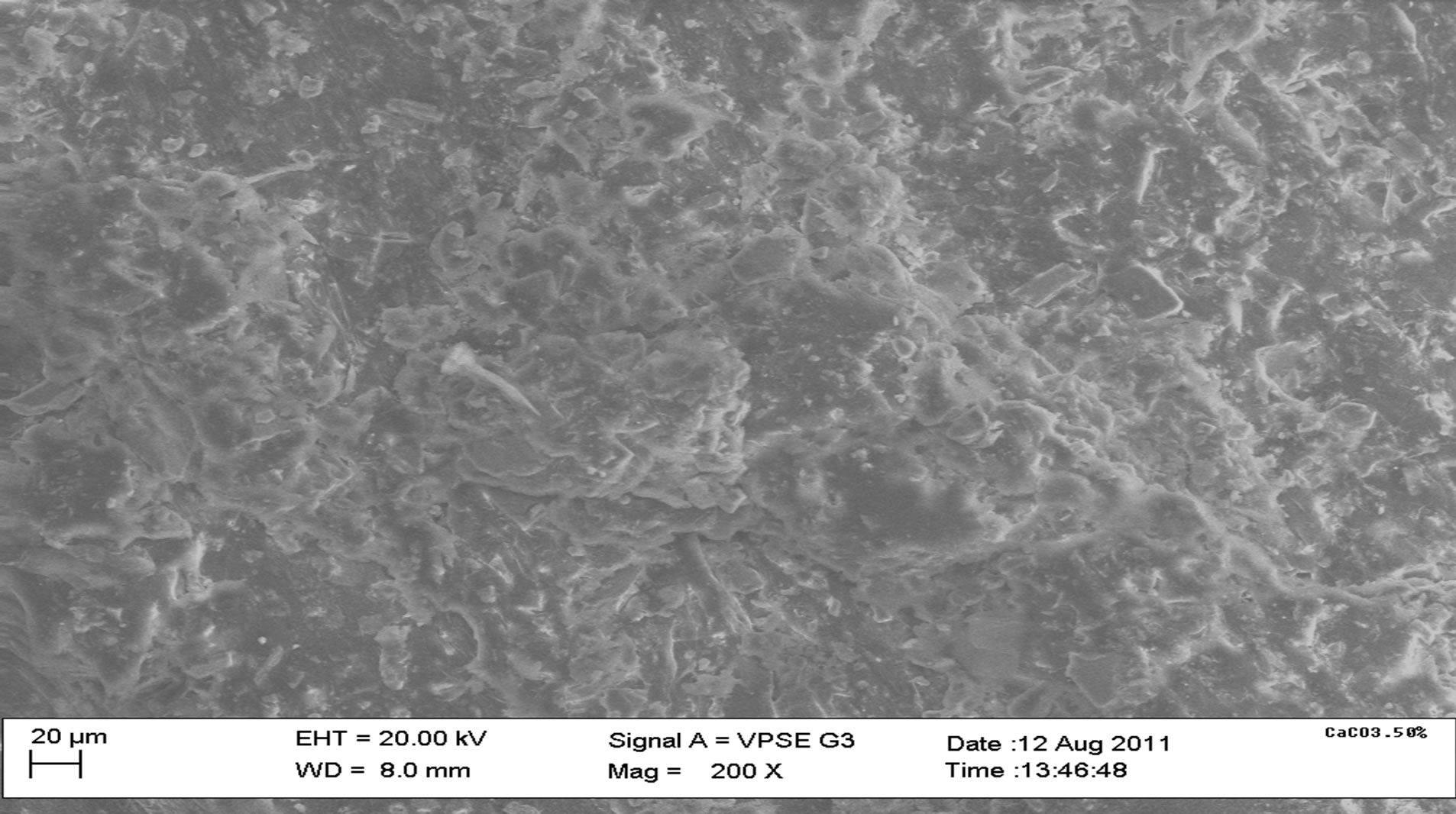 (c)
(c)
Figure 9. SEM microstructures of some composite samples. 100% PP, 10% CaCO3, 40% CaCO3.
tion that gave an optimum physical and mechanical property was attained at 25% CaCO3 addition.
REFERENCES
- K. Dey, N. Sharmin, R. A. Khan, S. Nahar, A. J. Parsons, and C. D. Rudd, “Effect of Iron Phosphate Glass on the Physico-Mechanical Properties of Jute Fabric-Reinforced Polypropylene-Based Composites,” Journal of Thermoplastic Composite Materials, Vol. 24, No. 5, 2011, pp. 695-711. doi:10.1177/0892705711401848
- K. Saminathan, P. Selvakumar and N. Bhatnagar, “Fracture Studies of Polypropylene/Nanoclay Composite. Part I: Effect of Loading Rates on Essential Work of Fracture,” Polymer Testing, Vol. 27, No. 3, 2008, pp. 296-307. doi:10.1016/j.polymertesting.2007.11.008
- Y. Zhao and H. X. Huang, “Dynamic Rheology and Microstructure of Polypropylene/Clay Nanocomposites Prepared under Sc-CO2 by Melt Compounding,” Polymer Testing, Vol. 27, No. 1, 2008, pp. 129-134. doi:10.1016/j.polymertesting.2007.11.006
- T. H. Zhou, W. H. Ruan, Y. L. Mai, M. Z. Rong and M. Q. Zhang, “Performance Improvement of Nano-Silica/ Polypropylene Composites through In-Situ Cross-Linking Approach,” Composite Science and Technology, Vol. 68, No. 14, 2008, pp. 2858-2863. doi:10.1016/j.compscitech.2007.10.002
- J. H. Chen, M. Z. Rong, W. H. Ruan and M. Q. Zhang, “Interfacial Enhancement of Nano-SiO2/Polypropylene,” Composite Science and Technology, Vol. 69, No. 2, 2009, pp. 252-259. doi:10.1016/j.compscitech.2008.10.013
- N. Muksing, M. Nithitanakul, B. P. Grady and R. Magaraphan, “Melt Rheology and Extrudate Swell of Organo Bentonite-Filled Polypropylene Nanocomposites,” Polymer Testing, Vol. 27, No. 4, 2008, pp. 470-479. doi:10.1016/j.polymertesting.2008.01.008
- V. Khunova, J. Hurst, I. Janigova and V. Smatko, “Plasma Treatment of Particulate Polymer Composites for Analyses by Scanning Electron Microscopy: II. A Study of Highly Filled Polypropylene/Calcium Carbonate Composites,” Polymer Testing, Vol. 18, No. 7, 1999, pp. 501- 509. doi:10.1016/S0142-9418(98)00038-5
- W. C. J. Zuiderduin, C. Westzaan, J. Huetink and R. J. Gaymans, “Toughening of Polypropylene with Calcium Carbonate Particles,” Polymer, Vol. 44, No. 1, 2003, pp. 261-275. doi:10.1016/S0032-3861(02)00769-3
- Y. S. Thio, A. S. Argon, R. E. Cohen and M. Weinberg, “Toughening of Isoactic Polypropylene with CaCO3 Particles,” Polymer, Vol. 43, No. 13, 2002, pp. 3661-3674. doi:10.1016/S0032-3861(02)00193-3
- Z. Demjen, B. Pukanszky and J. Nagy, “Evaluation of Interfacial Interaction in Polypropylene/Surface Treated CaCO3 Composites,” Composites A: Applied science and manufacturing, Vol. 29, No. 3, 1998, pp. 323-329.
- G. Guerrica-Echevarria, J. I. Eguiazabal and J. Nazabal, “Influence of Moulding Conditions and Talc Content on the Properties of Polypropylene Composites,” European Polymer Journal, Vol. 34, No. 8, 1998, pp. 1213-1219. doi:10.1016/S0014-3057(97)00228-0
- T. Xu, H. Lei and C. S. Xie, “Investigation of Impact Fracture Process with Particle-Filled Polymer Materials by Acoustic Emission,” Polymer Testing, Vol. 21, No. 3, 2002, pp. 319-324. doi:10.1016/S0142-9418(01)00091-5
- K. J. Kim and J. L. White, “Particle Orientation in TalcFilled Thermoplastics Extruded through Cylindrical, Rectangular and Annular Dies,” Journal of Non-Newtonian Fluid Mechanics, Vol. 66, No. 2-3, 1996, p. 257. doi:10.1016/S0377-0257(96)01464-4
- M. Y. Ahmed Fuad, J. Mustafah, M. S. Mansor, Z. A. Mohd Ishak and A. K. Mohd Omar, “Thermal Properties of Polypropylene/Rice Husk Ash Composites,” Polymer International, Vol. 38, No. 1, 1995, pp. 33-43.
- L. Jilken, G. Malhammar and R. Selden, “The Effect of Mineral Fillers on Impact and Tensile Properties of Polypropylene,” Polymer Testing, Vol. 10, No. 5, 1991, pp. 329-344. doi:10.1016/0142-9418(91)90011-L
- P. Mareri, S. Bastide, N. Binda and A. Crespy, “Mechanical Behaviour of Polypropylene Composites Containing Finer Mineral Filler: Effect of Filler Surface Treatment,” Composite Science and Technology, Vol. 58, No. 5, 1998, pp. 747-752. doi:10.1016/S0266-3538(97)00156-5
- A. M. Riley, C. D. Paynter, P. M. McGenity and J. M. Adams, “Factors Affecting the Impact Properties of Mineral Filled Polypropylene,” Plastics and Rubber Processing and Applications, Vol. 14, No. 2, 1990, pp. 85-93.
- B. M. Sole and A. Ball, “On the Abrasive Wear Behaviour of Mineral Filled Polypropylene,” Tribology International, Vol. 29, No. 6, 1996, pp. 457-465. doi:10.1016/0301-679X(95)00098-O
- J. Gassan and A. K. Bledzki, “The Influence of FiberSurface Treatment on the Mechanical Properties of JutePolypropylene Composites,” Composites A: Applied Science and Manufacturing, Vol. 28, No. 12, 1997, pp. 1001-1005.
- N. E. Zafeiropoulos, D. R. Williams, C. A. Baillie and F. L. Matthews, “Engineering and Characterization of the Interface of Flax Fibre/Polypropylene Composite Materials. Part I: Development and Investigation of Surface Treatments,” Composites A: Applied Science and Manufacturing, Vol. 33, No. 8, 2002, pp. 1083-1093.
- Y. W. Leong, M. B. Abu Bakar, Z. A. Mohd Ishak and A. Ariffin, “Effects of Filler Treatments on the Mechanical, Flow, Thermal, and Morphological Properties of Talc and Calcium Carbonate Filled Polypropylene Hybrid Composites,” Journal of Applied Polymer Science, Vol. 98, 2005, pp. 413-426. doi:10.1002/app.21507
- Y. W. Leong, M. B. Abu Bakar, Z. A. Mohd Ishak, A. Ariffin and B. Pukanszky, “Comparison of the Mechanical Properties and Interfacial Interactions between Talc, Kaolin, and Calcium Carbonate Filled Polypropylene Composites,” Journal of Applied Polymer Science, Vol. 91, 2004, pp. 3315-3326. doi:10.1002/app.13542
- J. Zhang, B. Han, N. Zhou, J. Fang, J. Wu, Z. Ma, H. Mo and J. Shen, “Preparation and Characterization of Nano/ Micro-Calcium Carbonate Particles/Polypropylene Composites,” Journal of Applied Polymer Science, Vol. 119, No. 6, 2011, pp. 3560-3565. doi:10.1002/app.33037
- S. K. Najafi, A. Bahra and M. Abdouss, “Effect of Oxidized Polypropylene as a New Compatibilizer on the Water Absorption and Mechanical Properties of Wood Flour-Polypropylene Composites,” Journal of Applied Polymer Science, Vol. 119, No. 1, 2011, pp. 438-442. doi:10.1002/app.32618
- I. I. Rubin, “Handbook of Plastic Materials and Technology,” New York, Wiley, 1990, p. 693.
- C. Chan, J. Wu, J. X. Li and Y. K. Cheung, “Polypropylene/Calcium Carbonate Nanocomposites,” Polymer, Vol. 43, No. 10, 2002, pp. 2981-2992. doi:10.1016/S0032-3861(02)00120-9
- W. C. Wake, “Fillers for Plastics,” Plastics Institute, 1971.
- L. E. Nielsen, “Mechanical Properties of Polymers and Composites,” 2nd Edition, Marcel Dekker, New York, 1994.
- T. T. L. Doan, S. L. Gao and E. Mader, “Jute/Polypropylene Composites; Effect of Matrix Modification,” Composite Science and Technology, Vol. 66, No. 7-8, 2006, pp. 952-963.
- H. D. Rozman, G. B. Peng and Z. A. Mohd Ishak, “The Effect of Compounding Technique on the Mechanical Properties of Oil Palm Empty Fruit Bunch-Polypropylene Composite,” Journal of Applied Polymer Science, Vol. 70, No. 13, 1998, pp. 2647-2655. doi:10.1002/(SICI)1097-4628(19981226)70:13<2647::AID-APP10>3.0.CO;2-2
NOTES
*Corresponding author.

