Food and Nutrition Sciences
Vol.5 No.16(2014), Article ID:49234,14 pages
DOI:10.4236/fns.2014.516171
Effect of Frying-Cooking on Nutritional and Bioactive Compounds of Innovative Ovo-Vegetarian Diets
Hassan Barakat
Department of Food Science, Faculty of Agriculture, Benha University, 13736 Moshtohor, Kaliuobia, Egypt
Email: hassan.barakat@fagr.bu.edu.eg
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 12 July 2014; revised 13 August 2014; accepted 21 August 2014
ABSTRACT
Vegetarian diets are becoming increasingly popular as meat prices as well as health concerns rise. Many people are cutting down or cutting out meat in favor of a full vegetarian diet. In present study, the applicability of different vegetables for producing ready-to-use and ready-to-eat chickpea-based ovo-vegetarian diets (OVDs) was investigated. Herein, six different vegetables (cauliflower, taro, green zucchini, pea, bean and spinach) were formulated with 25% chickpea and additional edible ingredients to produce ready-to-use OVDs. Subsequently, chemical composition, minerals content, bioactive compounds and antioxidant activity of those OVDs were investigated. However, ready-to-eat OVDs were organoleptically evaluated after frying as common cooking method. Results of composite analysis indicated 67.73% to 73.17%, 23.20% to 37.12%, 1.86% to 2.63%, 7.63% to 9.53%, 9.06% to 9.82% and 39.24% to 55.28% for moisture, crude protein, lipids, ash, fiber, and carbohydrates contents in ready-to-use OVDs, respectively. After frying, lipid content was increased in fried diets which changed the chemical composition and caloric value. Significant differences (p < 0.05) were found between macroand micro-nutrients content of readyto-use and ready-to-eat OVDs. The ready-to-use OVDs exhibit appropriate content of ascorbic acid, chlorophylls, carotenoids, flavonoids, and flavonols which basically depends on their ingredients. Frying process dramatically affected the ascorbic acid, chlorophylls, flavonoids, flavonols, and carotenoids contents. High organoleptic acceptability of ready-to-eat OVDs was recorded to confirm the consumer attractiveness further. In conclusion, the possibility of producing healthy ready-to-eat and ready-to-use OVDs incorporated with common consumed vegetables could provide a promising approach for improving human health and dietary pattern as well as for selecting the optimum processing conditions for innovative OVDs.
Keywords:Frying, Phytochemicals, Bioactive Compounds, Antioxidant Activity, Ovo-Vegetarian Diets, Health Benefits

1. Introduction
Vegetarian diets are often heterogeneous formulated in composition, involving a wide range of dietary sources for numerous and individual dietary requirements. Practically, adopting a vegetarian dietary pattern is traditionally interpreted to mean an absence of meat [1] . Basically, variations of vegetarian diet were classified into 1) lacto-ovo-vegetarians (includes dairy and eggs), 2) lacto-vegetarians (includes dairy), 3) ovo-vegetarians (includes eggs), and 4) vegan which have further restrictions imposed and exclude all animal origin foods. Additionally, vegetarians are characterised by high consumption of fruit, vegetables, legumes, nuts, grains and soy protein-food components, and each of these may independently be associated with positive health outcomes [2] - [5] . Interestingly, the meat substituting industry was highly encouraged to reduce the meat consumption and thereby reduce the risk of related disease. Purely, substituting consumption of meat by alternative protein rich products made from plant proteins, so-called Novel Protein Foods, would be an attractive option [6] . The University of Oxford suggests that a vegetarian diet could significantly reduce people’s risk of heart disease, finding that vegetarians have up to 32% less risk of developing heart disease than comparable to non-vegetarians [1] [7] [8] . This finding could encourage the processed meat consumers to change their nutritional behavior and prevent themselves from 42% higher risk of heart disease, a 19% higher risk of type 2 diabetes and bladder cancer as mentioned by [4] [9] .
Interestingly, in a new study from Harvard School of Public Health (HSPH) researchers has found that red meat consumption is associated with an increased risk of total, cardiovascular, and cancer mortality. The results also showed that substituting other healthy protein sources, such as fish, poultry, nuts, and legumes, was associated with a lower risk of mortality [10] . Additionally, vegetarians tend to have adjustable blood pressure, a lower body mass index (BMI), lower overall cancer rates, lower in saturated fats, have higher levels of dietary fiber, magnesium, iron and potassium, vitamins E and folate, carotenoids, flavonoids and other phytochemicals [4] [8] [9] [11] [12] . Practically, vegetables are commonly eaten as fresh or cooked for improving its sensory characteristics. The phytochemicals not only contribute to the vegetable’s color and taste, but also have been described to possess antimutagenic or even anticarcinogenic activity [11] -[13] . The Egyptian cuisine is notably conducive to vegetarian diets, as it relies heavily on vegetable dishes. However, several commonly consumed vegetables such as cauliflower, green pea, green bean, spinach and green zucchini were favorable for Egyptian consumers over the years ago. There are many studies that review the health benefits of mentioned vegetables considering their phytochemicals content and potential antioxidant, antimutagenic, anticarcinogenic, antimicrobial activities [14] -[21] . Indeed, carefully planned vegetarian and vegan diets can provide adequate nutrients for optimum health [2] . Evidence suggests that infants and children can be also successfully reared on vegan and vegetarian diets [22] [23] . However, all dietary practices, including non-vegetarian diets, can be deleterious for health if essential nutrients are not consumed. Therefore, vegetarian and vegan diets need to ensure a balance of nutrients from a wide variety of foods, especially for vulnerable groups. Improving dietary habits is a societal, not just an individual problem. Thus it demands a population-based, multisectoral, multi-disciplinary, and culturally relevant approach. Frying-cooking is one of the most important and commonly popular cooking methods. It helps to bring some desired changes in taste, texture, flavor and color. It is a method of cooking where beloved among Egyptians. Thus, in presented study the frying-cooking was taken as a cooking method module to be a cooking method for OVDs.
Accordingly, the main objectives of this study were to investigate the possibility to prepare innovative OVDs from different vegetables incorporated with chickpea as protein source. Moreover, to study the effect of frying cooking method on nutritional and bioactive compounds of innovative ovo-vegetarian diets as well. To achieve this purpose, six ovo-vegetarian diet formulas have been developed by incorporating 6 different vegetables with chickpea as well as some other edible ingredients. Proximate chemical composition, minerals, bioactive compounds and their antioxidant activity as well as the organoleptic properties for prepared diets were carried out.
2. Materials and Methods
2.1. Ingredients
Six different vegetables such as cauliflower (Brassica oleracea var.), pea (Pisum sativum L.), green zucchini (Cucurbita pepo L.), taro corms (Colocasia esculenta L.), green bean (Phaseolus vulgaris L.) and green spinach (Spinacia oleracea L.) were obtained in fresh status from the central local vegetable market at El-Obour City, Egypt. Chickpea (Cicer arietinum L.), sweet potato (Ipomoea batatas L.), whole barley, carrot (Daucus carota L.), green leafy vegetables mix [fresh coriander (Coriandrum sativum L.), dill (Anethum graveolens L.), parsley (Petroselinum crispum L.)], red pepper (Capsicum annuum L.), onion (Allium cepa L.), garlic (Allium sativum L.) eggs and edible salt were obtained from local supermarket, Egypt. In addition, traditional spices mixture was formulate as [25% black pepper, 25% cumin, 25% relish “Baharat; ready-mix of specific spices”, 10% dry coriander, 10% dry ginger and 5% dry chili] which were brought from Ragab El-Attar’s local spices supermarket, Egypt.
2.2. Preparation of Different Ingredients
All vegetables were washed, sorted and prepared as follow: green leaves of cauliflower were removed then edible part was cut into 1 - 1.5 cm, green pea was peeled and the end parties of green zucchini and green beans were removed then chopped in 2 cm pieces. The taro corms were peeled manually by sharp knife then chopped into 1 × 1 × 1 cm cubes. The yellow and undesired spinach leaves were removes after removing the pink rots of green spinach then, ripped by 1 - 2 cm. All prepared vegetables were washed and blanched for appropriate time (3, 3, 3.5, 4, 5 and 2.5 min, respectively) using live steam in blanching pot then cooled down and kept until use under freezing conditions.
Unpeeled chickpeas were washed and soaked in water for 12 h. Then excessive water was drained and chickpeas were peeled manually and ground for 3 min using a conventional kitchen machine to produce homogeneous puree. Sweet potato and carrots were peeled, washed, chopped in 1 cm slices, and blanched using live steam in blanching pot for 6 and 4 min, respectively. Adding sweet potato and carrot could increase the carotenoids contents. Subsequently, the blanched materials were immediately cooled down and homogenized to a puree. The whole naked barley kernels were milled twice to obtain homogeneous and fine barley flour. Sweet red pepper was washed and chopped in small cubes after removing the initial seeds. Further ingredients such as fresh onion and garlic were peeled, washed and then chopped immediately before preparing the vegetarian diets. To prepare the green leafy vegetables mix, fresh coriander, dill and parsley were washed, ripped then mixed as (2:1:1) respectively. The spices were ground and mixed as [25 g black pepper, 25 g cumin, 25 g relish (“Baharat”), 10 g dry coriander, 10 g dry ginger, and 5 g dry hot chili] to prepare 100 g spices mix and used immediately.
2.3. Preparation of Different Innovative Ready-to-Use OVDs
Six ovo-vegetarian diet formulas were prepared as a mix from the previously prepared ingredients according to recipes in Table1 One and half kilograms from each formula were prepared using a kitchen machine. Each ready-to-use ovo-vegetarian diet formula was filled in 2 polyethylene bags as 0.3 kg for chemical analysis of fresh diet and 1.2 kg for frying process and chemical analysis of fried diets. For preparing diets for sensory analysis, diets bags were kept for homogeneity of all ingredients for 12 - 18 hr under cooling conditions before frying, while 0.3-kg diet bags were subjected immediately for analysis. The whole experiment and analysis were done in triplicates.
2.4. Preparation of Different Innovative Ready-to-Eat OVDs
Ready-to-eat OVDs were left at room temperature for 5 min then mixed with 0.1% sodium bicarbonate amount immediately before frying [carbon dioxide producer, making the OVDs more porous and puffy during frying]. The vegetarian diet mixture was shaped using a frame and wide knife which were designed especially for this purpose. Appropriate amounts of each prepared vegetarian mixture was put into the frame, and then cut with the knife as bars in sequences of 10 × 0.8 × 0.6 cm prior to frying. For frying, sunflower oil was filled in a deepfrying skillet to a height of 1 cm for covering the whole OVDs then preheated to 160˚C before starting. The vegetarian bars were fried at 180˚C - 190˚C for 3 - 5 min with constant stirring. Excessive oil was removed by
Table 1 . Novel chickpea-based ovo-vegetarian diet recipes.
COVD: cauliflower ovo-vegetarian diet, POVD: pea ovo-vegetarian diet, ZOVD: zucchini ovo-vegetarian diet, TOVD: taro ovo-vegetarian diet, BOVD: bean ovo-vegetarian diet, SOVD: spinach ovo-vegetarian diet. a: all mentioned ingredients were obtained on fresh status from the different local markets in Egypt (see materials), b: green leafy vegetables mix (coriander:dill:parsley: 2:1:1), c: traditional species were obtained from spices supermarket and mixed as (25% pepper, 25% cumin, 25% relish (Boharat), 10% dry coriander, 10% dry ginger and 5% dry chili).
kitchen papers then OVDs were served immediately after frying for the panelists to evaluate its organoleptic characteristics. However, appropriate samples have been taken for chemical and phytochemicals analysis.
2.5. Analytical Methods
2.5.1. Proximate Chemical Composition and Minerals Content
Ready-to-use and ready-to-eat OVDs were subjected to chemical analysis (moisture, crude protein, crude lipids, ash, crude fibre according to methods of A.O.A.C. [24] . However, the carbohydrates content was determined by defference according to Merrill and Watt [25] . The minerals content including sodium, potassium, calcium was determined in both prepared fresh and fried vegetarian diets using flame photometry while magnesium, iron, copper, manganese and zinc contents were determined by atomic absorption spectroscopy according to A.O.A.C. [24] . A standard colorimetric method was employed for phosphorus as mentioned by Borah et al. [26] .
2.5.2. Ascorbic Acid Determination
The ascorbic acid content in various OVDs either ready-to-use and ready-to-eat OVDs was determined according to A.O.A.C. [24] applying the 2,6-dichloroin-dophenol titrimetric method. Vitamin C content is expressed as mg 100 g−1·fw. A pure ascorbic acid (Sigma) was used to prepare a standard solution as (1 mg∙ml−1).
2.5.3. Total Phenolic Content (TPC)
One g of freeze-dried ready-to-use and ready-to-eat OVDs was mixed with 25 ml of 70% methanol (v/v). The mixes were shaken vigorously in a dark bottle for 100 min at 100 rpm. After centrifugation at 3225 x g for 10 min, the supernatant was collected and the residue was re-extracted twice with 15 ml 70% methanol for total phenolic content and antioxidant activity determination. To avoid oxidation, all extracts were stored in the dark at −20˚C and analyses were performed within 48 h. The TPC of ready-to-use OVDs as well as ready-to-eat OVDs was determined according to Folin-Ciocalteu spectrophotometric method [27] . The measurements were compared to a standard curve of prepared gallic acid (GA) solution, and the total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per gram of dried sample (mg of GAE·g−1·dw).
2.5.4. Analysis of Antioxidant Activity by DPPH Assay
The radical scavenging activity using DPPH reagent (1,1-Diphenyl-2-picrylhydrazyl) for ready-to-use and ready-to-eat OVD extracts have been carried out using modified method by Lu et al. [27] . Each extract from fresh and fried diets (0.1 ml) was added to 2.9 ml of 6 × 10−5 mol methanolic solution of DPPH. The absorbance at 517 nm was measured after the solution had been allowed to stand in the dark for 60 min. The Trolox calibration curve was plotted as a function of the percentage of DPPH radical scavenging activity. The final results were expressed as micromoles of Trolox equivalents (TE) per gram (μmol·TE·g−1·dw).
2.5.5. Analysis of Phytochemicals
1) Total Carotenoids Determination
According to Yuan et al. [28] , five grams of each freeze-dried OVD was extracted with a mixture of acetone and petroleum ether (1:1, v/v) repeatedly using the mortar and pestle until a colorless residue was obtained. The upper phase was collected and combined with crude extracts after washed for several times with water. The extracts were made up to a known volume with petroleum ether. Total carotenoids content was determined by recording the absorbance at 451 nm with a spectrophotometer. Total carotenoids were estimated as mg·g−1·dw.
2) Flavonoids and Flavonols Determination
The total flavonoids content of the ready-to-use and ready-to-eat OVDs were determined according to the method of Mohdaly et al. [29] . A 0.5 ml aliquot of 2% AlCl3 ethanolic solution was added to 0.5 ml of the extracts and mixed well. After keeping for 1 h at room temperature, the absorbance at 420 nm was measured. A yellow color indicates the presence of flavonoids. The total flavonoids content were expressed as mg quercetin equivalents (QE) per 100 g dw. The total flavonols content were determined according to Kumaran and Karunakaran [12] . A 0.6 ml aliquot of 2% AlCl3 ethanolic solution was added to 0.6 ml of each extract and 0.8 ml of a 5% aqueous sodium acetate solution were added. After mixing and keeping for 2.5 h at room temperature, the absorbance at 440 nm was measured. Total flavonols content were expressed as mg quercetin equivalents (QE) per 100 g·dw.
2.5.6. Sensory Evaluation
Sensory evaluation of the ready-to-eat OVDs immediately after preparation of the six different OVDs was carried out. Twenty five panelists of the staff members and students from the Food Science Department, Faculty of Agriculture, Benha University, in the age range of 19 and 55 years were asked to evaluate the fried vegetarian bars towards appearance, color, taste, odor, texture, oiliness, and overall acceptability. A 7-point hedonic scale (7 being like extremely, 4 like accepted and 1 dislike extremely) was used to select the best recipe for a wide scale production. Results were subjected to analysis of variance and average of the mean values of the aforementioned attributes and their standard error were calculated according to Wilson et al. [30] .
2.5.7. Statistical Analysis
The statistical analysis was carried out using SPSS program with multi-function utility regarding to the experimental design under significance level of 0.05 for the whole results and multiple comparisons were carried out applying LSD with Duncana,B according to Steel et al. [31] .
3. Results
3.1. Proximate Chemical Composition of Innovative Ready-to-Use and Ready-to-Eat OVDs
Proximate chemical composition and caloric value of 6 ready-to-use and ready-to-eat OVDs prepared from different vegetables are presented in Table2 Significant difference (p > 0.05) was found between both ready-touse and ready-to-eat OVDs in all chemical composition parameters and caloric value. The moisture content of ready-to-use OVDs was in range of 67.73% in POVD to a high of 73.17% in COVD, whereas a low of 39.65% in BOVD to a high of 43.48% in COVD for ready-to-use OVDs was recorded. Significant difference (p < 0.05) was found between (POVD and TOVD) and (COVD, ZOVD and BOVD and SOVD), which was not similarly found after cooking. As shown, about 41.5% reduction in the moisture content of deep-fried vegetarian diets was recorded when calculated on means average of fresh and fried diets. The crude protein content of the six OVDs varied from 23.20% in SOVD to 37.12% in BOVD and from 17.93% in SOVD to 23.45% in POVD for readyto-use and ready-to-eat OVDs, respectively. The protein content in fresh POVD and BOVD was significantly higher than the other formulated OVDs, Table2 In the same context, lipid content was varied from 1.86% in COVD to 2.63% in ZOVD and from 17.60% in BOVD to 21.28% in SOVD for ready-to-use and ready-to-eat OVDs, respectively. Frying increased the lipid content in all fried diets by 8.1-times when compared to fresh diets, especially in COVD and SOVD. Data presented in Table 2 showed that ash content ranged from 7.63% in COVD to 9.53% in SOVD for fresh diets while it ranged from 6.21% in BOVD to 7.68% in SOVD for fried diets. However, no significant difference (p > 0.05) has been found between formulated diets after frying in both ash and crude fiber contents. The carbohydrates content varied from 39.24% in BOVD to 55.64% in COVD for fresh diets while ranging from 39.46% in SOVD to 45.23% for COVD for fried diets, Table2 The caloric value ranged from 88.6 kcal 100 g−1 in COVD to 99 kcal 108.6 g−1 in POVD and 228.1 kcal 100 g−1 in ZOVD and 257.7 kcal 100 g−1 in POVD calculated on fw in fresh and fried diets, respectively.
3.2. Minerals Content of Innovative Ready-to-Use and Ready-to-Eat OVDs
The minerals content (sodium, potassium, calcium, phosphorus, magnesium, iron, copper, manganese and zinc) in ppm of ready-to-use and ready-to-eat OVDs are given in Table3 Generally, minerals content was changed after frying with different reduction rates. Significant difference (p > 0.05) was found between both ready-to-use and ready-to-eat OVDs in all measured minerals. Formulated vegetarian diet with taro (TOVD) showed higher sodium content while the lowest content was recorded in formulated vegetarian diet with green bean (BOVD). No significant difference (p > 0.05) was found between the most of ready-to-use OVDs, however the sodium content of BOVD and SOVD diets was significantly low. After frying, the sodium content was ranged from 505 ppm in BOVD to 611 ppm in TOVD. Potassium content in formulated vegetables with different vegetables was ranged from a low of 434 ppm in SOVD to a high of 760 ppm in TOVD. In ready-to-eat diets, potassium content was changed with different reduction levels to a low of 370 ppm in SOVD to a high of 657 ppm in TOVD. The
Table 2 . Chemical composition of different innovative ready-to-use and ready-to-eat chickpea-based OVDs (mean ± SE).
¥: see materials and methods, Table 1, dw: values were calculated on dry weight basis, fw: values were calculated on fresh weight basis, a,b,c,...: Means with the same letter in the same raw into each parameter are not significantly different (p > 0.05), A,B,C,...: Means with the same letter in the same column are not significantly different (p > 0.05).
Table 3.Minerals content of different innovative ready-to-use and ready-to-eat chickpea-based OVDs(mean±SE)
TOVD showed the highest calcium content in both ready-to-use and ready-to-eat OVDs, while the lowest calcium content was recorded in both fresh and fried POVDs. Phosphorus was also determined in both ready-to-use and ready-to-eat OVDs and results were tabulated in Table3 Formulated chickpeas with green zucchini seem to be having higher phosphorus content than other formulated vegetables with same protein sources while the lowest phosphorus content had been recorded in both fresh and fried BOVDs. Magnesium content of 6 OVDs was assayed before and after frying, Table3 As previously shown, similar results of phosphorus content was found with magnesium content in fresh and fried diets. Iron content in different formulated vegetarian diets is given in the same table, which was ranged from 2.06 ppm in POVD and BOVD to 2.53 ppm in COVD while, it was ranged from 1.81 ppm in SOVD to 2.13 ppm in COVD in fried OVDs. Formulated chickpea with spinach (SOVD) exhibit higher copper content while formulated chickpea with taro (TOVD) exhibit lower content in both fresh and fried diets, Table3 The highest manganese and zinc contents were observed in COVD while the lowest manganese and zinc contents were observed in BOVD and SOVD, respectively, Table3
3.3. Ascorbic acid, Total Phenolic Compounds (TPC) and Antioxidant Activity of Innovative Ready-to-Use and Ready-to-Eat OVDs
Data in Table 4 shows the content of vitamin C [mg·100·g−1·fw], TPC [mg·g−1·dw] and antioxidant activity [μmol·TE·g−1·dw] of various innovative ready-to-use and ready-to-eat OVDs. All fresh diets demonstrated appropriate content of vitamin C which basically depends on the initial ingredients. However, the average levels of vitamin C were dramatically decreased in ready-to-eat OVDs which were influenced by frying cooking. Moreover, TPC and antioxidant activity of ready-to-use and ready-to-eat OVDs are presented in Table4 TPC of fresh prepared OVDs was ranged from a low of 73.01 mg·GAE·g−1 for POVD to a high of 81.26 mg·GAE·g−1 for COVD, whereas a low of 63.05 mg·GAE·g−1 for BOVD to a high of 79.75 mg·GAE·g−1 for COVD for fried diets were noticed. The evolution of DPPH radical scavenging activity of various OVDs was assayed using the common DPPH assay before and after frying and results are referred to Trolox equivalents g−1 [μmol·TE·g−1] and given in Table4 The antioxidant activity ranged from low of 125.5 μmol·TE·g−1 in POVD to high of 171.1 μmol·TE·g−1 in COVD for ready-to-use OVDs. The antioxidant activity decreased after frying to be in range of 102.8 μmol·TE·g−1 in TOVD to 149.1 μmol·TE·g−1 in COVD for ready-to-eat OVDs. The content of TPC and relative antioxidant activity in COVD was significantly higher than other formulated OVDs in both ready-to-use and ready-to-eat OVDs.
3.4. Phytochemicals Profile of Innovative Ready-to-Use and Ready-to-Eat OVDs
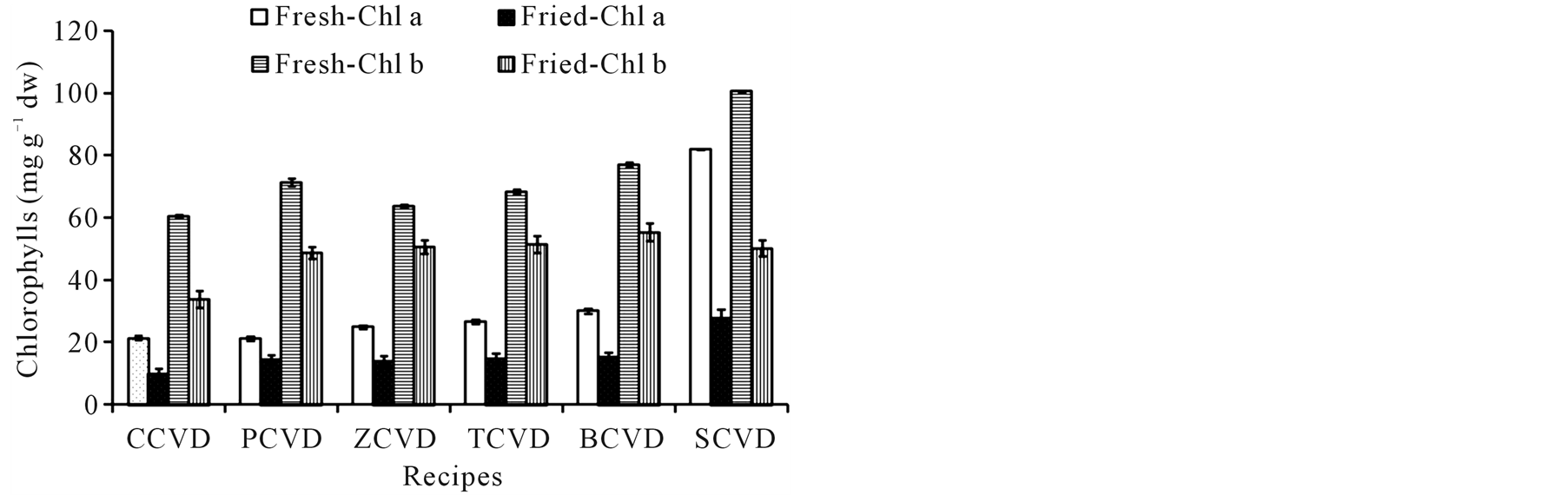
The phytochemicals such as chlorophylls, carotenoids, flavonoids, and flavonols of ready-to-use and ready-toeat OVDs have been investigated and data are given in Figure 1(a), Figure 1(b), Figure 1(c) and Figure 1(d). The frying cooking treatments caused a significant loss of chlorophyll a and b. The content of chlorophyll a in ready-to-eat OVDs was significantly reduced by 54%, 31%, 43%, 44%, 49% and 66% for COVD, POVD
Table 4. Ascorbic acid, total phenolic compounds and antioxidant activity of different innovative ready-to-use and readyto-eat chickpea-based OVDs (mean ± SE).
¥: see materials and methods, Table1 fw: values were calculated on fresh weight basis, dw: values were calculated on dry weight basis, a,b,c,...: Means with the same letter in the same raw are not significantly different (p > 0.05), A,B,C,...: Means with the same letter in the same column are not significantly different (p > 0.05).
 (a)
(a)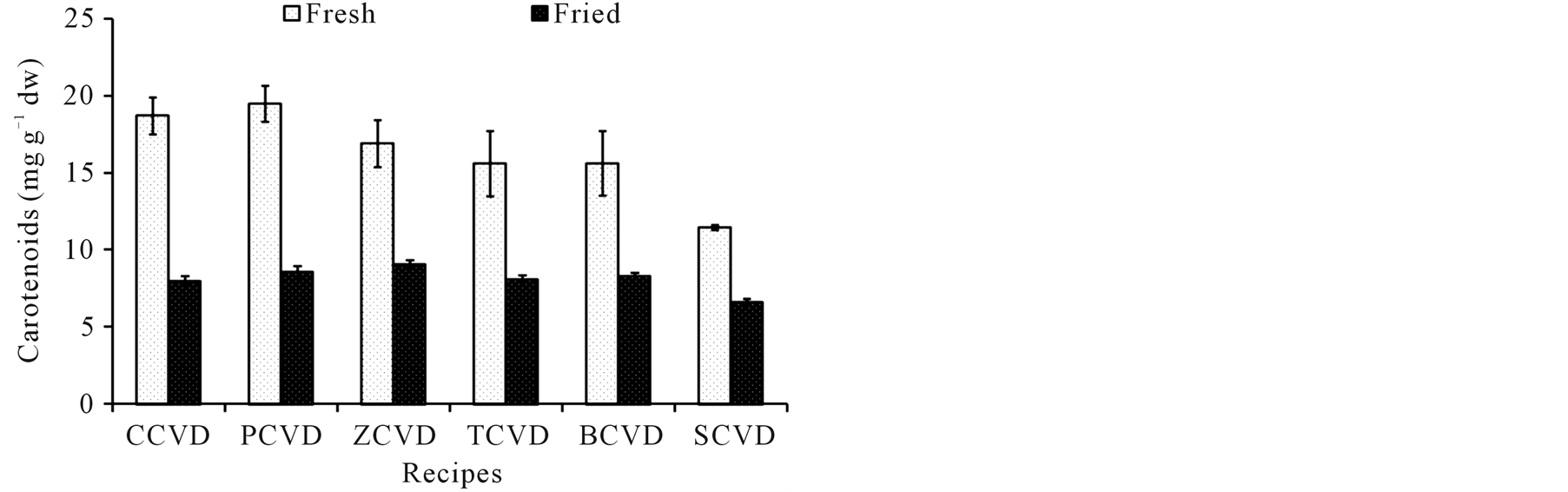 (b)
(b) (c)
(c)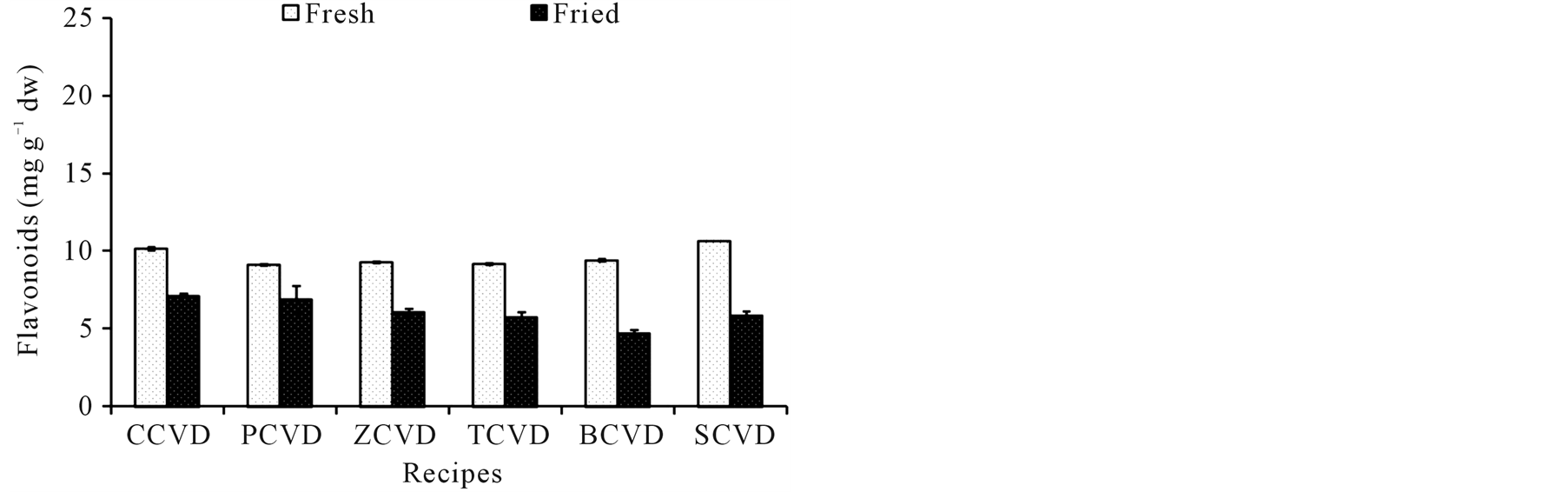 (d)
(d)
Figure 1. Effect of frying on phytochemicals content of innovative ovo-vegetarian diets, COVD: cauliflower ovo-vegetarian diet, POVD: pea ovo-vegetarian diet, ZOVD: zucchini ovo-vegetarian diet, TOVD: taro ovo-vegetarian diet, BOVD: bean ovo-vegetarian diet, SOVD: spinach ovo-vegetarian diet. Results expressed as mg∙g−1 calculated on dry matter.
ZOVD, TOVD, BOVD, and SOVD, respectively (Figure 1(a)). Moreover, the content of chlorophyll b was also significantly reduced by 44%, 32%, 20%, 25%, 28% and 50% for COVD, POVD, ZOVD, TOVD, BOVD, and SOVD, respectively. The frying cooking of innovate OVDs caused a loss of total carotenoids which ranged from 42% in SOVD to 57% in COVD, Figure 1(b). A significant loss of flavonoids content was observed in readyto-eat OVDs when compared to ready-to-use ones. The total flavonoids loss was ranged from 16% to 40% in COVD and SOVD, respectively Figure 1(c). The results for total flavonols illustrated in Figure 1(d) shows that frying had also pernicious effect on total flavonols content, where 25% - 50% loss was observed in POVD and BOVD, respectively.
3.5. Organoleptic Properties of Innovative Ready-to-Eat Chickpea-Based OVDs
Sensory evaluation of food products is an important criterion by which its consumer acceptability can be assessed. The sensory evaluation of ready-to-eat chickpea-based OVDs based on a seven-point hedonic scale showed that all fried diets recorded mean scores higher than 4 for all tested parameters except in color and overall acceptability of SOVD, Table5 The appearance of ready-to-eat OVDs showed higher mean scores for COVD, TOVD and ZOVD significantly. The most preferable color for the panelists was recorded for ZOVD and TOVD diets while lowest score was recorded for SOVD. Results for taste, as the most important organoleptic property showed that ZOVD and TOVD were preferred significantly. Odor attracts the consumer and is able to increase his appetite. The highest score was recorded for COVD, ZOVD and TOVD. The cooking method affected the texture of innovative diets, where the texture of POVD and TOVD was significantly preferred while the lowest texture score was recorded for BOVD. Oiliness reflects the oil retaining after cooking and panelists were asked to give higher score for lower oil content after pressing the vegetarian bars between their fingers. The lowest retaining oil level had been recorded for TOVD while the highest retaining oil level bad been recorded for BOVD, significantly. The statistical analysis had been classified the diets in three groups significantly
Table 5 . Organoleptic properties of different innovative ready-to-eat chickpea-based OVDs (mean ± SE).
¥: see materials and methods, Table 1, a,b,c,...: Means with the same letter in the same column are not significantly different (p > 0.05).
according to the overall acceptability, Table5 Moreover, the overall acceptability scores indicated that the different diets could be arranged as TOVD ˃ ZOVD ˃ POVD ˃ COVD ˃ BOVD ˃ SOVD.
4. Discussion
Vegetarian diets are associated with reduced risk of many diseases in health-conscious individuals. The major problem with recommending vegetarian diets for improving health is that a vegetarian diet is inadequately defined in terms of nutrient and food contents. Following the vegetarianism is not only eating plant origins but also formulating a balanced vegetarian diet. Findings from cohort studies to date indicate that increased meat consumption, especially processed meat, is positively and strongly associated with incident cardiovascular, type 2 diabetes, perhaps colon cancer and all-cause mortality which are independent of other lifestyle factors [10] . Additionally, it is not known whether a particular food, dietary compound or a combination of dietary or lifestyle/behavioural factors in the vegetarian diet provides optimal protection against chronic disease development. It is recognised that over-reliance on one single food, or food group, will not provide the range of nutrients required for optimum health and well-being.
Therefore, present study aimed to formulate different vegetables with chickpea and other ingredients to serve a balanced diet for consumers as ready-to-use and ready-to-eat OVDs. Practically, obtained results of proximate chemical composition concluded that prepared diets are considered as valuable source of crude protein, lipid, fiber and carbohydrates both ready-to-use and ready-to-use OVDs which may have appropriate health benefits [1] [4] [14] [32] -[34] . Formulated diets with legume vegetables such as green pea and bean increased the crude protein content as a result of its initial protein content [6] [17] . However, applying the deep frying cooking methods could increase the lipid content which influenced the nutritional composition. Thus, deep-frying can be changed to microwaving, steaming or baking which could lead to drastic reduction in lipid content in the diets (further study). Also, ready-to-eat OVDs exhibited valuable lipid content which increased the caloric value of the prepared diets. According to Dietary Reference Intakes [35] , the Recommended Dietary Allowances (RDA) of protein is ranged from 34 - 56 g∙d−1 for age ranging from 9 - 70 years of both genders, which increased to 71 g∙d−1 for females in pregnancy and lactation. The formulated OVDs, 100 g·dw could provide at least 40% of the RDA for adults and at least 29% of the RDA for pregnant and lactating women daily. In context, Adequate Intake (AI) of dietary fiber could be compensated by at least 37% when consuming 100 g·dw of CVD daily. Moreover, RDA of carbohydrates is 130 g·d−1 for age ranging from 9 - 70 years of both genders, which increased to 210 g·d−1 for females in pregnancy and lactation. Consuming about 100 g·dw CVD could provide at least 30% of the RDA for adults and at least 18% of the RDA for pregnant and lactating women (about 90% absorbance efficiency). Accordingly as shown, 100 g·fw of ready-to-eat OVDs could provide about 228 - 257 kcal which is cover adult person (70 kg) requirements for about 2.5 - 3.5 h [35] .
Formulated diets with chickpea and different vegetables demonstrated appropriate minerals content, Table3 This result may be basically depends on depression or increase of these minerals content in vegetable or protein sources as main ingredients in the diet. However, the minerals content had minus changes after frying in the different vegetarian diets. This may be due to the influence of frying method which increasing the absorbed oil and consequently increasing the lipids content. These results were in agreement with [5] [26] [36] -[38] . Furthermore, studies have been concluded that chickpea contain various minerals and could be efficiently successful to slows glycemic response and to combat global micronutrient malnutrition [35] . For human requirments, the presented minerals content in 100 g·dw of prepared CVDs could provide 46%, 12%, 6%, 14%, 19%, 7%, 48%, 50% and 13% from the daily AI and RDA of Na, K, Ca, P, Mg, Fe, Cu, Mn and Zn. As mentioned in Dietary Reference Intakes [35] , the AIs for sodium, potasium, calcium, and manganese are 1000 - 1500, 3000 - 5100, 500 - 1300, and 1.2 - 1.3 mg∙d−1, whereas the RDAs for phosphorus, magnesium, iron, copper, and zince are 460 - 1250, 80 - 420, 8 - 27, 0.34 - 1.3, and 3 - 13 mg∙d−1. The presented CVDs seem to be low in some minerals content and supplementing experiment is needed further.
Recently, research has confirmed a strong relationship between the amount of available biologically active compounds in vegetables and their antioxidant properties [39] -[47] . A drastic reduction of vitamin C was remarked after oil frying of all diets as influenced by cooking temperature to be in agreement with [48] . In the present study, the effect of frying cooking on TPC of OVDs and their antioxidant properties have been determined (Table 4). Appropriate data of TPC for many vegetables are available but a few are available on similar vegetarian diets [49] [50] which more or less confirmed the results of the present study. The antioxidant activity of vegetarian diets correlates with the TPC. However, it is known that phenolic compounds are not stable under thermal conditions and may be transformed into other compounds. Obtained results illustrated that scavenging activity was decreased upon the effect of frying process [39] [50] .
Phytochemicals are found virtually in plant-based foods and promoted to prevent and treat many related health diseases. In present study, chlorophylls, carotenoids, flavonoids, and flavonols contents of ready-to-use ovo-vegetarian could provide rich phytochemicals content, Figure 1. The applied cooking method was drastically affecting the phytochemicals content in fried diets. The chlorophyll’s content is responsible for the degree of greenness and is important for the determination of a vegetable’s quality [51] . Further, it was reported that chlorophyll and its derivatives exert beneficial effects such as anticarcinogenic and antimutagenic activities [52] . However, green vegetables exhibit poor color quality and the chlorophyll’s content decreases after being thermally processed [53] . In the present study, frying led to a significant loss of chlorophylls. This result is in agreement with a study described by Yuan et al. [28] . Chlorophyll b exhibits more heat resistance compared with chlorophyll a. The chlorophyll a and b were retained by 34% - 69% and 50% - 80% in ready-to-eat OVDs, respectively. In contrast, Turkmen et al. [52] observed that chlorophyll a is more heat resistant compared with chlorophyll b in five of six vegetables, except in peas.
Carotenoids have been extensively studied for their potential protection against numerous cancer diseases. In recent years, several reports on the retention of total carotenoids in cooked vegetables are available [28] [47] [54] . In all diets, formulation of chickpea with different vegetables sources exhibit the highest carotenoids content, a result of increasing the carotenoids content in chickpeas grains [55] . It is presented herein that, total carotenoids, flavonoids and flavonols were retained by 43% - 57%, 60% - 84%, and 55% - 75%, respectively. The retained content may depends on initial carotenoids, flavonoids and flavonols content, vegetable structure or diet matrix, and leaching of the carotenoids into the oil followed by thermal degradation during frying cooking, being similar to reports by [28] [47] [49] [56] .
In our previous study, the given sensorial data by most panelists confirmed that chickpea could be the best protein source for preparing OVDs when compared statistically with soy and faba bean [49] . Thus, the chickpea was used as main protein source in formulated ovo-vegetarian diet in present study. Regarding to the organoleptic properties, the panelists provided high scores for all prepared diets especially for TOVD and ZOVD. This may be due to the reflected organoleptic characteristics of those diets which might be the most preferred. In contrary, low score has been obtained in SOVD which might be due to the effect of thermal processing on green color of spinach and its disproportionate structure [57] . Reducing the water content with a corresponding denaturation of proteins and browning reactions are reasons for a good texture [30] [58] . Therefore, formulation of chickpea and different vegetables in combination with rich bioactive ingredients is hereby recommended as edible ovo-vegetarian diet, particularly during off-seasons when other conventional vegetables are scarce, expensive or not available.
5. Conclusion
With growing of urbanization, changes in consumer awareness for food habits occur. The current study concluded the potential applicability of different innovative chickpea-based OVDs which incorporated with different vegetables. The obtained results could provide sufficient information about macroand micronutrients, minerals, phytochemicals content and their antioxidant activity as well as sensory attractiveness of prepared OVDs. Highly consumer acceptability could be an encouraging motive for large scale applications. Therefore, new Egyptian standards for regulate ready-to-use and ready-to-eat OVDs could be required. However, studies about formulating different functional diets as well as shelf-life stability should be investigated further. In conclusion, all dietary practices should aim to meet current nutritional guidelines to reduce risk of chronic disease development. Accordingly, moderation and variety in individual diets is recommended. The current study could also provide valuable impact of thermal treatment for optimizing the cooking conditions as well as designing new functional foods.
Acknowledgements
The authors acknowledge the staff members and postgraduate students from Food Science Department, Faculty of Agriculture, Benha University, Egypt for evaluating the sensory aspects of the innovative ovo-vegetarian diets.
References
- Fraser, G.E. (2009) Vegetarian Diets: What Do We Know of Their Effects on Common Chronic Diseases? The American Journal of Clinical Nutrition, 89, 1607S-1612S. http://dx.doi.org/10.3945/ajcn.2009.26736K
- Craig, W.J. and Mangels, A.R. (2009) Position of the American Dietetic Association: Vegetarian Diets. Journal of the American Dietetic Association, 109, 1266-1282.
- Messina, M.J. (1999) Legumes and Soybeans: Overview of Their Nutritional Profiles and Health Effects. The American Journal of Clinical Nutrition, 70, 439S-450S.
- McEvoy, C.T., Temple-Woodside, J.V. and Woodside, J.V. (2012) Vegetarian Diets, Low-Meat Diets and Health: A Review. Public Health Nutrition, 15, 2287-2294. http://dx.doi.org/10.1017/S1368980012000936
- Hunt, J.R. (2003) Bioavailability of Iron, Zinc, and Other Trace Minerals from Vegetarian Diets. The American Journal of Clinical Nutrition, 78, 633S-639S.
- Jongen, W.M. and Meerdink, G. (2001) Pea Proteins Based Food Products as Meat Replacers: The Profetas Concept. Nahrung, 45, 402-404. http://dx.doi.org/10.1002/1521-3803(20011001)45:6<402::AID-FOOD402>3.0.CO;2-N
- Jakszyn, P., Gonzalez, C.A., Lujan-Barroso, L., Ros, M.M., Bueno-de-Mesquita, H.B., et al. (2011) Red Meat, Dietary Nitrosamines, and Heme Iron and Risk of Bladder Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiology, Biomarkers & Prevention, 20, 555-559. http://dx.doi.org/10.1158/1055-9965.EPI-10-0971
- Sacks, F.M. and Kass, E.H. (1988) Low Blood Pressure in Vegetarians: Effects of Specific Foods and Nutrients. The American Journal of Clinical Nutrition, 48, 795-800.
- Micha, R., Wallace, D. and Mozaffarian, D. (2010) Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Circulation, 121, 2271-2283. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.924977
- Pan, A., Sun, Q., Bernstein, A.M., Schulze, M.B., Manson, J.E., Stampfer, M.J., Willett, W.C. and Hu, F.B. (2012) Red Meat Consumption and Mortality. Archives of Internal Medicine, online March 12, 2012.
- Al-Duais, M., Hohbein, J., Werner, S., Böhm, V. and Jetschke, G. (2009) Contents of Vitamin C, Carotenoids, Tocopherols, and Tocotrienols in the Subtropical Plant Species Cyphostemma digitatum as Affected by Processing. Journal of Agricultural and Food Chemistry, 57, 5420-5427. http://dx.doi.org/10.1021/jf9003626
- Kumaran, A. and Karunakaran, R.J. (2007) In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT, Food Science and Technology, 40, 344-352.
- Gorinstein, S., Park, Y., Heo, B., Namiesnik, J., Leontowicz, H., Leontowicz, M., Ham, K., Cho, J. and Kang, S. (2009) A Comparative Study of Phenolic Compounds and Antioxidant and Antiproliferative Activities in Frequently Consumed Raw Vegetables. European Food Research and Technology, 228, 903-911. http://dx.doi.org/10.1007/s00217-008-1003-y
- Krumbein, A., Schonhof, I. and Brückner, B. (2006) Flavour and Health-Promoting Compounds in Broccoli and Cauliflower—An Inconsistency? Developments in Food Science, 43, 249-252.
- Swiatecka, D., Swiatecki, A., Kostyra, H., Marciniak-Darmochwal, K. and Kostyra, E. (2010) The Impact of Pea Protein Hydrolysates on Bacterial Physiological Activity—An in Vitro Study. International Journal of Food Microbiology, 140, 263-270. http://dx.doi.org/10.1016/j.ijfoodmicro.2010.03.015
- Nilsson, J., Stegmark, R. and Åkesson, B. (2004) Total Antioxidant Capacity in Different Pea (Pisum sativum) Varieties after Blanching and Freezing. Food Chemistry, 86, 501-507. http://dx.doi.org/10.1016/j.foodchem.2003.09.002
- Mitchell, D.C., Lawrence, F.R., Hartman, T.J. and Curran, J.M. (2009) Consumption of Dry Beans, Peas, and Lentils Could Improve Diet Quality in the US Population. Journal of the American Dietetic Association, 109, 909-913.http://dx.doi.org/10.1016/j.jada.2009.02.029
- Limón, R.I., Peñas, E., Martínez-Villaluenga, C. and Frias, J. (2014) Role of Elicitation on the Health-Promoting Properties of Kidney Bean Sprouts. LWT, Food Science and Technology, 56, 328-334.
- Doria, E., Campion, B., Sparvoli, F., Tava, A. and Nielsen, E. (2012) Anti-Nutrient Components and Metabolites with Health Implications in Seeds of 10 Common Bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) Landraces Cultivated in Southern Italy. Journal of Food Composition and Analysis, 26, 72-80. http://dx.doi.org/10.1016/j.jfca.2012.03.005
- Tang, G. (2010) Chapter 25, Spinach and Carrots: Vitamin A and Health. In: Watson, R.R. and Preedy, V.R., Eds., Bioactive Foods in Promoting Health, Academic Press, San Diego, 381-392.http://dx.doi.org/10.1016/B978-0-12-374628-3.00025-6
- Jacobo-Valenzuela, N., Maróstica-Junior, M., Zazueta-Morales, J. and Gallegos-Infante, J. (2011) Physicochemical, Technological Properties, and Health-Benefits of Cucurbita moschata Duchense vs. Cehualca: A Review. Food Research International, 44, 2587-2593. http://dx.doi.org/10.1016/j.foodres.2011.04.039
- Mangels, A.R. and Messina, V. (2001) Considerations in Planning Vegan Diets: Infants. Journal of the American Dietetic Association, 101, 670-677. http://dx.doi.org/10.1016/S0002-8223(01)00169-9
- Messina, V. and Mangels, A.R. (2001) Considerations in Planning Vegan Diets: Children. Journal of the American Dietetic Association, 101, 661-669. http://dx.doi.org/10.1016/S0002-8223(01)00167-5
- AOAC (2000) Official Methods of Analysis of the AOAC. 17th Edition, Association of Official Analytical Chemists.
- Merrill, A.L. and Watt, B.K. (1973) Energy Value of Foods: Basis and Derivation. Agriculture Handbook No. 74. Washington DC, ARS United States Department of Agriculture.
- Borah, S., Baruah, A., Das, A. and Borah, J. (2009) Determination of Mineral Content in Commonly Consumed Leafy Vegetables. Food Analytical Methods, 2, 226-230. http://dx.doi.org/10.1007/s12161-008-9062-z
- Lu, J., Zhao, H., Chen, J., Fan, W., Dong, J., Kong, W., Sun, J., Cao, Y. and Cai, G. (2007) Evolution of Phenolic Compounds and Antioxidant Activity during Malting. Journal of Agricultural and Food Chemistry, 55, 10994-11001.http://dx.doi.org/10.1021/jf0722710
- Yuan, G.F., Sun, J., Yuan, Q. and Wang, Q.M. (2009) Effects of Different Cooking Methods on Health-Promoting Compounds of Broccoli. Journal of Zhejiang University-SCIENCE B, 10, 580-588. http://dx.doi.org/10.1631/jzus.B0920051
- Mohdaly, A.A.A., Hassanien, M.F.R., Mahmoud, A., Sarhan, M.A. and Smetanska, I. (2012) Phenolics Extracted from Potato, Sugar Beet, and Sesame Processing By-Products. International Journal of Food Properties, 16, 1148-1168.http://dx.doi.org/10.1080/10942912.2011.578318
- Wilson, C.D., Pace, E., Bromfield, G., Jones, J.Y. and Lu, J.Y. (1998) Consumer Acceptance of Vegetarian Sweet Potato Products Intended for Space Missions. Life Support & Biosphere Science, 5, 339-345.
- Steel, R., Torrie, J. and Dickey, D. (1997) Principles and Procedures of Statistics: A Biometrical Approach. 3rd Edition, McGraw-Hill, New York.
- Messina, M.J. (1991) Legumes and Soybeans: Overview of Their Nutritional Profiles and Health Effects. The American Journal of Clinical Nutrition, 70, 439S-450S.
- Chiplonkar, S.A., Tarwadi, K.V., Kavedia, R.B., Mengale, S.S., Paknikar, K.M. and Agte, V.V. (1999) Fortification of Vegetarian Diets for Increasing Bioavailable Iron Density Using Green Leafy Vegetables. Food Research International, 32, 169-174. http://dx.doi.org/10.1016/0308-8146(93)90031-A
- Turner-McGrievy, G. (2010) Nutrient Adequacy of Vegetarian Diets. Journal of the American Dietetic Association, 110, 1450. http://dx.doi.org/10.1016/0308-8146(93)90031-A
- DRI (2002) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Food and Nutrition Board, Institute of Medicine, The National Academic Press, Washington DC.
- Gibson, R.S. (1994) Content and Bioavailability of Trace Elements in Vegetarian Diets. The American Journal of Clinical Nutrition, 95, 1223S-1232S.
- Srikumar, T.S. (1993) The Mineral and Trace Element Composition of Vegetables, Pulses and Cereals of Southern India. Food Chemistry, 46, 163-167. http://dx.doi.org/10.1016/0308-8146(93)90031-A
- Lightowler, H.J. and Davies, G.J. (2000) Micronutrient Intakes in a Group of UK Vegans and the Contribution of Self-Selected Dietary Supplements. Journal of the Royal Society for the Promotion of Health, 120, 117-124. http://dx.doi.org/10.1177/146642400012000210
- Gertz, C., Klostermann, S. and Kochhar, S.P. (2000) Testing and Comparing Oxidative Stability of Vegetable Oils and Fats at Frying Temperature. European Journal of Lipid Science and Technology, 102, 543-551.http://dx.doi.org/10.1002/1438-9312(200009)102:8/9<543::AID-EJLT543>3.0.CO;2-V
- Deng, G., Lin, X., Xu, X., Gao, L., Xie, J. and Li, H. (2013) Antioxidant Capacities and Total Phenolic Contents of 56 Vegetables. Journal of Functional Foods, 5, 260-266. http://dx.doi.org/10.1016/j.jff.2012.10.015
- Houghton, C.A., Fassett, R.G. and Coombes, J.S. (2013) Sulforaphane: Translational Research from Laboratory Bench to Clinic. Nutrition Reviews, 71, 709-726. http://dx.doi.org/10.1111/nure.12060
- Ismail, A., Marjan, Z.M. and Foong, C.W. (2004) Total Antioxidant Activity and Phenolic Content in Selected Vegetables. Food Chemistry, 87, 581-586. http://dx.doi.org/10.1016/j.foodchem.2004.01.010
- Lamy, E., Scholtes, C., Herz, V. and Mersch-Sundermann, V. (2011) Pharmacokinetics and Pharmacodynamics of Isothiocyanates. Drug Metabolism Reviews, 43, 387-407. http://dx.doi.org/10.3109/03602532.2011.569551
- Sikora, E., Cieslik, E., Leszczyńska, T., Filipiak-Florkiewicz, A. and Pisulewski, P.M. (2008) The Antioxidant Activity of Selected Cruciferous Vegetables Subjected to Aquathermal Processing. Food Chemistry, 107, 55-59.http://dx.doi.org/10.1016/j.foodchem.2007.07.023
- Verkerk, R., Schreiner, M., Krumbein, A., Ciska, E., Holst, B., Rowland, I., De Schrijver, R., Hansen, M., Gerhäuser, C., Mithen, R. and Dekker, M. (2009) Glucosinolates in Brassica Vegetables: The Influence of the Food Supply Chain on Intake, Bioavailability and Human Health. Molecular Nutrition & Food Research, 53, S219.http://dx.doi.org/10.1002/mnfr.200800065
- Volden, J., Borge, G.I.A., Bengtsson, G.B., Hansen, M., Thygesen, I.E. and Wicklund, T. (2008) Effect of Thermal Treatment on Glucosinolates and Antioxidant-Related Parameters in Red Cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chemistry, 109, 595-605. http://dx.doi.org/10.1016/j.foodchem.2008.01.010
- Zhang, D. and Hamauzu, Y. (2004) Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and Their Changes during Conventional and Microwave Cooking. Food Chemistry, 88, 503-509.http://dx.doi.org/10.1016/j.foodchem.2004.01.065
- Francisco, M., Velasco, P., Moreno, D.A., García-Viguera, C. and Cartea, M.E. (2010) Cooking Methods of Brassica rapa Affect the Preservation of Glucosinolates, Phenolics and Vitamin C. Food Research International, 43, 1455-1463. http://dx.doi.org/10.1016/j.foodchem.2004.12.038
- Barakat, H. (2013) Characterization and Evaluation of Kohlrabi (Brassica oleracea L. gongylodes) and Kohlrabibased Ovo-Vegetarian Diets as New Food Recipes. Egyptian Journal of Food Science, 41, 35-61.
- Turkmen, N., Sari, F. and Velioglu, Y.S. (2005) The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chemistry, 93, 713-718. http://dx.doi.org/10.1016/j.foodchem.2004.12.038
- Nisha, P., Singhal, R.S. and Pandit, A.B. (2004) A Study on the Degradation Kinetics of Visual Green Colour in Spinach (Spinacea oleracea L.) and the Effect of Salt Therein. Journal of Food Engineering, 64, 135-142.http://dx.doi.org/10.1016/j.jfoodeng.2003.09.021
- Turkmen, N., Poyrazoglu, E.S., Sari, F. and Velioglu, Y.S. (2006) Effects of Cooking Methods on Chlorophylls, Pheophytins and Colour of Selected Green Vegetables. International Journal of Food Science & Technology, 41, 281-288. http://dx.doi.org/10.1111/j.1365-2621.2006.01420.x
- Adebooye, O.C., Vijayalakshmi, R. and Singh, V. (2008) Peroxidase Activity, Chlorophylls and Antioxidant Profile of Two Leaf Vegetables (Solanum nigrum L. and Amaranthus cruentus L.) under Six Pretreatment Methods before Cooking. International Journal of Food Science & Technology, 43, 173-178. http://dx.doi.org/10.1111/j.1365-2621.2006.01420.x
- Gliszczyńska-Swiglo, A., Ciska, E., Pawlak-Lemańska, K., Chmielewski, J., Borkowski, T. and Tyrakowska, B. (2006) Changes in the Content of Health-Promoting Compounds and Antioxidant Activity of Broccoli after Domestic Processing. Food Additives and Contamination, 23, 1088-1098. http://dx.doi.org/10.1080/02652030600887594
- Segev, A. (2011) Total Phenolic Content and Antioxidant Activity of Chickpea (Cicer arietinum L.) as Affected by Soaking and Cooking Conditions. Food and Nutrition Sciences, 2, 724-730. http://dx.doi.org/10.4236/fns.2011.27099
- Buchner, N., Krumbein, A., Rohn, S. and Kroh, L.W. (2006) Effect of Thermal Processing on the Flavonols Rutin and Quercetin. Rapid Communications in Mass Spectrometry, 20, 3229-3235. http://dx.doi.org/10.1002/rcm.2720
- Delchier, N., Reich, M. and Renard, C.M.G.C. (2012) Impact of Cooking Methods on Folates, Ascorbic Acid and Lutein in Green Beans (Phaseolus vulgaris) and Spinach (Spinacea oleracea). LWT, Food Science and Technology, 49, 197-201.
- Heenan, C.N., Adams, M.C., Hosken, R.W. and Fleet, G.H. (2004) Survival and Sensory Acceptability of Probiotic Microorganisms in a Nonfermented Frozen Vegetarian Dessert. LWT, Food Science and Technology, 37, 461-466.


