Food and Nutrition Sciences
Vol.4 No.6A(2013), Article ID:33015,5 pages DOI:10.4236/fns.2013.46A005
“HoneySweet” Plum—A Valuable Genetically Engineered Fruit-Tree Cultivar*
![]()
1UMR 1332-BFP, Biologie du Fruit et Pathologie, INRA-Bordeaux, Villenave d’Ornon Cedex, France; 2USDA-ARS, Appalachian Fruit Research Station, Kearneysville, USA; 3Department of Virology, Division of Plant Health, Crop Research Institute, Prague, Czech Republic; 4Mendel University Brno, Horticultural Faculty Lednice, Lednice, Czech Republic.
Email: ravelona@bordeaux.inra.fr
Copyright © 2013 Michel Ravelonandro et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 21st, 2013; revised March 24th, 2013; accepted April 8th, 2013
Keywords: Genetic Engineering; Prunus Domestica; Sharka; Fruit Quality
ABSTRACT
“HoneySweet” is a plum variety developed through genetic engineering to be highly resistant to plum pox potyvirus (PPV) the causal agent of sharka disease that threatens stone-fruit industries world-wide, and most specifically in Europe. Field testing for over 15 years in Europe has demonstrated the stable and durable PPV resistance of “HoneySweet”. Resistance is based on gene silencing whereby the inserted gene induces a natural plant defense mechanism against viruses. This resistance has been transferred to seedlings through cross-hybridization as a single locus dominant trait making it useful as a parent for developing new plum varieties for specific growing areas and markets. “HoneySweet” plums are of high quality and compare well to the quality and nutritional value of conventional plums. “HoneySweet” demonstrates the utilization of genetic engineering to provide safe and effective solutions to important agricultural challenges facing growers, and ultimately consumers.
1. Introduction
Prunus domestica L. is one of the tree fruits threatened by Plum pox potyvirus (PPV) a quarantine disease that causes fruit loss to plums and other stone fruits (Prunus) [1]. Symptoms of the disease include reduced fruit quality, fruit deformation, premature fruit drop, and leaf chlorosis. Sharka [2] has been a concern to European fruit production for more than one century and the total estimated losses exceed 10,000 million Euros [1]. Since its identification it has spread from eastern to western Europe and more recently to North and South America [3] and Asia [4]. Control of sharka through methods such as the control of the aphid vector and through quarantine have been only marginally effective. While the use of resistant cultivars represents the most effective solution to control sharka, a century of breeding has produced few highly resistant cultivars and the genetic basis of resistance remains elusive. As an enhancement to classical breeding, genetic engineering was used to produce transgenic clones that contain the PPV coat protein (CP) gene, applying the principle of pathogen-derived resisntance [5]. A result of this effort was the development of a transgenic clone designated as C5 (cv. HoneySweet) [6]. “HoneySweet” plum has proven to be highly resistant to PPV [7] and this high level of resistance has remained stable in European field tests for over 15 years under high PPV infection pressure [8]. Resistance in “HoneySweet” is based on gene silencing or RNA interference (RNAi) which is a natural virus defense system in plants [9]. RNAi in “HoneySweet” produces a high level of resistance that prevents systemic virus infection [10]. In European field tests, no “HoneySweet” tree has ever been infected through natural aphid transmission.
The resistance of “HoneySweet” can be transferred to seedlings through cross-hybridization. When “HoneySweet” is hybridized with conventional plum (P. domestica) 50% of the seedlings carry the PPV resistance trait, demonstrating that resistance is inherited in a Mendelian fashion as a single locus dominant trait [11,12] making it a useful parent in PPV resistance breeding programs.
Based on laboratory and greenhouse research and on data from US and European field tests “HoneySweet” plum was approved for food and cultivation in the US [13]. Here we provide summary information resulting from environmental safety studies of “HoneySweet”. We present analyses of fruit quality, and demonstrate that the nutritional qualities of “HoneySweet” are comparable with the qualities of conventional plums.
2. Environmental Risk
To verify the PPV resistance of “HoneySweet”, field tests were initiated in Europe under the appropriate permits and restrictions adopted by the European Commission. At the same time trees were field evaluated in the US under Animal and Plant Health Inspection Service
(APHIS) permit. Field tests in the US evaluated tree and fruit characteristics but not PPV resistance since no fieldwork involving PPV inoculations could be undertaken in the US under quarantine regulations. Field tests in Europe evaluated PPV resistance, tree growth, fruit production, fruit quality, and environmental safety. While each field test had a different focus, much of the information gathered was complementary and all together provided useful information characterizing the genetically modified trees. In terms of environmental risk, studies in Romania and Spain demonstrated that the genetically modified trees containing a PPV genome segment did not promote the emergence of variant PPV strains [14,15]. Studies in Spain demonstrated that aphid and other non-
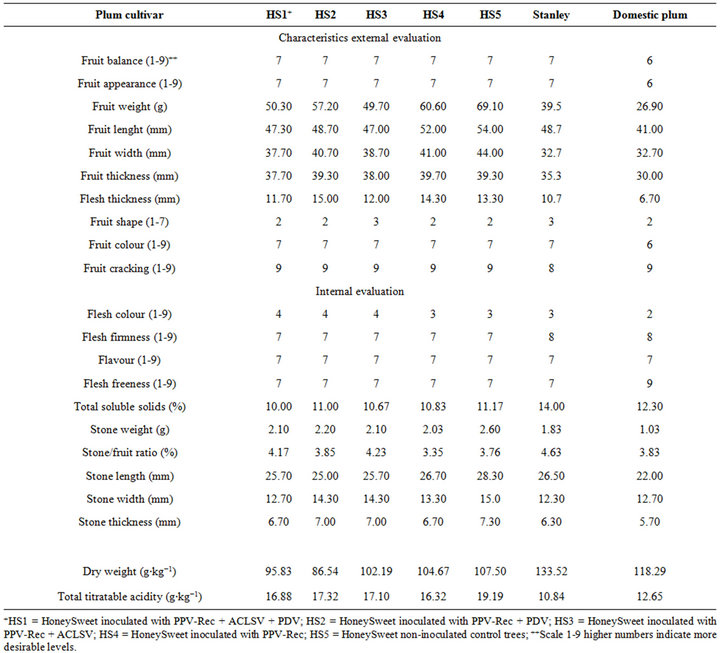
Table 1. Pomological evaluation of external and internal characteristics of fruit “HoneySweet” and conventional plums Praha, Czech Republic.
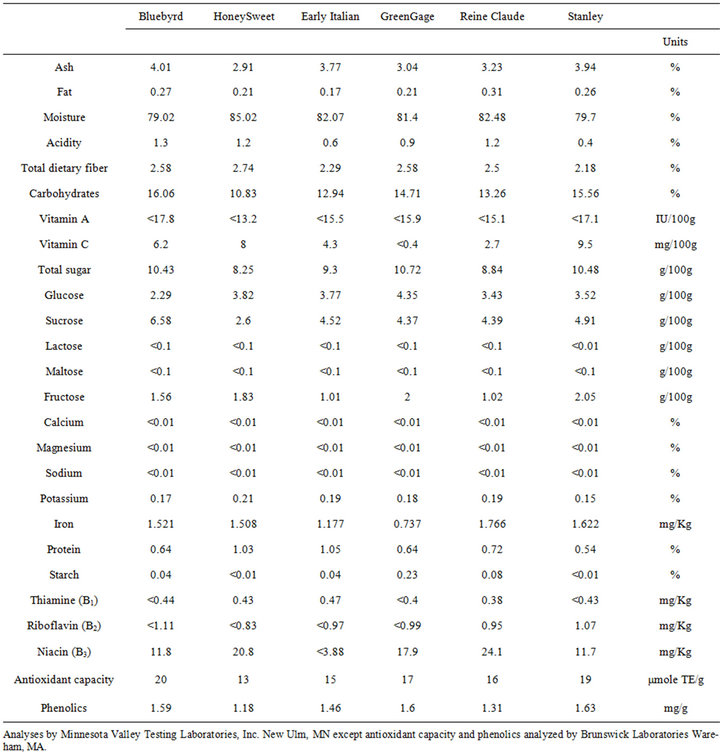
Table 2. Plum fruit compositional analysis, Kearneysville, West Virginia, US.
target insect populations were not affected by genetically engineered plum trees [14,16]. Preliminary studies of transgene flow in the US indicated that transgene flow was low and subsequent studies conducted over an 11 year period confirmed the low level of pollen-mediated gene flow. Seed mediated gene flow into the cultivated and natural environment was not at a detectable level (Scorza et al. in preparation). Gene flow in plums and other fruit trees differs from gene flow in crops such as soybean (Glycine max), maize (Zea mays) and other crops that are seed propagated and where seed is the consumed product. In the case of many fruit species such as plum and other stone fruits, the seed is not used for food or feed but is discarded. The seeds are not generally planted to reproduce trees but instead trees are propagated through grafting onto rootstocks. Rootstocks are in many cases produced from seed but rootstocks are generally not allowed to produce flowers. These factors further limit the potential for spread of transgenic plums or other fruit trees.
Field tests over more than 15 years in 4 countries in Europe and in the US have provided critical information that demonstrate the stable and long-term nature of PPV resistance provided by “HoneySweet”, its potential as a resistant parent in breeding programs, and its environmental safety. Studies with “HoneySweet” and additional resistant clones are continuing to provide additional information on resistance and environmental interactions to evaluate the potential effects of climate change, new virus strains, and other Prunus viruses on RNAi-based resistance. These studies are taking advantage of technological advances including DNA and RNA sequencing.
3. Fruit Quality
In addition to virus resistance and environmental risk studies genetically engineered plum trees released into the environment were evaluated for fruit quality. An example of these studies is the fruit sampling spanning from July to August 2010, in Praha, Czech Republic. Analyses considered overall fruit uniformity, attractiveness, weight, length, width and fruit thickeness, flesh thickeness, fruit shape, skin colour, flesh colour, flesh firmness, flavour, flesh freeness, total soluble solids (brix, determined by refractometer), total titratable acidity, stone size, weight and stone/flesh ratio, and dry weight of fruits harvested from “HoneySweet” artificially inoculated with PPV-Rec (HS1), and uninoculated control trees of cvs. HoneySweet, Domácí švestka, and Stanley [17]. These analyses demonstrated the high quality of “HoneySweet” fruits (Table 1).
In the US fruit composition was evaluated comparing “HoneySweet” to a range of conventional plum cultivars (Table 2). These analyses showed that “HoneySweet” fruit composition is generally in the range of the other plum cultivars tested. Fruit compositional studies are continuing in the US and Europe since quality and nutrient composition is affected by the time of harvest and environmental factors that may vary within and between years. Nevertheless, the studies to date show that “HoneySweet” fruit are of high quality and nutritious.
“HoneySweet” is the first perennial fruit tree to be approved for food and cultivation in the US (or in any country) and is the second genetically modified fruit tree behind papaya [18]. Both species have been developed to resist virus infection. Both are unique in having been developed by public institutions and both provide useful approaches to solving important agricultural problems. The safety and efficacy, and in the case of the earlier developed papaya, the commercial success of these crops indicates the value of the technology to growers and consumers.
REFERENCES
- M. Cambra, N. Capote, A. Myrta and G. Llácer, “Plum Pox Virus and Estimated Costs Associated to Sharka Disease,” OEPP/EPPO Bulletin, Vol. 36, 2006, pp. 202- 204.
- D. Atanassov, “Plum Pox. A New Virus Disease,” Ann Univ Sofia, Fac Agric Silvic, Vol. 11, 1932, pp. 49-69.
- “Current Status of Plum Pox Virus and Sharka Disease Worldwide,” OEPP/EPPO Bulletin, Vol. 36, 2006, pp. 205-218.
- K. Maejima, H. Hoshi, M. Hashimoto, M. Himeno, T. Kawanishi, K. Komatsu, Y. Yamaji, H. Hamamoto and S. Namba, “First Report of Plum Pox Virus Infecting Japannese Apricot (Prunus mume Sieb. Et Zucc.) in Japan,” Journal of General Plant Pathology, Vol. 76, No. 3, 2010, pp. 229-231. doi:10.1007/s10327-010-0233-6
- R. Scorza, M. Ravelonandro, A. M. Callahan, J. M. Cordts, M. Fuchs, J. Dunez and D. Gonsalvez, “Transgenic Plums (Prunus domestica L.) Express the Plum Pox Virus Coat Protein Gene,” Plant Cell Reports, Vol. 14, No. 1, 1994, pp. 18-22. doi:10.1007/BF00233291
- R. Scorza, M. Ravelonandro and D. Gonsalves, “Plum Tree Named ‘HoneySweet’,” United States of America Plant patent number PP15, 154, 2004, p. 2.
- M. Ravelonandro, R. Scorza, J. C. Bachelier, G. Labbone, L. Lery, V. Damsteegt, A. M. Callahan and J. Dunez, “Resistance of Transgenic Plums (Prunus domestica L.) to Plum Pox Virus Infection,” Plant Disease, Vol. 81, No. 11, 1997, pp. 1231-1235. doi:10.1094/PDIS.1997.81.11.1231
- T. Malinowski, M. Cambra, N. Capote, B. Zawadzka, M. T. Gorris, R. Scorza and M. Ravelonandro, “Field Trials of Plum Clones Transformed with the Plum Pox Virus Coat Protein (PPV-CP) Gene,” Plant Disease, Vol. 90, No. 8, 2006, pp. 1012-1018. doi:10.1094/PD-90-1012
- A. S. Zvereva and M. M. Pooggin, “Silencing and Innate Immunity in Plant Defense against Viral and Non-Viral Pathogens,” Viruses, Vol. 4, No. 1, 2012, pp. 2578-2597. doi:10.3390/v4112578
- J.-M. Hily, R. Scorza, K. Webb and M. Ravelonandro, “Accumulation of the Long Class of siRNA Is Associated with Resistance to Plum pox virus in a Transgenic Woody Perennial Plum Tree,” Molecular Plant-Microbe Interactions, Vol. 18, No. 8, 2005, pp. 794-799. doi:10.1094/MPMI-18-0794
- R. Scorza, A. M. Callahan, L. Levy, V. Damsteegt and M. Ravelonandro, “Transferring Potyvirus Coat Protein Genes through Hybridization of Transgenic Plants to Produce Plum Pox Virus Resistant Plums (Prunus domestica),” Acta Horticulturae, Vol. 472, 1998, pp. 421-427.
- M. Ravelonandro, R. Scorza, P. Briard, B. Lafargue and R. Renaud, “Inheritance of Silencing in Transgenic Plums,” Acta Horticulturae, Vol. 899, 2011, pp. 139-144.
- R. Scorza, A. Callahan, M. Ravelonandro and M. Braverman, “Development and Regulation of the Plum Pox Virus Resistant Transgenic Plum ‘HoneySweet’,” In: C. A. Wozniak and A. McHughen, Eds., Regulation of Agricultural Biotechnology: The United States and Canada, Springer, Dordrecht, 2013, pp. 269-280. doi:10.1007/978-94-007-2156-2_12
- N. Capote, J. Perez-Panades, C. Monzo, E. Carbonell, A. Urbaneja, R. Scorza, M. Ravelonandro and M. Cambra, “Assessment of the Diversity and Dynamics of Plum Pox Virus and Aphid Populations in Transgenic European Plums under Mediterranean Conditions,” Transgenic Research, Vol. 17, 2008, pp. 367-377. doi:10.1007/s11248-007-9112-0
- I. Zagrai, M. Ravelonandro, I. Gaboreanu, B. Ferencz, R. Scorza, L. Zagrai, B. Kelemen, D. Pamfil and O. Popescu, “Transgenic Plums Expressing the Plum Pox Virus (PPV) Coat Protein Gene Do Not Assist the Development of PPV Recombinants under Field Conditions,” Journal of Plant Pathology, Vol. 93, No. 1, 2011, pp. 159-165.
- N. Capote, C. Monzó, A. Urbaneja, J. Pérez-Panadés, E. Carbonell, M. Ravelonandro, R. Scorza and M. Cambra, “Evaluación del Riesgo Ambiental del Cultivo en Campo de Ciruelos Europeos Transgénicos Sensibles y Resistentes a Plum Pox Virus,” Revista ITEA, Vol. 103, No. 3, 2007, pp. 156-167.
- J. Polák, J. Pívalová, J. Kumar-Kundu, M. Jokeš, R. Scorza and M. Ravelonandro, “Behaviour of Transgenic Plum Pox Virus-Resistant Prunus domestica L. Clone C5 Grown in the Open Field under a High and Permanent Infection Pressure of the PPV-Rec Strain,” Journal of Plant Pathology, Vol. 90, Suppl. 1, 2008, pp. S1.33- S1.36.
- D. Gonsalves, “Control of Papaya Ringspot Virus in Papaya—A Case Study,” Annual Review of Phytopathology, Vol. 36, 1998, pp. 415-437. doi:10.1146/annurev.phyto.36.1.415
NOTES
*This work was supported in part by grants from the European Union, FP7-IRSES-Interest n 269292 (2011-2014).

