Food and Nutrition Sciences
Vol. 3 No. 10 (2012) , Article ID: 23385 , 7 pages DOI:10.4236/fns.2012.310180
Prebiotic-Like Effects of SweetPearl® Maltitol through Changes in Caecal and Fecal Parameters*
![]()
Biology and Nutrition Department, ROQUETTE, France.
Email: #clementine.thabuis@roquette.com
Received July 18th, 2012; revised August 18th, 2012; accepted August 25th, 2012
Keywords: Prebiotic; Polyol; Maltitol; α-Glucosidase; Colonic Fermentation
ABSTRACT
Prebiotic-like effects of maltitol were investigated supplementing two groups of rats with either 5% maltodextrin (control group) or 5% maltitol (maltitol group). A third group was supplemented with 5% maltitol at first and then with 5% maltodextrin (maltitol/maltodextrin group). Faecal parameters were monitored throughout the experiment and caecal parameters at the end. The weights of caecal content and caecal wall were significantly higher in the maltitol group than in the control group, but not in the maltitol/maltodextrin group. Propionic acid concentration was significantly higher in the maltitol group compared to both control and maltitol/maltodextrin group. Faecal parameters were also influenced by the dietary supplementation with maltitol: the amount of dry matter in feces decreased and alpha-glucosidase activity increased. These effects lasted 28 days in the maltitol only group, whereas they stopped some days after the switch to maltodextrin in the maltitol/maltodextrin group. Maltitol could induce prebiotic-like effects.
1. Introduction
Sugar alcohols or polyols are characterized as low digestible carbohydrates, because they are incompletely digested in the small intestine and fermented in the colon at various extents depending on the polyol type. Maltitol is a polyol resulting from the hydrogenation of starch hydrolysates. Maltitol metabolism follows a well-known mechanism [1-3]. This polyol is only partially absorbed in the proximal intestine and reaches the lower bowel and the colon. Consequently, the digestive tolerance of maltitol has been previously studied in chocolate in healthy adult volunteers [4-6]. It has been published that adults could consume up to 40 grams of maltitol per day with no major symptoms and children were able to consume 15 grams per intake [4,7].
Prebiotic were defined as “a non-digestible food ingredient that beneficially affect the health of the host by selectively stimulating the growth and activity of beneficial bacteria and/or by decreasing harmful bacteria in the gut, by decreasing intestinal pH, by producing short chain fatty acids (SCFA) and by changing bacteria enzyme concentrations” [8,9]. Typically, prebiotics are carbohydrates similar to oligosaccharides. The most prevalent forms of prebiotic are nutritionally classified as soluble fibres [8,10]. More recently, it has been shown that fructooligosaccharides such as oligofructose, inulin and galacto-oligosaccharides fully meet the prebiotic criteria, whereas mannan oligosaccharides have been called prebiotics but could more correctly be called immunosaccharides [11]. The distinction between short-chain, longchain, and full-spectrum prebiotics is now a matter of concern for scientists: “short-chain” prebiotics—e.g. oligofructose—contain 2 - 8 links per saccharide molecule, whereas longer-chain prebiotics—e.g. inulin—contain 9 - 64 links per sucrose molecule, and tend to be fermented more slowly [12]. Prebiotics were shown to be involved in body weight management, the prevention of cardiovascular diseases and a decrease in colon cancer risk [13- 15]. In addition, for technological reasons, the food industry would be interested in new ingredients with prebiotic properties.
Polyols such as maltitol are interesting molecules for the food industry because of their technological and nutritional properties. At the technological level, maltitol is a bulking sweetener allowing a 1/1 (w/w) substitution of sucrose in food product. At the nutritional level, maltitol has been described as safe for teeth thanks to its nonacidogenic properties [16,17]. Since maltitol is only partly absorbed by the proximal intestine, it provides less energy per mass unit than sucrose [18], and consequently triggers a lower glycaemic response than sucrose [19]. Moreover, maltitol displays beneficial effects on gastrointestinal health similar to that of a prebiotic. In a recent clinical study, a maltitol-polydextrose chocolate consumption led to gut prebiotic effects but these results could not clearly allocated because of the mix of putative functional ingredients in the experimental diet [20].
Thus, in the present study, we aimed at assessing the prebiotic potential of maltitol studying faecal and caecal parameters in a rat model. Rats were supplemented with maltitol during either 17 or 28 days in order to identify short and long lasting effects in the same study.
2. Materials and Methods
2.1. Animals, Diet and Experimental Design
All the experiments were conducted according to the French Regulations for Animal Experimentation (decree of., Oct. 19th, 1987, Ministry of Agriculture) and in compliance with the European Community Council Directive 86/609/EEC. Sprague-Dawley rats, purchased at 300 - 325 g of weight from Charles River (L’Abresle, France), were housed in an environmentally controlled room (temperature at 20˚C ± 2˚C, hygrometry at 50% ± 10% with a 12 h light/dark cycle) and fed ad libitum on maintaining diet A04C (Table 1, SAFE, Augy, France). Subsequently, 30 rats were blocked by body weight and randomly split into 3 groups (N = 10 per group) before entering the 4 week-experimental phase. Two groups of rats were given SweetPearl® maltitol (ROQUETTE, Lestrem, France) (50 g per kg of food) in their diet, while the third group received the same concentration of a totally digestible maltodextrin (control group). The first maltitol group was supplemented during the 4-week experimental period (maltitol only group), the second one was supplemented with 5% maltitol during 17 days and was then given the control diet (maltitol/maltodextrin group). Food intake, water consumption and body weight gain were monitored on a weekly basis. Feces were collected during 12 hours thanks to metabolic cages at baseline and after 17, 19, 21, 25 and 28 days of supplementation.
2.2. Sampling
At the end of the experimental period, rats were euthanized by CO2 inhalation. Caecal mucosa were removed, emptied, washed, weighed and stored at −80˚C. Feces and caecal content samples were stored at −20˚C until analyzed.
2.3. Feces and Caecal Analysis
Wet weight, dry matter and pH were measured at the beginning of feces preparation. Freeze-dried feces were
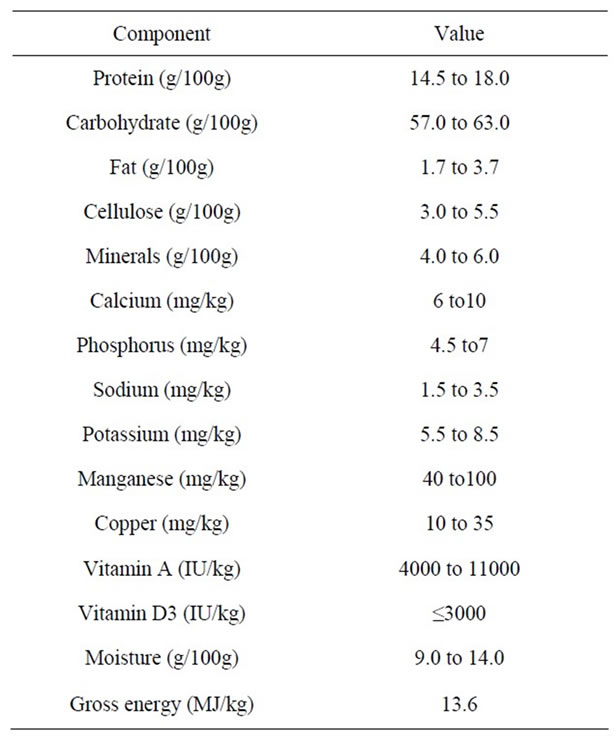
Table 1. Composition of the basal control diet.
suspended into distilled water and mixed for one minute. Then they were centrifuged (3 min at 15,000 rpm). Supernatants were used to test enzyme activity. The activity of β- and α-glucosidases was determined as previously described [21]. The activity of β-galactosidase, β-glucuronidase, esterase and protease enzymes were measured on freeze-dried feces samples using commercial purified enzymes as standard (β-galactosidase, β-glucuronidase, esterase from Bacillus stearothermophilus, trypsin type I from bovin pancreas from Sigma Aldrich, USA). P-nitrophenyl-b-D-glucuronide, P-nitrophenyl-bD-galactopyranoside, P-nitrophenyl acetate, N-α-benzoyl-DL-arginine-4-nitroanilide hydrochloride were used as colorimetric substrates. Optical densities were red at 405 nm and enzyme activities were expressed in absorbance Unit/min/g of dry feces (U abs/min/g of dry feces).
Caecal contents were removed after euthanasia. Wet weight and pH were measured and samples were frozen at −20˚C. For SCFA analysis, a 2 g sample was homogenized in 5 ml double distilled water and then centrifuged at 2500 g for 5 min. The supernatant was acidified to pH 2 using 25 ml and injected into a BP21 gas chromatography column (length: 30 m, inner diameter: 530 mm, film: 1 mm) along with an internal standard (4-hydroxy- 4-methyl-2-pentanone). H2 was supplied as the carrier gas at a flow rate of 1.5 ml/min. The initial oven temperature was 135˚C and was kept there for 6 min, and then raised to 180˚C by 25˚C/min and held there for 1 min, and then further increased to 230˚C by 25˚C/min, and finally held at 230˚C for 1 min. Glass wool (Supelco) was inserted in the glass liner of the split injection port. The temperatures of the flame ionization detector and the injection port were 240˚C and 280˚C, respectively. The flow rates of H2 and air as make-up gas were 40 and 400 ml/min, respectively. The injected sample volume for gas chromatography (AutoSystem XL; PerkinElmer) analysis was 1 ml, and the running time for each analysis was about 10 min. The different SCFA were identified according to the retention time of the various elution peaks. Quantification was obtained by comparison to a standard curve [22].
2.4. Statistical Analysis
Results are presented as means ± standard error of the means (SEM). The statistical analysis of physiological parameters was performed on Statistica software (StatSoft, Paris, France). Variance homogeneity was checked using Bartlett’s test. If variance was homogenous, experimental groups were compared using non parametric tests (Kruskall & Wallis test and Mann & Whitney test). On the contrary, if variance was not homogenous, experimental groups were compared using one-way ANOVA and Scheffe test. For overall results, statistical significance was set up at the p < 0.05 level.
3. Results
Global animal behaviour, clinical observations, weekly food and water consumptions and changes in body weight were identical in all groups throughout the experimental period.
3.1. Effect of Maltitol Supplementation on Caecal Parameters
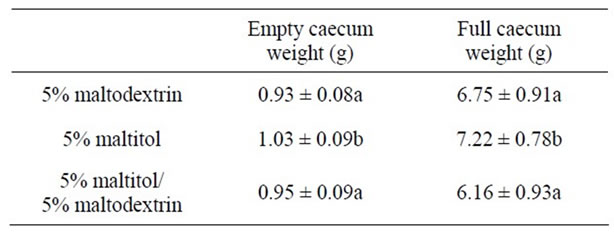
After 28 days of maltitol supplementation, the weights of full and empty caeca of the maltitol only group were significantly higher than those of the control group (see Table 2). Interestingly, the maltitol/maltodextrin group displayed caecal parameters similar to that of the control group. Maltitol supplementation did not change the pH value of the caecal content. Concerning SCFAs the maltitol group displayed no significant difference in the concentration of butyric acid in the caecal content as compared with the control group and with the maltitol/maltodextrin group. Acetic acid was significantly decreased in both maltitol and maltitol/maltodextrin groups. But the maltitol group significantly displayed a higher concentration in propionic acid within the caecum (+18%, p < 0.05) than in the control group and in the maltitol/maltodextrin group (which exhibited a significantly lower propionic concentration than the control).
 (A)
(A)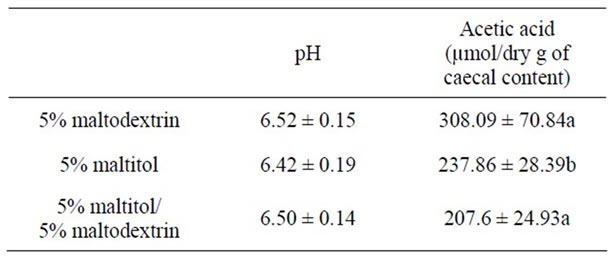 (B)
(B) (C)
(C)
(A), (B), (C): Figures that do not share the same letter are statistically different (p ≤ 0.05).
Table 2. Caecal parameters after an experimental period of 4 weeks.
3.2. Effect of Maltitol Supplementation on Faecal Parameters
Feces samples were collected throughout the experimental period in order to identify the effects of both supplementations on faecal parameters. An effect of maltitol supplementation was observed during the first 17 days: the amount of dry matter in feces was significantly lower in both supplemented groups (maltitol only and maltitol/ maltodextrin groups, −3.5%) than in the control group. After 17 days, dry matter amounts in the maltitol only group kept on decreasing significantly until the end of the experimental period, whereas the amount of dry matter of the feces collected in the maltitol/maltodextrin group went back up to the level of the control group after 28 days (see Figure 1). Feces pH was not modified by maltitol supplementation.
The activity of different enzymes was measured in feces suspensions throughout the experimental period. The activity of β-glucosidase, β-galactosidase, β-glucuronidase, protease and esterase was not significantly impacted by maltitol supplementation, whereas α-glucosedase activity was significantly increased (approximately threefold in comparison to the control group after 17 days of supplementation). The level of α-glucosidase activity remained high in the maltitol only group whereas it went down to control group level in the maltitol/maltodextrin group once the maltitol supplementation was over (see Figure 2).
4. Discussion
Prebiotics are defined as “a non-digestible food ingredient that beneficially affect the health of the host by selectively stimulating the growth and activity of beneficial bacteria and/or by decreasing harmful bacteria in the gut, by decreasing intestinal pH, by producing SCFA and by changing bacterial enzyme concentrations” [8]. In the present study, we aimed at investigating the prebiotic potential of maltitol supplementation in a rat model.
In the feces, the supplementation with maltitol was associated with a decrease of dry matter amount showing clearly that maltitol, as a low digestible carbohydrate, could reach the large intestine. Indeed, the fermentation.
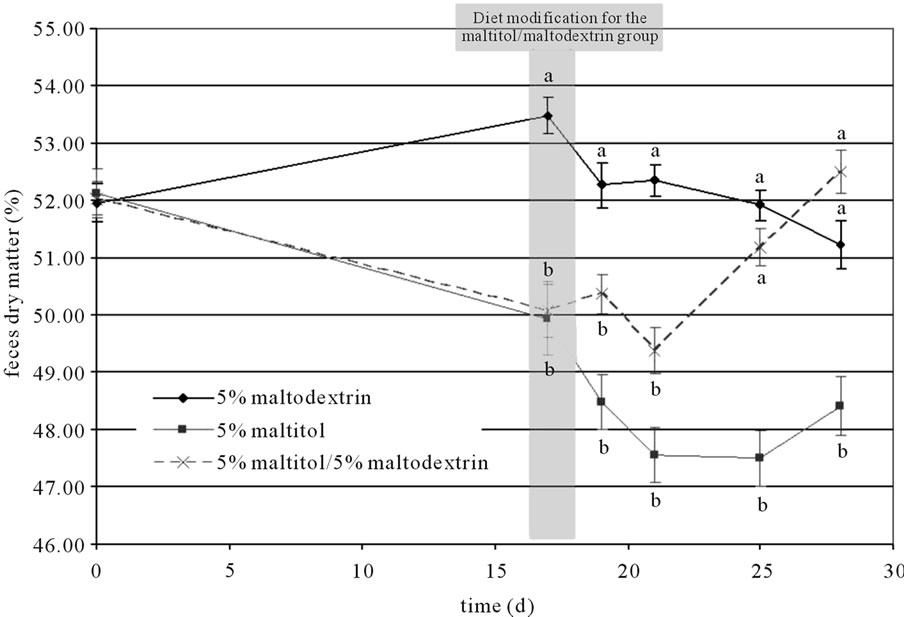
Figure 1. Changes in amount of dry matter in feces throughout the experimental period. Results are mean ± SEM, N = 10 per group. Plots that do not share the same letter (a, b, c) are statistically different (p ≤ 0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation curve.
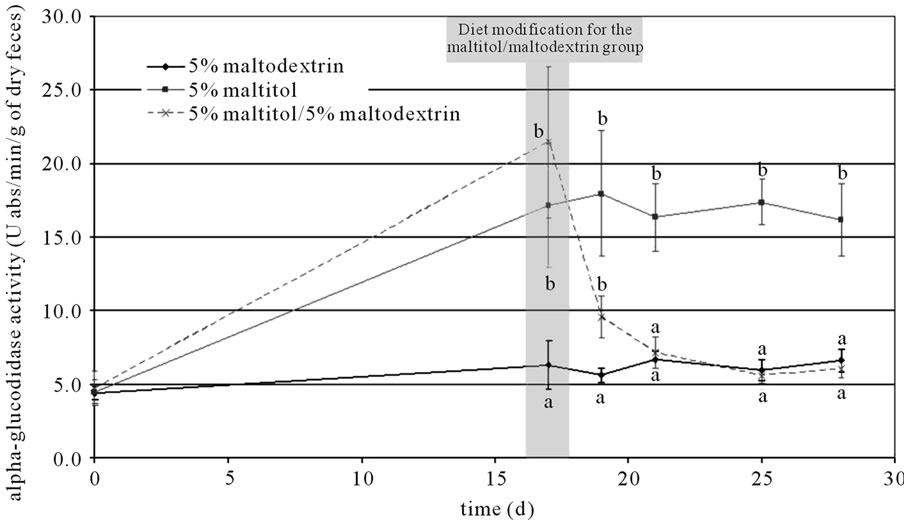
Figure 2. Changes in α-glucosidase activity throughout the experimental period. Results are mean ± SEM, N = 10 per group. Plots that do not share the same letter (a, b, c) are statistically different (p ≤ 0.05). Letters mentioning the statistical significance were given the colour and style corresponding to their allocation curve.
of prebiotic substrates in the large intestine by colonic bacteria produces SCFA that are known to increase osmotic pressure in the colon, sometimes triggering diarrhea after inulin or oligofructose fermentation [23,24] These effects were often linked to the high molecular weight of SCFA. Therefore, the decrease in faecal dry matter after maltitol consumption could be linked to an osmotic phenomenon due to either maltitol reaching the colon or to maltitol fermentation products (propionate) in the colon. Moreover, the increase in the α-glucosidase activity during maltitol supplementation clearly shows that maltitol stimulates saccharolytic colonic bacteria. In addition, α-glucosidase activity has been partially attributed to Bacteroides [25], which was reported to be potentially beneficial for the host [26]. We confirmed, as previously described, that the major part of the ingested maltitol can reach the caecum and the colon where it is fermented [1,2,27].
In rats, the major part of the fermentation process occurs in the caecum. Here, the maltitol caecal fermentation was demonstrated through the increase in caecal content weight and through the production of propionic acid. Indeed, the caecal weight increase must be linked to the increase in caecal bacterial mass and not to the increase in osmotic pressure as dry matter of the caecal contents was not significantly modified. In the caecum, we also observed a significant increase in caecal wall weight linked to maltitol consumption. This increase could be explained by maltitol fermentation and the properties of SCFAs that favour colonocytes growth [28]. It has been reported that the SCFA pattern produced by gut bacteria was highly correlated to the substrate [29]. Here we showed that maltitol was mainly fermented into propionic acid. Propionate production is linked to the metabolism of specific substrates (rhamnose, tagatose, resistant starch…) by specific bacteria (Bacteroides, propionibacteria…) depending on different fermentation pathways [30]. Maltitol fermentation seems to be linked to Bacteroides activity through propionate production and α-glucosidase activity [25,30]. A diet containing propionate was found to be associated with lower fasting glycaemia in rats [31]. Propionate could also decrease gluconeogenesis, while on the other hand it stimulates glycolysis in rat adipocyte cultures [32]. Its influence on lipid metabolism was also reported [30]. Another study in humans showed that the addition of propionate to bread inhibited the activity of amylase [33]. Thus the observed increase in propionate production linked to maltitol supplementation leads one to expect an effect of this polyol on glycaemia. A decrease in acetic acid was also observed, and could be explained by the fact that maltitol as a fermentation substrate did favour propionate producing bacteria more than acetate producing bacteria.
No modification of the caecal pH value was observed during maltitol supplementation. It is well-known that a decrease in caecal pH, consecutively to SCFA production, could prevent the colonization of potentially pathogenic microorganisms such as Enterobacteria [8] and could stimulate the growth of probiotic bacteria [14]. In the present study, the caecal pH value was not significantly modified by a 5% maltitol feed. We can hypothesize that the dose of maltitol supplementation was not sufficient to significantly decrease the pH in the caecum. Indeed a significant decrease in caecal content pH and in faeces pH was observed after the consumption of 10% maltitol in the feed during 36 days (data not shown).
Finally, the prebiotic-like effects of maltitol were observable after 17 days of supplementation and lasted at least until the 28th day of supplementation. Nevertheless, the caecal and colonic parameters of the group that stopped maltitol supplementation (the maltitol/maltodextrin group) went back to the level of the control maltodextrin group. These transient effects on large intestine parameters showed that the observed modulation of the biomarkers was clearly due to maltitol supplementation. When supplementation is stopped, the caecal and faecal parameters went back to baseline as the composition and the activity of the gut microbiota became as initially.
In conclusion, this work reports for the first time the prebiotic-like effects of SweetPearl® maltitol on faecal and caecal parameters in a rat model as it had an impact on caecal mucosa and caecal bacterial mass, and promoted gut saccharolytic bacteria displayed by α-glucosidase activity. Maltitol also increased propionate production in the caecum and decreased faecal dry matter underlying changes in the ecosystem of the large intestine as expected with prebiotics. Nevertheless further studies on the effects of this polyol on the composition of intestinal microbiota would be necessary to confirm its prebiotic effects.
5. Acknowledgements
This study was sponsored by Roquette and conducted on their premises in Lestrem, France. C. Thabuis wrote the paper. D. Wils and L. Guerin-Deremaux designed the experiment. A.-C. Herbomez, F. Desailly and F. Ringard performed the biochemical and in vivo studies.
REFERENCES
- G. Livesey, “Health Potential of Polyols as Sugar Replacers, with Emphasis on Low Glycaemic Properties,” Nutrition Research Reviews, Vol. 16, No. 2, 2003, pp. 163- 191. doi:10.1079/NRR200371
- T. Matsuo, “Lactic Acid Production from Sugar Alcohol, Maltitol and Lactitol, in Human Whole Saliva,” Shigaku, Vol. 60, No. 6, 2003, pp. 760-775.
- T. Oku, M. Akiba, M. H. Lee, S. J. Moon and N. Hosoya, “Metabolic Fate of Ingested [14C]-Maltitol in Man,” Journal of Nutritional Science and Vitaminology (Tokyo), Vol. 37, No. 5, 1991, pp. 529-544. doi:10.3177/jnsv.37.529
- G. A. Koutsou, D. M. Storey, A. Lee, A. Zumbe, B. Flourie, Y. leBot and P. Olivier, “Dose-Related Gastrointestinal Response to the Ingestion of Either Isomalt, Lactitol or Maltitol in Milk Chocolate,” European Journal of Clinical Nutrition, Vol. 50, No. 1, 1996, pp. 17-21.
- A. Ruskone-Fourmestraux, A. Attar, D. Chassard, B. Coffin, F. Bornet and Y. Bouhnik, “A Digestive Tolerance Study of Maltitol after Occasional and Regular Consumption in Healthy Humans,” European Journal of Clinical Nutrition, Vol. 57, No. 1, 2003, pp. 26-30. doi:10.1038/sj.ejcn.1601516
- D. M. Storey, G. A. Koutsou, A. Lee, A. Zumbe, P. Olivier, Y. Le Bot and B. Flourie, “Tolerance and Breath Hydrogen Excretion Following Ingestion of Maltitol Incorporated at Two Levels into Milk Chocolate Consumed by Healthy Young Adults with and without Fasting,” Journal of Nutrition, Vol. 128, No. 3, 1996, pp. 587-592.
- C. Thabuis, M. Cazaubiel, M. Pichelin, D. Wils and L. Guerin-Deremaux, “Short-Term Digestive Tolerance of Chocolate Formulated with Maltitol in Children,” International Journal of Food Sciences and Nutrition, Vol. 61, No. 7, 2010, pp. 728-738. doi:10.3109/09637481003766812
- G. R. Gibson and M. B. Roberfroid, “Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics,” Journal of Nutrition, Vol. 125, No. 6, 1995, pp. 1401-1412.
- M. N. Woods and S. L. Gorbach, “Influences of Fibres on the Ecology of Intestinal Flora,” In: CRC Handbook of Dietary Fiber in Human Nutrition, 3rd Edition, CRC Press, Boca Raton, pp. 257-269.
- M. B. Roberfroid, “Functional Effects of Food Components and the Gastrointestinal System: Chicory Fructooligosaccharides,” Nutrition Reviews, Vol. 54, No. 11, 1996, pp. S38-S42. doi:10.1111/j.1753-4887.1996.tb03817.x
- M. B. Roberfroid, “Prebiotics: The Concept Revisited,” Journal of Nutrition, Vol. 137, No. 3, 2007, pp. 830S- 837S.
- B. Kleessen, L. Hartmann and M. Blaut, “Oligofructose and Long-Chain Inulin: Influence on the Gut Microbial Ecology of Rats Associated with a Human Faecal Flora,” British Journal of Nutrition, Vol. 86, No. 2, 2001, pp. 291-300. doi:10.1079/BJN2001403
- F. R. Bornet, “Undigestible Sugars in Food Products,” American Journal of Clinical Nutrition, Vol. 59, No. 3, 1994, pp. 763S-769S.
- C. Duggan, J. Gannon and W. A. Walker, “Protective Nutrients and Functional Foods for the Gastrointestinal Tract,” American Journal of Clinical Nutrition, Vol. 75, No. 5, 2002, pp. 789-808.
- M. S. Geier, R. N. Butler and G. S. Howarth, “Probiotics, Prebiotics and Synbiotics: A Role in Chemoprevention for Colorectal Cancer?” Cancer Biology and Therapy, Vol. 5, No. 10, 2006, pp. 1265-1269. doi:10.4161/cbt.5.10.3296
- T. N. Imfeld, “Clinical Caries Studies with Polyalcohols. A Literature Review,” Schweiz Monatsschreitung Zahnmedecine, Vol. 104, No. 8, 1994, pp. 941-945.
- E. J. Lee, B. H. Jin, D. I. Paik and I. K. Hwang, “Preventive Effects of Sugar-Free Chewing Gum Containing Maltitol on Dental Caries in Situ,” Food Science and Biotechnology, Vol. 18, No. 2, 2009, pp. 432-435.
- F. Bornet, C. Alamowitch and G. Slama, “Volatile Fatty Acids. Effect on Glucose Metabolism?” Revue des Praticiens, Vol. 44, No. 8, 1994, pp. 1051-1055.
- K. C. Ellwood, “Methods Available to Estimate the Energy Values of Sugar Alcohols,” American Journal of Clinical Nutrition, Vol. 62, No. 5, 1995, pp. 1169S-1174S.
- E. Beards, K. Tuohy and G. Gibson, “A Human Volunteer Study to Assess the Impact of Confectionery Sweeteners on the Gut Microbiota Composition,” British Journal of Nutrition, Vol. 104, No. 5, 2012, pp. 701-708. doi:10.1017/S0007114510001078
- E. G. van den Heuvel, D. Wils, W. J. Pasman, M. H. Saniez and A. F. Kardinaal, “Dietary Supplementation of Different Doses of NUTRIOSE FB, a Fermentable Dextrin, Alters the Activity of Faecal Enzymes in Healthy Men,” European Journal of Nutrition, Vol. 44, No. 7, 2005, pp. 445-451. doi:10.1007/s00394-005-0552-0
- J. M. Lecerf, F. Depeint, E. Clerc, Y. Dugenet, C. N. Niamba, L. Rhazi, A. Cayzeele, G. Abdelnour, A. Jaruga, H. Younes, H. Jacobs, G. Lambrey, A. M. M. Abdelnour and P. R. Pouillart, “Xylo-Oligosaccharide (XOS) in Combination with Inulin Modulates Both the Intestinal Environment and Immune Status in Healthy Subjects, While XOS Alone Only Shows Prebiotic Properties,” British Journal of Nutrition, in press.
- J. H. Cummings, G. T. Macfarlane and H. N. Englyst, “Prebiotic Digestion and Fermentation,” American Journal of Clinical Nutrition, Vol. 73, No. 2, 2001, pp. 415S- 420S.
- G. R. Gibson, E. R. Beatty, X. Wang and J. H. Cummings, “Selective Stimulation of Bifidobacteria in the Human Colon by Oligofructose and Inulin,” Gastroenterology, Vol. 108, No. 4, 1995, pp. 975-982. doi:10.1016/0016-5085(95)90192-2
- T. M. Gloster, J. P. Turkenburg, J. R. Potts, B. Henrissat and G. J. Davies, “Divergence of Catalytic Mechanism within a Glycosidase Family Provides Insight into Evolution of Carbohydrate Metabolism by Human Gut Flora,” Chemical Biology, Vol. 15, No. 10, 2008, pp. 1058-1067. doi:10.1016/j.chembiol.2008.09.005
- J. A. Parnell and R. A. Reimer, “Prebiotic Fibres DoseDependently Increase Satiety Hormones and Alter Bacteroidetes and Firmicutes in Lean and Obese JCR:LA-cp Rats,” British Journal of Nutrition, Vol. 107, No. 4, 2012, pp. 601-613. doi:10.1017/S0007114511003163
- T. Oku, R. Hongo and S. Nakamura, “Suppressive Effect of Cellulose on Osmotic Diarrhea Caused by Maltitol in Healthy Female Subjects,” Journal of Nutritional Science and Vitaminology (Tokyo), Vol. 54, No. 4, pp. 309-314.
- J. M. Campbell, G. C. Fahey Jr. and B. W. Wolf, “Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, pH and Microflora in Rats,” Journal of Nutrition, Vol. 127, No. 1, 1997, pp. 130-136.
- M. Nyman, “Fermentation and Bulking Capacity of Indigestible Carbohydrates: The Case of Inulin and Oligofructose,” British Journal of Nutrition, Vol. 87, No. 2, 2002, pp. S163-S168. doi:10.1079/BJN/2002533
- E. Hosseini, C. Grootaert, W. Verstraete and T. van de Wiele, “Propionate as a Health-Promoting Microbial Metabolite in the Human Gut,” Nutrition Reviews, Vol. 69, No. 5, 2011, pp. 245-258. doi:10.1111/j.1753-4887.2011.00388.x
- J. Boillot, C. Alamowitch, A. M. Berger, J. Luo, F. Bruzzo, F. R. Bornet and G. Slama, “Effects of Dietary Propionate on Hepatic Glucose Production, Whole-Body Glucose Utilization, Carbohydrate and Lipid Metabolism in Normal Rats,” British Journal of Nutrition, Vol. 73, No. 2, 1995, pp. 241-251. doi:10.1079/BJN19950026
- J. W. Anderson and S. R. Bridges, “Short-Chain Fatty Acid Fermentation Products of Plant Fiber Affect Glucose Metabolism of Isolated Rat Hepatocytes,” Proceedings of Society for Experimental Biology and Medecine, Vol. 177, No. 2, 1984, pp. 372-376.
- T. Todesco, A. V. Rao, O. Bosello and D. J. Jenkins, “Propionate Lowers Blood Glucose and Alters Lipid Metabolism in Healthy Subjects,” American Journal of Clinical Nutrition, Vol. 54, No. 5, 1991, pp. 860-865.
NOTES
*All the authors declared there is no conflict of interest.
#Corresponding author.

