Food and Nutrition Sciences
Vol. 2 No. 2 (2011) , Article ID: 4524 , 4 pages DOI:10.4236/fns.2011.22011
1-Deoxynojirimycin Content and Alfa-Glucosidase Inhibitory Activity and Heat Stability of 1-Deoxynojirimycin in Silkworm Powder
![]()
1Faculty of Agriculture, Tamagawa University, Tokyo, Japan; 2Shimane Institute of Health Science 223-7 Enya-cho, Izumo, Japan; 3Kanazawa University, Graduate School of Medical Science, Takaramachi, Kanazawa, Japan; 4European University Viadrina, Frankfurt (Oder), Germany; 5Meiji University of Integrative Medicine, Nantan, Kyoto, Japan; 6Nagasaki University Graduate School of Biomedical Science, Nagasaki, Japan.
E-mail: yatsunam@agr.tamagawa.ac.jp
Received December 20th, 2010; revised January 16th, 2011; accepted February 10th, 2011.
Keywords: Silkworm, Bombyx Mori, 1-Deoxynojirimycin, Alfa-Glucosidase, Inhibitor, Heat Stability
ABSTRACT
Silkworm powder containing 1-deoxynojirimycin (DNJ) has α-glucosidase inhibitory activity and is promising as a complementary and alternative medicine (CAM) agent in Japan. Silkworm powder produced in Korea was extracted with 75% ethanol. The extract was derivatized with 9-fluorenylmethyl chloroformate (FMOC-Cl), and DNJ-FMOC content was measured by HPLC. Then, alfa-glucosidase inhibition by silkworm and mulberry powder in pig liver crude enzyme was assayed using 4-nitrophenyl-alfa-D-glucopyranoside as a substrate. Silkworm powder DNJ content (0.39% to 0.58%) was higher than that in mulberry powder (0.08% to 0.12%). The alfa-glucosidase inhibitory activity of silkworm powder was more potent than that of mulberry leaves and green tea. Silkworm powder DNJ was stable upon heating to 121˚C for up to 15 min.
1. Introduction
Silkworm and silkworm droppings have long been used in China and Korea as a folk remedy for the treatment of diabetes. Silkworm powder has blood glucose-lowering effects [1], and mulberry leaves, the diet of silkworms, effectively inhibit alfa-glucosidase in the human small intestine [2]. Mulberry leaves contain about 0.11% (dry weight) 1-deoxynojirimycin (DNJ), a potent alfa-glucosidase inhibitor [3]. Comparisons have shown that DNJ content is 2.7-fold higher in silkworms than in mulberrys [4]. Thus, silkworm powder, with higher expected DNJ content than mulberry leaves, is considered to be a promising CAM agent in Japan. However, direct comparison of DNJ content between the silkworm powder available in Japan and mulberry leaves has not previously been performed. One of the pharmacologic effects of DNJ is a decrease in infections due to type A influenza and human parainfluenza viruses [5,6]. The mechanism of this antiviral activity is related to DNJ inhibition of microsomal alfa-glucosidase I and II, which are involved in viral protein synthesis and glycoprotein processing.
This blocks the synthesis of viral surface envelope proteins, thus preventing viral replication. Trimming glucosidase, involved in protein synthesis, has been isolated from pig liver [7,8]. Crude alfa-glucosidase was prepared from liver, and using mulberry powder and green tea powder (common beverage in Japan) as standards, inhibitory activity of silkworm powder was measured. In addition, when silkworm powder is used as a supplement, sterilization of the raw materials is often performed for hygienic reasons. However, the stability of DNJ when heated to 121˚C has not previously been reported. Therefore, we evaluated the stability of DNJ for potential use as an antidiabetic and antiviral CAM agent in Japan.
2. Materials and Methods
Silkworm powder (SwP1, Wwp2) produced in Korea was purchased from Bombyx Co. in Japan, while mulberry powder (MulP1-3) and green tea powder (GTP) were purchased as commercially distributed products in Japan.
After heating at 121˚C for 5 to 60 min for SwP1 and Wwp2, they were used for heat stability assay. Each powder was extracted with 75% ethanol, and test extracts were stored at 5˚C. Extracts were ultrafiltrated and derivatized with 9-fluorenylmethyl chloroformate (FMOCCl), and DNJ content was then measured by HPLC [9]. Fresh pig liver was homogenized in phosphate buffer (pH 6.5) containing 100 µM phenanthroline monohydrate and 0.25 M sucrose, and was then centrifuged (4˚C, 10,000 rpm, 30 min) to obtain the supernatant. This was centrifuged (4˚C, 18,000 rpm, 30 min) to obtain the precipitate. Triton X-100 was added to a final concentration of 0.1%, and the extract was used as crude alfa-glucosidase. We assayed alfa-glucosidase inhibitory activity using 4-nitrophenyl-alfa-D-glucopyranoside as a substrate. After reacting at pH 6.4 and 37˚C for 60 min, we measured the increase in absorbance at 405 nm to determine enzyme activity and calculate inhibition.
3. Results and Discussion
DNJ content in silkworm powder ranged from 0.39% to 0.58%, and that in mulberry powder ranged from 0.08% to 0.12% (Figure 1). DNJ content in silkworm powder, as compared to mulberry powder, was 3 to 7 times higher.
In order to compare whether DNJ content in silkworm powder is higher than in mulberry powder and mulberry food products, it was compared with previously reported DNJ content in mulberry powder and mulberry food products [10-12]. Silkworm powder clearly has higher DNJ content than in mulberry powder produced in Japan or China, and contains an equal or greater amount of DNJ than mulberry leaf tablets made in Japan. DNJ content in silkworm powder is lower than propolis mixed with mulberry extract, with reported antidiabetic effects [13]. However, because daily ingestion is likely to be higher, silkworm powder can be used as a supplement for effective glycemic control in type 2 diabetic patients. DNJ content is higher in silkworm powder than in mulberry powder because when mulberry leaves are damaged by insects, a milky sap is secreted, and this milky sap contains high DNJ content, ranging from 0.32 to 0.47% [14].
Bioconcentration is thought to occur when silkworms consume the milky sap together with the leaves. Mulberry DNJ content is correlated with alfa-glucosidase inhibitory activity in the small intestine [9], and thus in silkworm powder, with higher DNJ content, more potent intestinal alfa-glucosidase inhibition is expected than that with mulberry leaves. To explore the potential of silkworm powder as an antiviral agent, inhibitory activity against liver-derived alfa-glucosidase was measured (Figure 2). Silkworm powder showed more potent inhibitory activity than mulberry or green tea powder. Japanese green tea extracts inhibit the growth of influenza A and B [15], but DNJ in silkworm powder inhibits the growth of influenza by a mechanism that differs from catechins; oral consumption of mulberry leaves containing DNJ results in DNJ transfer to human blood [16]. When transferred to blood, DNJ acts on infected cells, and in combination, for example, with green tea, effective anti-influenza activity is likely.
In order to evaluate heat stability (Figure 3), we heated silkworm powder to 121˚C for 5 to 60 min and mea-
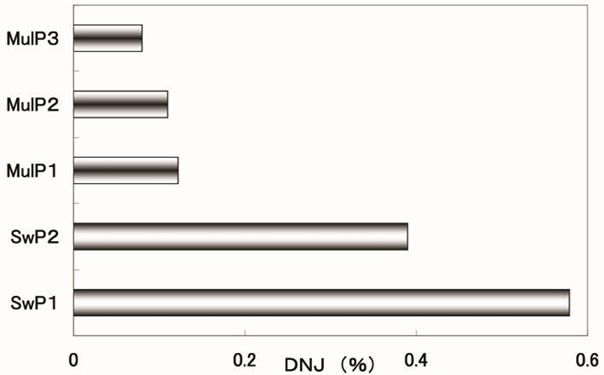
Figure 1. 1-deoxynojirimycin (DNJ) content in powders of silkworm (SwP) and mulberry (MulP).

Figure 2. Alfa-glucosidase inhibitory activity in powders of silkworm(SwP), mulberry (MulP) and green tea (GtP).
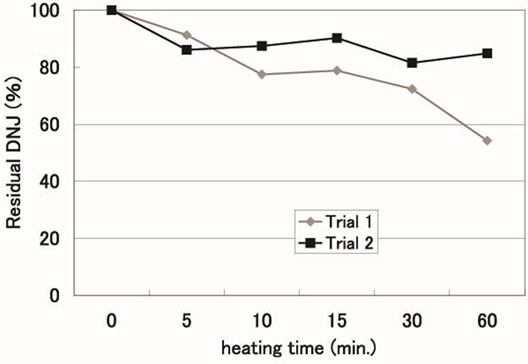
Figure 3. Heat stability of 1-1-deoxynojirimycin in Silkworm powder.
sured residual DNJ. The DNJ was relatively heat stable for up to 15 min. Therefore, sterilized silkworm powder, as a supplement raw material, has a DNJ content similar to that when not heated.
In conclusion, the use of silkworm powder as an antidiabetic CAM agent in Japan may also prevent influenza viral infections. This makes silkworm powder a promising CAM agent.
REFERENCES
- K.-S. Ryu, H.-S. Lee and I. Kim, “Effects and Mechanisms of Silkworm Powder as a Blood Glucose-Lowering Agent,” International Journal of Industrial Entomology, Vol. 4, 2002, pp. 93-100.
- T. Oku, M. Yamada, M. Nakamura, N. Sadamori and S. Nakamura, “Inhibitory Effects of Extractives from Leaves of Morus Alba on Human and Rat Small Intestinal Disaccharidase Activity,” British Journal of Nutrition, Vol. 95, No. 5, 2006, pp. 933-938. doi:10.1079/BJN20061746
- N. Asano, E. Tomioka, H. Kizu and K. Matsui, “Sugars with Nitrogen in the Ring Isolated from the Leaves of Morus Bombycis,” Carbohydrate Research, Vol. 253, 1994, pp. 235-245. doi:10.1016/0008-6215(94)80068-5
- N. Asano, T. Yamashita, K. Yasuda, K. Ikeda, H. Kizu, Y. Kameda, A. Kato, R.-J. Nash, H.-S. Lee and K.-S. Ryu, “Polyhydroxylated Alkaloids Isolated from Mulberry Trees (Morus Alba l.) and Ailkworms (Bombyx Mori l.),” Journal of Agricultural and Food Chemistry, Vol. 49, 2001, pp. 4208-4213. doi:10.1021/jf010567e
- T. Saito and I. Yamaguchi, “Effect of Glycosylation and Glucose Trimming Inhibitors on the Influenza Avirus Glycoproteins,” Journal of Veterinary Medical Science, Vol. 62, 2000, pp. 575-581. doi:10.1292/jvms.62.575
- Y. Tanaka, J. Kato, M. Kohara and M.-S. Galinski, “Antiviral Effects of Glycosylation and Glucose Trimming Inhibitors on Human Parainfluenza Virus Type 3,” Antiviral Research, Vol. 72, No. 1, 2006, pp. 1-9. doi:10.1016/j.antiviral.2006.03.016
- E. Bause, J. Schweden, A. Gross and B. Orthen, “Purification and Characterization of Trimming Glucosidase I from Pig Liver,” European Journal of Biochemistry, Vol. 183, No. 3, 1989, pp. 661-669. doi:10.1111/j.1432-1033.1989.tb21096.x
- J. Schweden and E. Bause, “Characterization of Trimming Man9-Mannosidase from Pig Liver,” Biochemistry Journal, Vol. 264, No. 2, 1989, pp. 347-355.
- K. Yatsunami, H. Ichida and S. Onodera, “The Relationship between 1-Deoxynojirimycin Content and α-Glucosi-dase Inhibitory Activity in Leaves of 276 Mulberry Cultivars (Morus Spp.) in Kyoto, Japan,” The Journal of Natural Medicines, Vol. 62, 2008, pp. 63-66. doi:10.1007/s11418-007-0185-0
- T. Anno, K. Tamura, H. Oono and H. Tomi, “Maltase, Sucrase and α-Amylase Inhibitory Activity of Morus Leaves Extract,” Food Preservation Sciences, Vol. 30, No. 5, 2004, pp. 223-229.
- T. Kimura, K. Nakagawa, Y. Saito, K. Yamaguchi, M. Suzuki, K. Yamaki, H. Shinmoto and T. Miyazawa, “Determination of 1-Deoxynojirimycin in Mulberry Leaves Using Hydrophilic Interaction Chromatography with Evaporative Light Scattering Detection,” Journal of Agricultural and Food Chemistry, Vol. 52, 2004, pp. 1415- 1419. doi:10.1021/jf0306901
- K. Murata, K. Yatsunami, E. Fukuda, S. Onodera, O. Mizukami, N. Suzuki and T. Kamei, “Effect of Propolis and Mulberry Leaf Extract (Quapolis Tm) on Type 2 Diabetes,” Food Function, Vol. 1, No. 1, 2005, pp. 26-30.
- K. Murata, K. Yatsunami, E. Fukuda, S. Onodera, O. Mizukami, G. Hoshino and K. Kamei, “Antihyperglycemic Effects of Propolis Mixed with Mulberry Leaf Extract on Patients with Type 2 Diabetes,” Alternative Therapies in Health and Medicine, Vol. 10, No. 3, 2004, pp. 78-79.
- K. Konno, H. Ono, M. Nakamura, K. Tateishi, C. Hirayama, Y. Tamura, M. Hattori, A. Koyama and K. Kohno, “Mulberry Latex Rich in Antidiabetic Sugar-Mimic Alkaloids Forces Dieting on Caterpillars,” Proceedings of National Academy of Sciences in USA, Vol. 103, 2006, pp. 1337-1341. doi:10.1073/pnas.0506944103
- N. Imanishi, Y. Tuji, Y. Katada, M. Maruhashi, S. Konosu, N. Mantani, K. Terasawa and H. Ochiai, “Additional Inhibitory Effect of Tea Extract on the Growth of Influenza A and B Viruses in MDCK Cells,” Microbiology and Immunology, Vol. 46, No. 7, 2002, pp. 491-494.
- K. Nakagawa, H. Kubota, T. Tsuzuki, J. Kariya, T. Kimura, S. Oikawa and T. Miyazawa, “Validation of an Ion Trap Tandem Mass Spectrometric Analysis of Mulberry 1-Deoxynojirimycin in Human Plasma: Application to Pharmacokinetic Studies,” Bioscience Biotechnology and Biochemistry, Vol. 72, No. 8, 2008, pp. 2210-2213. doi:10.1271/bbb.80200

