American Journal of Plant Sciences
Vol.5 No.17(2014), Article ID:48419,8 pages
DOI:10.4236/ajps.2014.517273
Drying, Storage and Osmotic Conditioning of Psidium guineense Swartz Seeds
Tathiana Elisa Masetto, Eliane Marques da Silva Neves, Silvana de Paula Quintão Scalon, Daiane Mugnol Dresch
Federal University of Grande Dourados in Dourados, Dourados, Brazil
Email: tmasetto@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

![]()

Received 27 May 2014; revised 29 June 2014; accepted 24 July 2014
ABSTRACT
This study aimed to evaluate the tolerance of Psidium guineense Swartz seeds to air-dry storage and priming. Desiccation tolerance was analyzed for seed moisture content of 15%, 10% and 5%. The longevity of seeds during storage was analyzed in seeds with 5% and 10% moisture content maintained in cold and dry chamber (16˚C ± 1˚C/40% RH), laboratory environment (25˚C ± 2˚C/60% RH), refrigerator (5˚C ± 1˚C) and freezer (−18˚C ± 1˚C) during 90 days, and the priming effect in seeds that were imbibed for 5 or 10 days in polyethylene glycol 6000 at osmotic potentials of −0.3 MPa, −0.5 MPa, −0.7 MPa and −1.3 MPa. The quality of seeds was evaluated after desiccation, storage, and priming by the seeds germination, fresh weight of seedlings, seedlings growth, and germination medium time. The experimental design was completely randomized, with four replications of 25 seeds each one. Although a reduction in seed germination and seedling growth was observed in seeds with 10% and 5% moisture content, drying did not cause complete loss of seed germination and seedling development. Sour guava seeds exhibited an orthodox response to desiccation tolerance and storage. Seed germination and seedling growth were reduced with increased osmotic potential of osmoconditioning. However, conditioning of 10 days increased the seeds germination and optimized the average germination time to 20 days. The seeds of Psidium guineense are able to tolerate desiccation and storage for up to 90 days at a temperature of 5˚C ± 1˚C and priming for 10 days is a promising technique for propagation of P. guineense.
Keywords:Myrtaceae, Brazilian Savannah, Longevity, Polyethylene Glycol

1. Introduction
The Myrtaceae Family includes several species of fruit-trees and shrubs including the genus Psidium and its species P. guineense Swartz that are native to the Savannah ecoregion in Brazil. Psidium guineense is commonly known as sour guava, and its raw fruits have a potential economic value. Its raw fruits are high in vitamin C content, up to four times more than citrus fruit, and the tree itself has a high fruiting capacity and high resistance to diseases and pests. The species is widely distributed, indicating species capability to adapt to diverse environments. Psidium guineense is a shrub about 3 m tall, very similar to the common guava, with inflorescences covered with reddish-brown trichomes during early growth, ranging from gray to yellowish; its fruits are oval to oblong, with yellow skin and clear and mucilaginous pulp. The dispersal is predominantly by seed, and vegetative propagation was not successful [1] .
A successful implementation of programs for restoration and revitalization of Brazilian Savannah areas requires detailed knowledge of factors affecting the survival and initial growth of seedlings in a nursery as well as in the field [2] . Studies of desiccation tolerance and seed storage of native species are important [3] , because they will help to preserve the seed quality until its sowing, assist the maintenance of the germplasm banks, and are valuable in the restocking of vegetation. If the seeds are intended for long-term storage, the period of their survival or their longevity is the most important feature [4] . In this context, information about the level of seed desiccation tolerance, the conditions of storage environment, and storage duration has both ecological and economic importance.
The storage capacity of many species is increased when the reduction in water content of the seeds is associated with a reduction of the environmental temperature [5] . However, some species do not tolerate sharp reductions in temperature due to the damage caused by freezing (−18˚C), which leads to a loss of viability [6] [7] . However, the success of seed storage depends on knowledge regarding the behavior of seeds during the process, which enables the use of appropriate conditions that maintain their viability [8] .
Seed germination begins with the uptake of water and ends with radicle protrusion. It depends on external factors such as humidity, light, temperature, and oxygen. In species with slow growth and development, as is the case with the majority of native Brazilian Savannah species, various methods are used to accelerate seedling establishment. Certain techniques such as priming are developed to increase the speed and uniformity of germination and seedling emergence. Priming consists of immersion of the seeds in an osmotic solution under predetermined temperature and for a length of time. The effects of priming provide greater uniformity and timing of germination, higher rate of emergence and seedling development, higher rate of shoot growth, and faster ripening.
Given the importance of sour guava in the regional economy and the need for development of techniques that will maximize its growth and development, this study aimed to evaluate the tolerance to desiccation, storage, and the effect of osmotic priming in P. guineense.
2. Materials and Methods
Fruits of P. guineense were collected from 10 trees located in Sitio Sao Francisco de Assis (21˚52'28.1"S and 54˚38'51.9"W), in the city of Rio Brilhante-MS, during the first fortnight of September 2009.
After collection, the fruits were brought to the Laboratory of Nutrition and Metabolism of Plants, Federal University of Grande Dourados in Dourados, MS, Brazil. The fruits were washed in running water and the damaged fruits were discarded. Subsequently, the fruits were processed manually to separate the seeds.
To determine the water content of seeds, four replications of 20 seeds each were dried in the oven at 105˚C ± 2˚C for 24 h. The results were expressed as percentage of weight of fresh seeds. For the germination test, the seeds were extracted from fresh fruits, immersed in 1% solution of sodium hypochlorite for 5 min, rinsed in tap water, and sown in plastic boxes lined with two sheets of paper Germitest®, previously humidified with distilled water and maintained in B.O.D (Biochemical Oxygen Demand) at 25˚C under constant white light. Three different experiments were conducted.
Experiment I—Desiccation tolerance: To evaluate the level of desiccation tolerance in seeds, the moisture level in seeds was reduced in five percentage points. The desired levels of humidity were obtained as follows: recently collected seeds were divided into sub-samples, and in a single layer subjected to slow drying at room temperature (25˚C ± 2˚C at 60% relative humidity (RH) for various lengths of time until reaching the desired moisture level (15%, 10%, and 5% moisture content). Successive weighing was performed until the weight coincided with the desired moisture content. Upon reaching the predetermined amount of water, seeds were disinfected in 1% sodium hypochlorite, rinsed in tap water, and sown on Germitest® following the aforementioned protocol.
Experiment II―Storage: Seeds with 10% and 5% water content were placed in transparent plastic bags with a thickness of 0.25 mm and kept under storage conditions: cold and dry chamber (16˚C ± 1˚C/40% RH), laboratory environment (25˚C ± 2˚C/60% RH), refrigerator (5˚C ± 1˚C) and freezer (−18˚C ± 1˚C) during 90 days.
Experiment III—Priming: The effect of priming on germination was studied using polyethylene glycol 6000 (PEG) as osmoticum. Solutions of PEG with osmotic potential of −0.3, −0.5, −0.7, −1.3 MPa, and a control (0 MPa) were prepared of according [9] . Initially, two sets of seeds were placed in a single layer in Petri dishes filled with moistened paper Germitest® and soaked with 12 mL of PEG 6000 for 5 or 10 days. After this soaking period, the seeds were washed in running water to remove the solution, and then dried until the initial seeds moisture content. The germination tests were performed following the protocol described above.
In all three experiments, the quality of seeds was evaluated after desiccation, storage, and priming by the number of germinated seeds (germination), fresh weight of seedlings, seedlings growth, and germination medium time. Germination was measured on Germitest® paper rolls with four replications of 25 seeds each, germinated with B.O.D. (Biochemical Oxygen Demand) at 25˚C under continuous white light. Assessments were conducted daily, and the root was considered protruded when it reached a length of 5 mm. The results were expressed in percentages (%). Fresh weights of seedlings were obtained by weighing the shoots and roots of 10 normal seedlings on an analytical balance, and the results were expressed in g∙seedling−1. Seedling growth was evaluated from the length of primary roots and shoots in 10 randomly chosen normal seedlings. The measurements were done with a millimeter ruler and the results were expressed in centimeters. Germination time was evaluated during the germination test by counting the number of germinated seeds in each repetition. The mean germination time (MGT) is the average time required for germination of seeds expressed in days. It was calculated using the equation cited by [10] .
The experimental design was completely randomized, with four replications of 25 seeds each. In the experiment I, three levels of seeds moisture content were evaluated; in the experiment II, two levels of seeds moisture content and four storage conditions and in the experiment III, two soaking periods and four concentrations of PEG were used. Data were analyzed using SISVAR: the analysis of variance with F test at 5% probability and significance level; the means were compared by Tukey’s test (to assess tolerance to desiccation and storage), and regression analysis (to assess the effect of osmotic conditioning).
3. Results and Discussion
3.1. Desiccation Tolerance
A significant difference in the seed moisture content was observed for all analyzed variables, except the fresh aerial mass and mean germination time (Table 1). Seeds with 15% moisture content had higher germination rate, root fresh weight and length compared to the seeds with 5% and 10% moisture content, which did not show significant difference. However, in seeds containing 15% moisture content, the aerial length was shorter than in seeds with 10% and 5% moisture content (Table 1). These results suggest that although the seed drying was negatively affected, the aerial part of seedlings showed higher tolerance to drying than the root length.
Although a reduction in seed germination and seedling growth was observed in seeds with 10% and 5% moisture content, drying did not cause complete loss of seed germination and seedling development (Table 1) suggesting that the seeds of P. guineense are tolerant to desiccation. The orthodox seeds (seeds that tolerate dehydration and are storable in this condition) remain viable until around 5% moisture content and tolerance is progressively acquired during its development before suffering a severe drop in water content [11] .
Seed desiccation tolerance can be mediated by protective systems that prevent fatal damage to various cellular components such as membrane proteins and the cytoplasm. Three major systems have been characterized: the accumulation of non-reducing sugars, the ability to prevent, tolerate, or repair free radical attacks, and the presence of LEA proteins (late embryogenesis abundant proteins) [11] -[14] . Possibly, at least one of these protective mechanisms is present or acting in synergy to the maintenance of seed germination and seedling growth in P. guineense after drying to 5% moisture content during storage.
Seeds of P. guineense with moisture content of 5% and 10% after storage for 90 days showed changes in their water levels. Among the tested environments, the conditions of refrigeration and freezing provided less variation in moisture content of seeds stored for 5% and 10%, respectively (Table 2). However, for seeds of P. guineense when stored in a freezer (−20˚C and 90% RH) wrapped in craft paper had moisture content of around 11.74%
Table 1. Germination percentage (G), root fresh mass (RFM), aerial part fresh mass (APFM), root length (RL), aerial part length (APL) and mean germination time (MGT) of seeds of Psidium guineense Swartz with different moisture contents.
*Means followed by the same letter in the column do not differ significantly by Tukey test at 5% probability.
Table 2. Moisture content of Psidium guineense Swartz seeds after storage in different environments during 90 days.
which indicated that the packaging permitted the replacement of water with the medium [1] . Because seeds are hygroscopic, there is a tendency towards the change in their moisture content during the storage in humid environments. Possibly, the use of airtight plastic containers will maintain the moisture content of seeds at the same level. For seeds of other species in the family Myrtaceae, such as Myrcia palustris DC. and M. glabra (O. Berg) Legrand D., there was no significant difference in moisture content after 150 days kept in a cold storage (5˚C ± 1˚C and 80% RH) [15] .
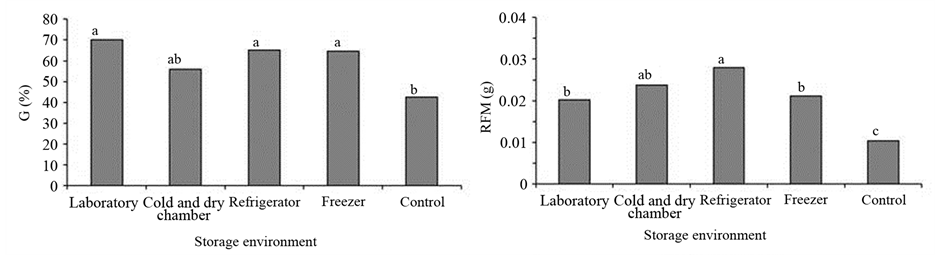
Significant effects of storage conditions on germination, fresh weight of roots and shoots, and the length of primary roots and shoots in P. guineense (Figures 1(a)-(e), respectively) were observed. Seeds that were stored in all tested conditions showed increased germination percentage compared to the control group. Germination of 70% was observed in seeds stored in the laboratory, independent of moisture content (Figure 1(a)), indicating the positive effects of storage on seeds vigor. Similar results were found in P. guineense who reported that the lab environment (26.5˚C ± 1˚C and 69.9% to 74.8% RH) was the most suitable for storage of seeds, although the initial twinning was 60% and the germination of the seeds during storage in such environment gradually decreased, dropping to 47% at 180 days [1] .
Increase in germination (Figure 1(a)), root fresh weight (Figure 1(b)) and root length (Figure 1(d)) in seedlings indicates the seeds of P. guineense are tolerant to the reduction of moisture content and storage. However, it was found that the tested storage conditions hampered the shoot growth, as seen by the reduction in fresh weight, except for the refrigeration environment (Figure 1(c)) and by the reduction in shoot length (Figure 1(e)). There was a significant effect of the seed moisture content on the fresh weight and seedling length (Table 3). Seeds with 5% moisture content after storage scored higher fresh weight and shoot length, whereas the opposite effect was observed in the root mass and root length, which were higher in seeds stored with 10% moisture content. These results indicate a response to desiccation tolerance associated with the storage of seeds.
There was significant interaction between the storage site and the moisture content of seeds and the germination time (Table 4). Seeds with 5% and 10% moisture content that were not subjected to storage showed the lowest MGT, and the seeds with 10% moisture content had higher MGT than seeds with 5%. The greatest MGT was observed in seeds with 5% moisture content stored in a cool and dry chamber (Table 4). These results are similar to those observed in seeds of Myrcianthes pungens (Berg) Legrand that showed an increase in MGT and loss of vigor after the third month in storage in a cold chamber (5˚C ± 1˚C and 80% RH) [16] .
Moreover, in seeds of P. guineense showed faster germination when stored under normal laboratory environment than the seeds stored in a freezer [1] . This is probably due to the fact that during the storage in a normal laboratory environment, the process of seed deterioration is slower and more gradual, which may have prevented severe damage and a sharp decline in the seed germination.
3.2. Priming
Significant effects of conditioning times on germination, fresh weight, and root length were observed (Table 5). Additionally, we observed significant effect of osmotic potential on the traits studied, with the exception of the germination of primed seeds. An increase in germination, root fresh weight and root length were observed in seeds that remained soaked for 10 days regardless of the concentrations of PEG 6000 (Table 5) suggesting that
 (a)
(a)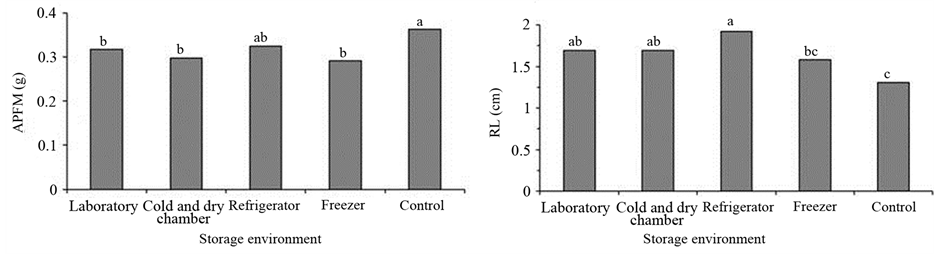 (b)
(b) (c)
(c)
Figure 1. (a) Germination (G); (b) Root fresh mass (RFM); (c) Aerial part fresh mass (APFM); (d) Root length (RL); (e) Aerial part length (APL) according to the storage environment of Psidium guineense Swartz seeds. Means followed by the same letter are not significantly different by Tukey test at 5% of probability.
Table 3. Root fresh mass (RFM), aerial part fresh mass (APFM), root length (RL) and aerial part length (APL) of seeds of Psidium guineense Swartz storaged with different moisture contents during 90 days.
*Means followed by the same letter in the column do not differ significantly by F test at 5% probability.
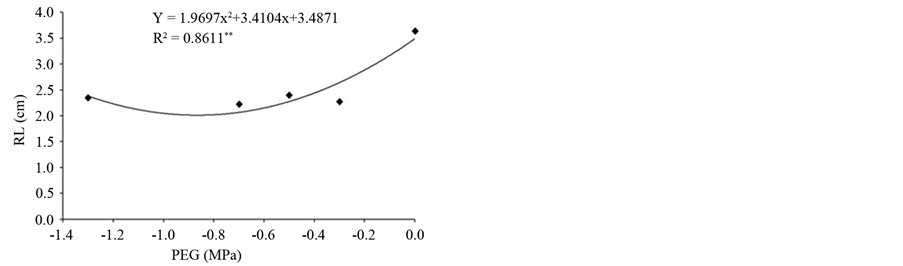
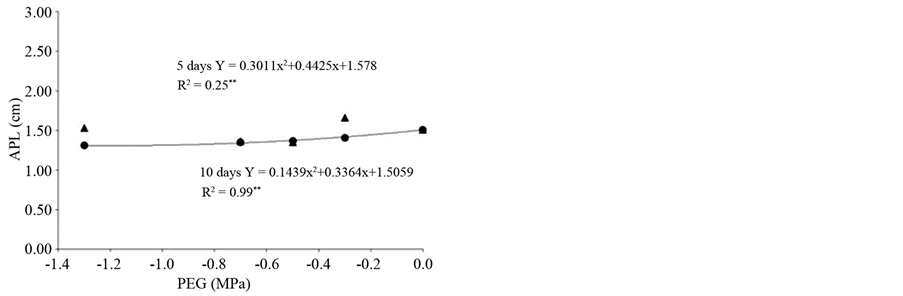
longer imbibition favored germination response and root growth. However, with increasing concentration of osmotic solutions root growth was reduced and reached a minimum (2.01 cm) at −0.87 MPa (Figure 2(a)) indicating that the increase in PEG conditioning impaired the formation of the root system in plants.
The average time of germination of primed seeds was significantly reduced across the different priming conditions and reached a minimum value of 21 days at −0.87 MPa. It is noteworthy that the unprimed seeds initiated germination only at 35 days after sowing, with the average germination time of 47 days (Figure 2(b)). Previous studies indicated that priming is a technique that, in addition to increasing the germination rate, it also reduces the average germination time. Thus, pre-soaking of seeds is enough to activate the metabolism, but insufficient to allow primary root protrusion.
There was a significant interaction between soaking times and concentrations of PEG and the length (Figure 3(a)) and fresh weight of shoots (Figure 3(b)). Although no regression adjustment in fresh mass was observed, the priming of seeds for 10 days resulted in higher values for measured characteristics than the 5 days conditioning for all solutions (Figure 3(b)). The aerial part length had adjusted regression curve only for the soaking time of 10 days and reached a minimum value (1.30 cm) at −1.17 MPa (Figure 3(a)). In their study of other brazilian savannah species, observed no interaction between the priming treatments and conditioning time in seeds of Stryphnodendron adstringens (Mart.) Coville and S. polyphyllum Mart. that were conditioned at −0.5 MPa. In these species, conditioning increased the percentage and germination rate [17] . In seeds of P. guineense, only the 10 days priming significantly increased the germination rate. However, priming was effective in reducing the average germination time by approximately 50%, thereby providing an effective technology for optimization of the propagation in this plant species.
Table 4. Mean germination time (MGT) (days) of seeds of Psidium guineense Swartz storaged with two moisture contents in different environments.
*Means followed by the same lower case letters in a column and capital letters on the lines do not differ significantly to the level of 5% probability.
Table 5. Germination percentage (G), root fresh mass (RFM) and root length (CL) of seeds of Psidium guineense Swartz according to the imbibition time in polyethylene glycol 6000.
*Means followed by the same letter in the column do not differ significantly by F test at 5% probability.
 (a)
(a)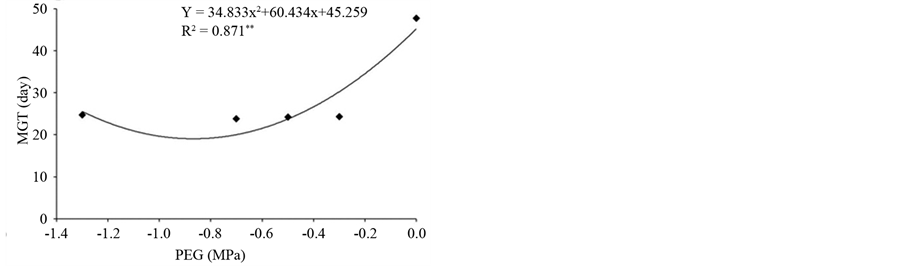 (b)
(b)
Figure 2. (a) Root length (RL); (b) Mean germination time (MGT) of osmoconditioned seeds of Psidium guineense Swartz at different osmotic potentials (MPa).
 (a)
(a)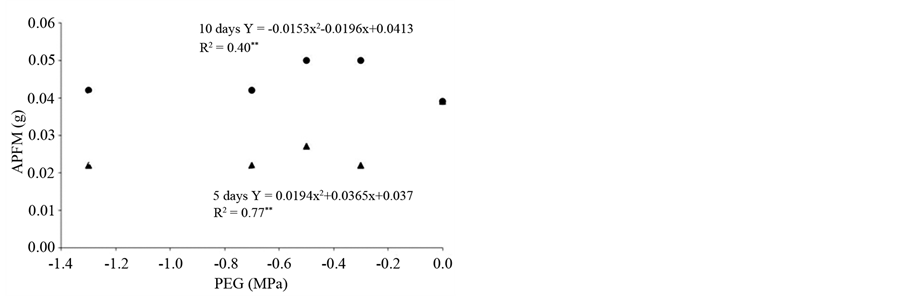 (b)
(b)
Figure 3. (a) Aerial part length (APL); (b) Aerial part fresh mass (APFM) of seedlings of Psidium guineense Swartz derived from osmoconditioned seeds at different osmotic potentials (MPa) and imbibitions times (days).
4. Conclusions
The seeds of Psidium guineense are able to tolerate desiccation and storage for up to 90 days at a temperature of 5˚C ± 1˚C. The seeds with 5% water content maintained the optimal germination and seedling growth.
Priming for 10 days increases the percentage of seed germination, root growth, and reduces by half the mean germination time. Therefore, it constitutes a promising technique for propagation of P. guineense.
Acknowledgements
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa Nacional de Pós-Doutorado (PNPD/CAPES—Projeto 2673/2011).
References
- Cisneiros, R.A., Matos, V.P., Lemos, M.A., Reis, O.V. and Queiroz, R.M. (2003) Physiological Quality of Seeds of Psidium Guineens Swartz during Storage. Revista Brasileira de Engenharia Agrícola e Ambiental, 7, 513-518. http://dx.doi.org/10.1590/S1415-43662003000300018
- Fonseca, E.P., Valeri, S.V., Migliorança, E., Fonseca, N.A.N. and Couto, L. (2002) Target Seedlings of Trema micrantha (L.) Blume Grown Under Different Periods of Shading. Journal of Brazilian Forest Science, 26, 515-523. http://dx.doi.org/10.1590/S0100-67622002000400015
- Kohoma, S., Maluf, A.M., Bilia, D.A.C. and Barbedo, C.J. (2006) Drying and Storage of Eugenia brasiliensis Lam. (“Grumixameira”) Seeds. Journal of Seed Science, 28, 72-78. http://dx.doi.org/10.1590/S0101-31222006000100010
- Hay, F.R., Smith, R.D., Ellis, R.H. and Butler, L.H. (2010) Developmental Changes in the Germinability, Desiccation Tolerance, Hardseededness, and Longevity of Individual Seeds of Trifolium ambiguum. Annals of Botany, 105, 1035-1052. www.ncbi.nlm.nih.gov/pubmed/20228084
- Walters, C. (1998) Understanding the Mechanisms and Kinetics of Seed Aging. Seed Science Research, 8, 223-244. http://dx.doi.org/10.1017/S096025850000413
- Chin, H.F., Krishnapillay, B. and Stanwood, P.C. (1989) Seed Moisture: Recalcitrant vs. Orthodox Seeds. In: Stanwood, P.C. and Mcdonald, M.B., Eds., Seed Moisture, Crop Science Society of America, Madison, 15-22.
- Fonseca, S.C.L. and Freire, H.B. (2003) Recalcitrants Seeds: Post-Harvest Problems. Bragantia, 62, 297-303. http://dx.doi.org/10.1590/S0006-87052003000200016
- Hong, T.D. and Ellis, R.H. (1996) A Protocol to Determine Seed Storage Behavior. International Plant Genetic Resources Institute, Rome, (IPGRI. Technical Bulletin, 1), 55 p.
- Michel, B.E. and Kaufmann, M.R. (1973) The Osmotic Potential of Poliethylene Glycol 6000. Plant Physiology, 51, 914-916. http://dx.doi.org/10.1104/pp.51.5.914
- Ranal, M.A. and Santana, D.G. (2006) How and Why to Measure the Germination Process? Brazilian Journal of Botany, 29, 1-11. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-84042006000100002
- Berjak, P. and Pammenter, N.W. (2008) From Avicennia to Zizania: Seed Recalcitrance in Perspective. Annals of Botany, 101, 213-228. http://doi.10.1093/aob/mcm168
- Guimarães, R.M., Vieira, M.G.G.C.; Fraga, A.C., Von Pinho, E.V.R. and Ferraz, V.P. (2002) Desiccation Tolerance in Coffee Seeds (Coffea arabica L.). Ciência e agrotecnologia, 26, 128-139. http://www.editora.ufla.br/site/_adm/upload/revista/26-1-2002_15.pdf
- Jia H., Suzuki, M. and Mccarty, D.R. (2014) Regulation of the Seed to Seedling Developmental Phase Transition by the LAFL and VAL Transcription Factor Networks. Developmental Biology, 3, 135-145. http://dx.doi.org/10.1002/wdev.126
- Maia, J., Dekkers, B.J., Dolle, M.J., Ligterink, W. and Hilhorst, H.W. (2014) Abscisic Acid (ABA) Sensitivity Regulates Desiccation Tolerance in Germinated Arabidopsis Seeds. New Phytologist, 203, 81-93. http://dx.doi.org/10.1111/nph.12785
- Leonhardt, C., Calil, A.C. and Fior, C.S. (2010) Myrcia glabra (O. Berg) D. Legrand and Myrcia palustris DC.— Myrtaceae Seed Germination in Cold Chamber Storage. IHERINGIA—Série Botanica, 65, 25-33. http://www.fzb.rs.gov.br/publicacoes/iheringia-botanica/Ih65-1-p025-034.pdf
- Fior, C.S., Rodrigues, L.R., Calil, A.C., Leonhardt, C., Souza, L.S. and Silva, V.S. (2010) Physiological Quality of Guabijuzeiro (Myrcianthes pungens (Berg) Legrand—Myrtaceae) Seeds Under Storage. Journal of Brazilian Forest Science, 34, 435-442. http://dx.doi.org/10.1590/S0100-67622010000300007
- Kissmann, C., Scalon, S.P.Q., Mota, L.H.S. and Vieira, M.C. (2010) Germination of Primed Seeds of Stryphnodendron Mart. Journal of Seed Science, 32, 26-35. http://dx.doi.org/10.1590/S0101-31222010000200003


