American Journal of Plant Sciences
Vol.5 No.9(2014), Article ID:44517,11 pages DOI:10.4236/ajps.2014.59129
Morphometry of Lipid Bodies in Embryo, Kernel and Mesocarp of Oil Palm: Its Relationship to Yield
Li Sim Ho, Anusha Nair, Hirzun Mohd Yusof, Harikrishna Kulaveerasingam, Mohamad Sanusi Jangi
Sime Darby Technology Centre Sdn Bhd, Sime Darby Plantation, Petaling Jaya, Malaysia
Email: ho.li.sim@simedarby.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 23 January 2014; revised 7 March 2014; accepted 19 March 2014
Abstract
Oil palm drupe which has thick fleshy mesocarp contains rich oil, where storage of oil in it can make up to 80% of its dry mass [1] . Ongoing research interest in oil palms has been focused on the mechanisms of oil production in oil palm drupes, while investigation on the ultrastructural morphometry of its oil storage entity, namely lipid body, has received limited attention. By employing microscopy techniques, this study investigated the morphometric of lipid bodies in the mesocarp, kernel and embryo of oil palms drupes at 22 week-after-anthesis (WAA). This study also investigated the relationship between the size of lipid bodies and oil yield of oil palms at the same state of maturity (22 WAA). The structural arrangements of lipid bodies were found to be different among embryo, kernel and mesocarp of oil palms. It was also found that the size of lipid bodies of mesocarp of oil palms was larger compared to other crops. Lipid bodies in both embryo and mesocarp were statistically shown to be larger in low oil-yielding palms.
Keywords:Oil Palm; Lipid Body; Oil Yield; Microscopy

1. Introduction
Lipid bodies are intracellular organelles comprising a matrix of triacylglycerol (TAG) surrounded by phospholipids monolayer embedded and covered with unique proteins, namely oleosin, caleosin and steroleosin [2] . They are often termed as oil bodies, lipid droplets, oil globules, oleosomes and spherosomes [3] . Lipid bodies are found in leaf [4] , embryo and cotyledon of plants and serve as energy supply during seed germination and early stages of growth [5] .
In general, seed lipid bodies are small and spherical in shape with diameters ranging from 0.5 - 2.5 µm. The size of the lipid bodies differs among plant species [6] and is also affected by nutrient supply and the environment [7] . Research has shown that lipid content and the size of lipid bodies are correlated, and the size of lipid bodies is controlled by the quantity of oleosin. Lipid bodies resist coalescence and remain small individual units, even during the final stages of seed maturation where desiccation occurs [8] .
Researches on lipid bodies had focused on the lipid bodies of seed origins as well as their related proteins and function [3] [7] [9] -[12] . Not only is the lipid-rich fleshy mesocarp tissue of the oil palm (Elaeis guineensis) drupes the main source of edible oil for the world, but it is also the richest dietary source of provitamin A, yet the lipid bodies of oil palm have received less scientific attention than other oil crops like Arabidopsis spp. and Zea mays.
This study examined the morphometry of the lipid bodies in different tissue of the fruit of oil palm by transmission electron microscope (TEM), confocal laser scanning microscope (CLSM) and light microscope (LM). Furthermore, it compared the differences in the morphometry of lipid bodies between predetermined high and low oil-yielding palms [13] .
2. Material and Methods
2.1. Plant material
Commercial crosses of oil palm, namely tenera, which are hybrid of Serdang Avenue dura and AVROS psifera were used in this study. These palms are cultivated in an oil palm plantation in Carey Island, Selangor, Malaysia, which is managed by Sime Darby Berhad (Kuala Lumpur, Malaysia).
2.2. Structural Examination
Drupes of oil palms (Elaeis guineensis) were harvested at 22 weeks after anthesis (WAA) for structural studies at maturation state. Mesocarp tissue was excised carefully with a razor blade. Kernel tissues and embryos were extracted from seed. The excised tissues were subjected to samples preparation process as described below.
2.3. Investigation of Relationship between Size of Lipid Bodies and Oil Yield
It was shown in a preliminary study (data not shown) that only lipid bodies of drupes from the proximal region exhibited differences of size when comparing between high oil and low oil-yielding palms, while drupes from the central and distal region did not show any differences (Figure 1). Thus, in the present study, only oil palm drupes at 22 WAA from the proximal region of the fresh fruit bunch (FFB) were used for further validation. For statistical validation, twelve oil palm trees were selected for the study. Six were categorized as high oil-yielding (Hy) and the other six as low oil-yielding (Ly). The Hy palms were identified by their relatively high oil yield with an average of 78.1 kg of palm oil per palm per year, over the past 7 years. In contrast, the Ly trees yielded an average of only 40.5 kg of palm oil per palm per year. Mesocarp tissues of oil palm drupes were excised and subjected to sample preparation process as described below.
2.4. Samples Preparation
Transmission electron microscopy (TEM, Hitachi H7100), light microscopy (Olympus, BX 51) and confocal laser scanning microscope (CLSM, Leica TCS P5) were employed to examine the morphometrics of the lipid bodies in embryo and mesocarp of oil palm. Tissue pieces of 1 - 2 mm3 were fixed in 4% glutaraldehyde, 0.01 M sodium cacodylate buffer (pH7.0) for 48 hours in vacuum at room temperature and washed three times in 0.01 M sodium cacodylate buffer. These samples were then post-fixed in 0.1% osmium tetraoxide in 0.01 M sodium cacodylate buffer for 2 hours and washed three times in 0.01 M sodium cacodylate buffer. Washed tissue samples were dehydrated through a graded acetone series, and then infiltrated and embedded in epoxy resin. For TEM observation, ultrathin sections of tissues with 60 - 90 nm thickness were stained with uranyl acetate for 15 minutes, followed by lead stain for 10 minutes. For light microscopy, semithin (1 - 2 µm) sections of mesocarp tissues were prepared and stained with toluidine blue, while for CLSM, samples were stained with LipidTOXTM Red Neutral Lipid stain. The ultrastructure and the size of lipid bodies in mesocarp and embryo were measured
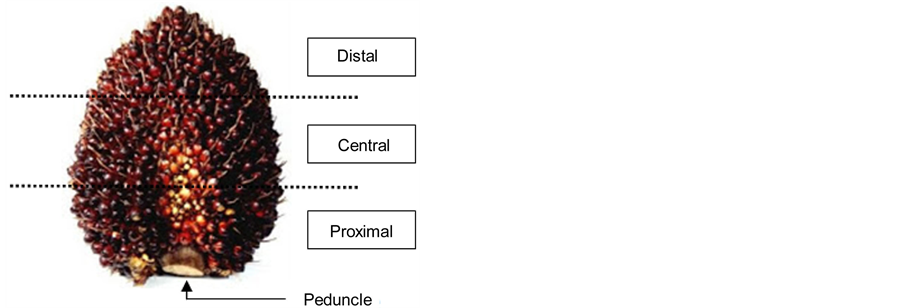
Figure 1. Fresh fruit bunch of oil palms. Drupes harvested from the proximal region were used in the study.
using the Analysis Five (Olympus) imaging software.
2.5. Statistical Analysis and Comparison of Lipid Bodies in High and Low Oil-Yielding Palms
For mesocarp lipid bodies, diameter of lipid bodies was measured for 31 randomly selected cells, from randomly selected oil palm drupes (from the proximal region) of each of the selected palms. Number of lipid bodies contained within a single cells as well as the size of the cells were also recorded. As for embryo lipid bodies, due to the small size of the lipid bodies, large size range of embryo cells (data not shown) and the micro-scaled observation field of the transmission electron microscope, lipid bodies were sampled from three randomly picked observation fields (with a defined area, µm2) for diameter measurement. Student’s t-test and Spearman’s Rank Correlation were performed using SSPS.
3. Results
3.1. Morphology of Lipid Bodies in Mesocarp, Kernel and Embryo of Oil Palms
It was found that the cells of mesocarp, kernel and embryo showed very different sub-cellular structure and position in the mother cell (Figure 2). The mesocarp parenchyma cells of oil palm are fully packed with lipid bodies with few small protein bodies (stained solid blue) embedded among the lipid bodies (Figures 2(a)-(c)). In kernel, cytoplasmic spaces were fully packed with lipid bodies and large storage vacuoles (Figure 2(d) and Figure 2(e)).
While in the embryo, lipid bodies are deposited along the periphery of cell and between sub-cellular organelle like protein bodies (Figure 2(f)). The lipid bodies found in mesocarp and embryo are generally spherical in shape. However, in kernel, the lipid bodies observed had irregular geometry which appeared fitting into available spaces (Figure 2(d) and Figure 2(e)). It was observed that the monolayer phospholipid membrane surrounding the lipid bodies appeared to have different morphology among the three different tissue types. Discontinuity of membrane was obvious for lipid body of mesocarp (Figure 2(b)) while the membrane of lipid body of kernel appeared to remain intact (Figure 2(d)). On the other hand, the membrane of the embryo lipid bodies were indefinable even at 60 k times magnification (Figure 2(g)) where neither a full unit membrane nor a half membrane was readily apparent.
For determination of lipid body sizes, ‘size’ was defined as the longest axis across the lipid body globules. In order to eliminate technical error, all measurements were done by a single individual. A total of 5243 mesocarp lipid bodies from 341 cells of 12 biological samples were examined. The sizes of mesocarp lipid bodies were found to be in the range of 4.09 - 31.77 µm, with a median of 10.86 µm (Figure 3). The observation of size of lipid bodies for kernel tissue was based on single biological sample, where a total of 154 lipid bodies were examined. It was found that the size of lipid bodies of kernel range between 4.47 - 24.68 µm with a median of 10.27 µm. Distribution curve was not plotted for lipid bodies of kernel due to the small data size (only 154 lipid bodies were observed).
On the other hand, examination and measurement of lipid bodies of embryonic cells were done under TEM
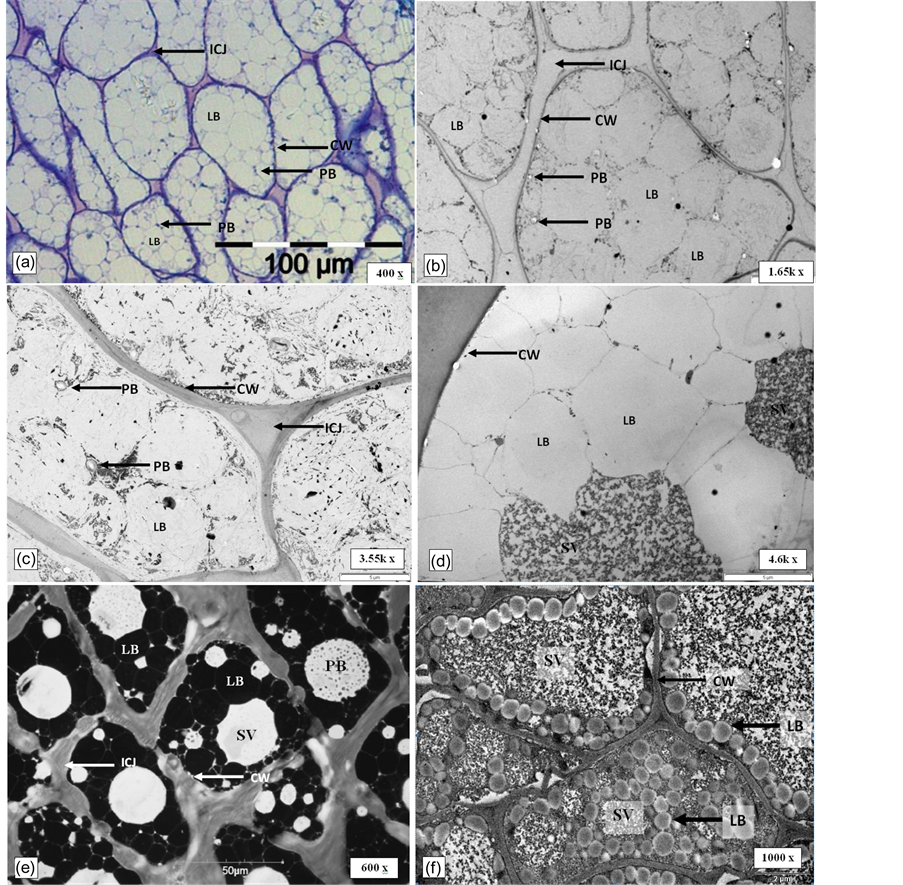
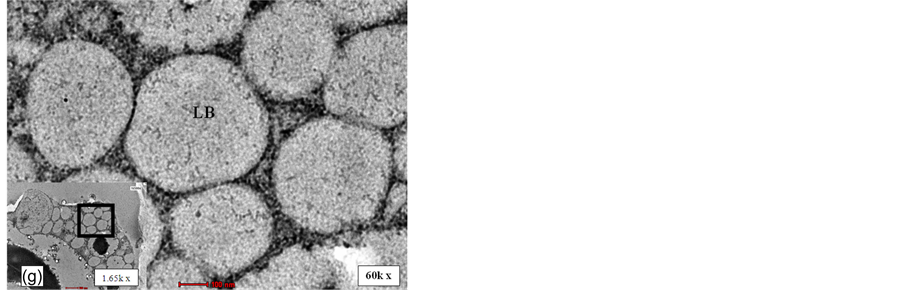
Figure 2. Distinct ultra-structure of different tissues of oil palm drupelets. (a): LM image for mesocarp tissue of oil palm (400 X). (b) and (c): TEM micrograph of mesocarp (1.65 and 3.55 kX). (d): TEM micrograph of kernel of oil palm (4.6 kX). (e): CLSE micrograph of kernel of oil palm (600 X). (f): TEM micrograph of embryo of oil palm (1000 X). (g): TEM micrograph of lipid bodies of embryo (60 kX). Bold square box was which the observation area enlarged for details examination (1.65 kX). LB: lipid body; SV: Storage vacuole; PB: Protein body; CW: Cell wall; V: vacuole; ICJ: intercellular junctions.
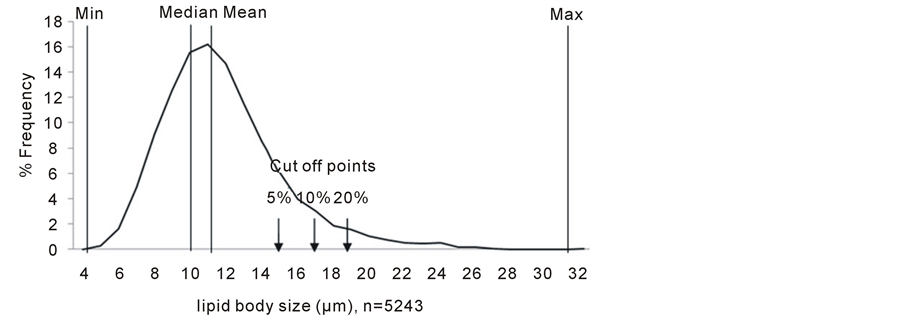
Figure 3. Distribution of mesocarp lipid bodies. A total of 5243 lipid bodies of mesocarp were examined. Mean: 11.34 µm; Max: 31.77 µm; Min: 4.09 µm: Median: 10.86 µm..
with very high resolution. Under each observation field, only a portion of intact cell can be seen. Hence, the total number of embryonic cells that were observed was disregarded. 2402 lipid bodies from 12 biological samples revealed that the sizes of embryonic lipid bodies were found to be ranged from 0.18 - 4.71 µm with a median of 0.68 µm (Figure 4).
3.2. Lipid Bodies in High and Low Oil-Yielding Palms
For the study of lipid bodies size of mesocarp tissue of oil palms, of the twelve palms examined in the present study, one outlier was detected statistically and was excluded in the following analysis and discussion. A total of 2854 and 2389 lipid bodies were observed for five high and six low oil-yielding palms respectively. Figure 5 illustrates the distribution of the size of mesocarp lipid bodies in high and low oil-yielding palms.
Based on Student’s t-test, it was found that the mean of the size of low oil and high oil-yielding was significantly different at 95% confident level (p < 0.05) and recorded 14.48% difference, where low oil-yielding palms has larger lipid bodies (mean = 12.17 µm) compared to high oil-yielding palms (mean = 10.63 µm).
In addition to that, correlation analyses were also performed. Three different yield traits were used in the Spearman’s Rank Correlation analyses, i.e., oil per palm per year (O/P; Kg), oil per dry mesocarp mass (O/DM; %) and mesocarp mass per drupe mass (M/F; %). Data for the respective yield traits were average of data collected over seven years (data not shown). For mesocarp lipid body size, average of each individual replicate was used in this analysis. The analyses have shown that in all three cases, mesocarp lipid body size is negatively correlated to oil yield (Table 1). These results again support the observation where high oil-yielding palms possess smaller size lipid bodies and vice versa.
While for lipid bodies in embryo, 1396 and 1243 lipid bodies were sampled from embryonic cells of six high and six low oil-yielding palms respectively. Distribution of size of lipid bodies in embryo tissue of high and low oil-yielding palms was presented in figure 6.
The mean of size of lipid bodies in embryo tissue of high oil-yielding palms and low oil-yielding palms was compared by Student’s t-test. P value of 0.0051 at 95% confidence level indicating significant different between means of lipid bodies in embryo tissue from the two different oil palm groups, where lipid bodies in embryo tissue of low oil-yielding palms (mean = 0.74 µm) are larger than lipid bodies in high oil-yielding palms (mean = 0.71 µm) by 4.2%.
3.3. Extra Large Lipid Droplets in Oil Palm Mesocarp
We also looked into lipid bodies of mesocarp with extra large size in high and low oil-yielding palms. We defined lipid droplets with a diameter larger than two times mean (mean = 11.34 µm), 22.68 µm as extra large lipid droplets. Overall, extra large lipid bodies accounted for 0.99% of all 5243 lipid bodies observed. When looking into the palms based on oil yielding capacity, extra large lipid bodies occurred at an average of 0.32% and 1.80% in high and low oil-yielding palms respectively (Figure 7). Frequency of occurrence of extra large
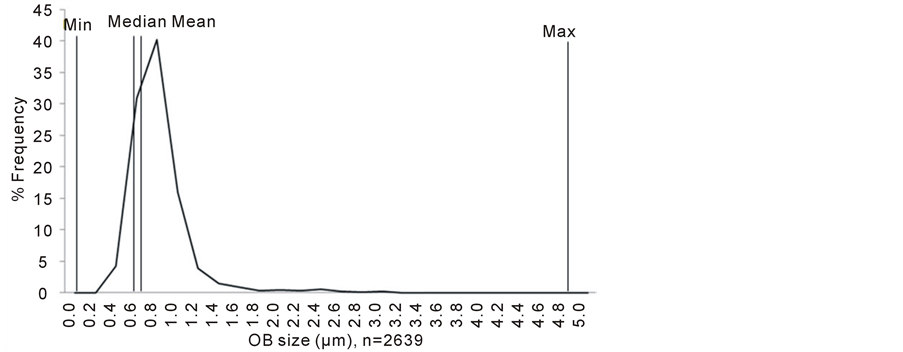
Figure 4. Distribution of embryonic lipid bodies. A total of 2639 lipid bodies of mesocarp were examined. Mean: 0.72; Max: 4.71 µm; Min: 0.18 µm: Median: 0.67 µm.
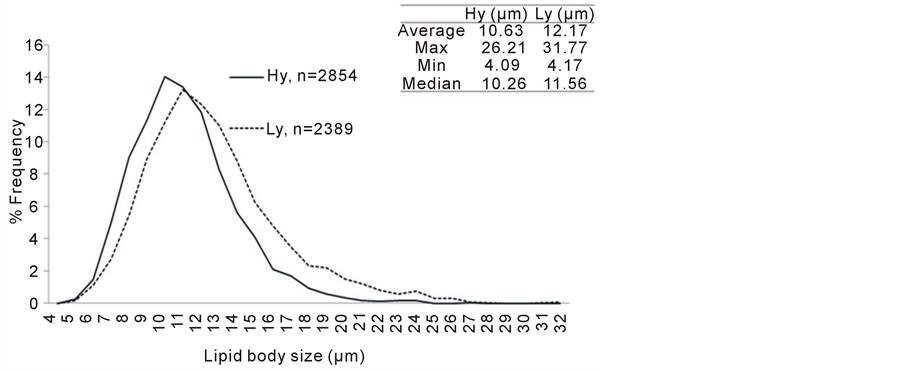
Figure 5. Distribution of embryonic lipid bodies. A total of 2639 lipid bodies of mesocarp were examined. Mean: 0.72; Max: 4.71 µm; Min: 0.18 µm: Median: 0.67 µm.
Table 1. Spearman’s Rank Correlation analyses on mesocarp lipid body size and oil yield..
lipid droplets in high oil-yielding palms ranged between 0% - 0.35%. On the other hand, frequency of occurrence of extra larger lipid droplets in low oil-yielding palms ranged between 0.22% - 5.56%. The percent occurrence of extra large lipid bodies, when compared by student t-test, was shown to be significantly different between the mean of high and low oil-yielding palms (P < 0.05).
To test the robustness of our observation where in the mesocarp of drupelets from proximal region of high oil-yielding palms, there were higher number of smaller size lipid bodies compared to the low oil-yielding palms, we compared the size of lipid bodies at different cut off points. In another word, based on the distribution curve of lipid bodies sizes (Figure 3), cut off points for sizes of interest were set at median as well as 5, 10, and 20% largest size (indicated by arrows in Figure 3). T-test showed significant difference when frequencies of occurrence of lipid bodies at defined cut off points were compared between high and low oil-yielding palms.

Figure 6. Distribution, min, max, median and mean of size of lipid bodies in embryo tissue of high oil-yielding palms and low oil-yielding palms. Hy: high oil-yielding palm; Ly: low oil-yielding palm.
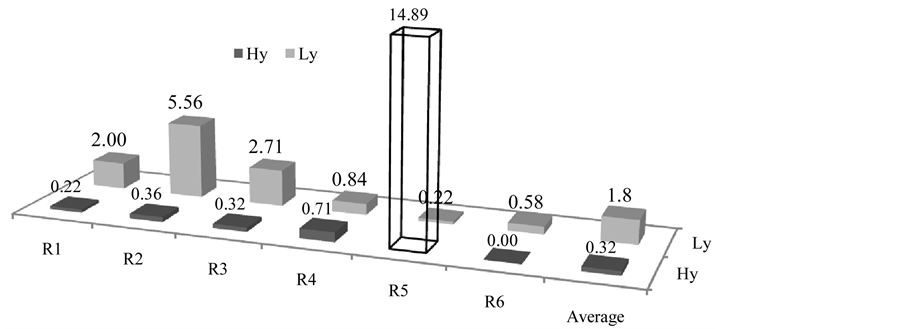
Figure 7. Frequency of occurrence of extra large lipid bodies. Y-axis indicated percent occurrence for each biological replicate. Percent was computed based on number of extra large lipid bodies over total number of lipid bodies observed for each individual biological replicates. Note that replicate number 5 of high oil-yielding palms was the outlier and was excluded in the study as well as the computation of average value. R: Replicates. Hy: High oil-yielding palms; Lylow oil-yielding palms.
Same trend was observed, i.e. low oil-yielding palms possessed more lipid bodies with larger sizes at all defined cut off points tested (Figure 8).
3.4. Number of Lipid Bodies per Cell and Cell Size (µm2)
Of the total 341 parenchymal lipid bodies bearing cells examined, data was collected for the number of lipid bodies per cell and size of cell size (µm2). The mesocarp cell of high oil-yielding palms has significantly more lipid bodies compare to low oil-yielding palms by 17.93% when tested at 95% confidence level (Table 2). There is also significant different when comparing the size of mesocarp cell of high and low oil-yielding palm at 95% confidence level (P = 0.041). The mean of size of mesocarp cell of high oil-yielding palms is larger than low oil-yielding palms by 0.91% (Table 2).
4. Discussion
Lipid bodies in various oil crops, such as Brassica spp., Zea mays and Arabidopsis spp have been extensively
Figure 8. . Frequency of occurrence of larger lipid bodies at different cut off points. Y—axis indicated percent occurrence larger lipid bodies for each category. Hy—High oil-yielding palms; Ly—low oil-yielding palms; LB—lipid bodies.
Table 2 No. of lipid bodies per cell and size of cell of high and low oil-yielding palms. Hy—High oil-yielding palms; Ly— Low oil-yielding palms.
studied, main focus were always lipid bodies in embryonic tissue, seed as well as in pollen. Research on the lipid bodies in mesocarp tissue has been very rare, not only in the similar field of research, but also rare in the past two\decades. Among all economically important oil crops, very few are accumulating oil in the endosperm, among which is olive, avocado and oil palm. In olive, mesocarp cells are large and contain one or more lipid bodies with diameter up to20 µm [14] . It was reported that in the ripening process of avocado, neither loss of organizational resistance nor degradation of the cytoplasmism was observed, where the structural integrity of mitochondria as well as other organelles appeared retained [15] . In addition, parenchyma cells of avocado mesocarp usually contain many lipid bodies, while the scattered idioblast of oil contains only one large lipid droplet [16] .
In another study of oil palm mesocarp ultrastucture, it was shown that in immature mesocarp (week 16 after anthesis), organelles like nucleus could be clearly seen without clear appearance of lipid bodies (data not shown), and this indicating that there was a loss of protoplasm organizational integrity approaching maturity. In the present study, mesocarp of oil palms at 22 weeks after anthesis were observed with cell containing multiple lipid bodies (size with a median 10.86 µm) and limited number of protein or starch (Figure 2(c)). There are also vascular bundles scattered in the parenchymal layer between the lipid bodies containing cell but they are rare in numbers. In addition to that, in another work done on tissue morphology, which was carried out using SEM, raphides crystal of calcium oxalate can be observed indicating presence idoblasts in the mesocarp tissue (data not shown). However, mesocarp cell containing only single lipid body, like the idioblast observed in avocado, was not observed from all the 341 cells examined.
On the other hand, olive which also produces oil in its mesocarp was reported to have very similar ultrastructure for its embryonic and kernel cells. The kernel cells and embryonic cells of oil palms have a very different cytoplasmic organization (Figures 2(d)-(f)). In embryonic cell of oil palms, space was occupied by relatively large storage vacuole where lipid bodies were deposited along its periphery. This observation is in agreement with the report by Ross and team [14] . In contrast, lipid bodies found in kernel cells of oil palms were relative large and many, while storage vacuole were relatively a minor component of the cytoplasmic matrix. They were irregular in outline and the content appearance varied from transparent to a fine flocculent or granular material refer to Figure 2(d) and Figure 2(e).
In terms of size, the current observation also recorded a larger range of size (4.09 - 31.77 µm) with a median of 10.86 µm, compared to the earlier report where the size of mesocarp lipid bodies of oil palm was reported to be ranged between 10 - 20µm [17] [18] . The size of lipid bodies in embryo tissue of oil palm ranged between 0.18 - 4.71 µm in diameter, which is also a larger range compared to that of reported for olive [14] and others crops [6] which was ranged between 0.5 and 2.0 µm.
The differences in size of lipid bodies between high oil-yielding palm and low oil-yielding palm have been the main interest of our study. There was no previous record found on this subject specifically on oil palm. In this study, it has been statistically shown that lipid bodies of low oil-yielding palm are larger than that of high oil-yielding palms, for both embryo and mesocarp. In mesocarp, Spearman’s Rank correlation analyses showed that lipid body sizes are negatively correlated to oil yield. Not only that, it was also shown that in low oil-yielding palm, possesses higher number of large lipid body compared to high oil-yielding palms at different cut off points (Figure 7 and Figure 8). This observation is found to be similar to that observed by Hu and team [9] reported that in embryo of Brassica napus, unusually large lipid bodies are highly correlated with low oil content. In another study, oil content of ole1ole2 of Arabidopsis thaliana was found to be much lower than the wild type but enlarger lipid bodies [19] , again, this finding is in agreement with the current study. However, in maize, lipid bodies of high oil-yielding kernel are large and spherical where this finding is in contrast with the current observation in oil palm mesocarp and embryo [20] . Various factors may contribute to the disagreement between observations, one of which is comparisons were always made between different genotypes, different stages of development or different tissues and organs (mainly seed and embryo).
When we observed that in general, lipid bodies size is inversely related to oil content, it is also relating to lipid body associated proteins like oleosin [9] [21] [22] where higher TAG (triacylglycerol)-to-oleosin ratio typically results in larger lipid bodies and higher oil content. In the work done by Siloto and team [21] , where the expression of oleosin in seed of Arabidopsis thaliana was manipulated, it was shown that OLEO1 suppression resulted in larger lipid droplets but lower TAG content accompanied by an increase of total protein in an almost compensatory manner comparing to the wild type. In the work done by Liu and team [22] , overexpression of soybean oleosin in the embryo of transgenic rice seed also resulted in increased oil content and smaller lipid bodies compared to wild type. Such an event was also observed in embryo and seed of Brassica spp [9] . While the presence of large lipid bodies related to the reduced level of oleosin is consistently reported for seed and embryo, there are no report of oleosin found in the mesocarp of olive and avocado where their lipid bodies are several times larger than those of seed and embryo [14] . In the work done by Shariza [23] , it was shown that the expression of oleosins are detectable in kernel and embryo but not mesocarp of oil palm; leaf and germinating seedling tissues indicating that oleosins are found only in tissues that undergo dehydration. Also, it is presumably that smaller lipid bodies with higher surface/volume ratio enhance the binding efficiency of cytoplasmic lipase during lipolysis and hence allowing rapid release of energy germination and growth [24] . In general, mesocarp lipids function to attract animals for seed dispersion and to date, there is yet any evidence showing involvement of mesocarp lipid bodies in seed germination. In another word, lipid bodies size are not restricted when rapid lease of energy is not required [24] . However, in one of our work investigating lipid bodies associated proteins; mRNA of oil palm oleosin was detected and was successfully isolated from the mesocarp of oil palm. Expression of the oil palm oleosin gene and western blot validation indicated the presence of oleosin in mesocarp of oil palm (data not shown). The investigation on the relationship of level of oleosin and oil yield as well as size of lipid bodies is in progress.
The size of the parenchymal cell of high and low oil-yielding palms recorded 0.9% difference. Given a similar size of space, the observation on the number of lipid bodies per cell was considerable in accordance with the observation of size of lipid bodies. In another words, the larger the lipid bodies are, the smaller number of lipid bodies can be fitted in space of similar size. Based on our observation, when the cytoplasmic matrix of mesocarp cells is fully occupied by lipid bodies with minor number of protein bodies, the oil content per cell of high and low oil-yielding palms are close if not the same. However, the oil yield of high oil-yielding palms is recorded at close to two fold higher than the low oil-yielding palms. As such, mesocarp thickness may be a determinative factor in oil yield. This observation affirming the work done by Teh and team, where amino acids which are required for cell division, were found accumulated in the mesocarp of high oil-yielding palms. The high oil yielding palms that were used in their study showed a 35% larger mesocarp mass per bunch on average compared to the low oil yielding palms suggesting that mesocarp development an important factor for oil yield [13] .
5. Conclusions
In conclusion, the mean size of lipid bodies of mesocarp tissue for high oil yield palm is significantly different from lipid bodies of mesocarp tissue for low oil-yielding palms. Mesocarp of low oil-yielding palms has larger lipid bodies than high oil-yielding palms. Mean of size of lipid bodies of embryonic cells for high oil yield palm is significantly different from lipid bodies of embryo tissue for low oil-yielding palms. Embryos of low oilyielding palms have larger lipid bodies than high oil-yielding palms. It was also statistically shown that low oilyielding palms possess higher number of larger size lipid bodies at all cut off points tested. In addition, high oilyielding palms have high number of lipid bodies per cell while the different between the sizes of the cell is small.
Microscopy techniques allow ultrastructural examination of mesocarp, kernel and embryonic tissue of oil palms. Knowing the differences in physical morphology and properties of lipid bodies between high oil-yielding palms and low oil-yielding palms based on the current study, further understanding the relationship between the lipid body associated proteins and oil yield of oil palm, as well as the structural organization at tissue level could help unfold the key factor in improving the crop performance.
Acknowledgements
The authors would like to thank their colleagues at SDTC and SDRC for their valued input and discussions, especially: the Breeding group and the harvesting team (SDRC), Encik Rafiuz Zaman from UPM EM unit, Cik Suhada from HUKM EM unit, Cik Suhaida from UKM EM unit, Ms Siaw Siew Siew from Hi-Tech Instruments Sdn Bhd and Mr David Lyn from Matrix Optics Sdn Bhd for microscopy technical advises and services provided.
References
- Murphy, D.J. (2009) Oil Palm: Future Prospects for Yield and Quality Improvements. Lipid Technology, 21, 257-260. http://dx.doi.org/10.1002/lite.200900067
- Murphy, D.J. and Vance, J. (1999) Mechanisms of Lipid-Body Formation. Trends in Biochemical Sciences, 24, 109- 115. http://dx.doi.org/10.1016/S0968-0004(98)01349-8
- Tzen, J.T.C. (2012) Integral Proteins in Plant Oil Bodies. ISRN Botany, 2012, 16. http://dx.doi.org/10.5402/2012/173954
- Lersten, N.R., Czlapinski, A.R., Curtis, J.D., Freckmann, R. and Horner, H.T. (2006) Oil Bodies in Leaf Mesophyll Cells of Angiosperms: Overview and a Selected Survey. American Journal of Botany, 93, 1731-1739. http://dx.doi.org/10.3732/ajb.93.12.1731
- Tzen, J. and Huang, A. (1992) Surface Structure and Properties of Plant Seed Oil Bodies. The Journal of Cell Biology, 117, 327-335. http://dx.doi.org/10.1083/jcb.117.2.327
- Tzen, J.T., Cao, Y., Laurent, P., Ratnayake, C. and Huang, A.H. (1993) Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiology, 101, 267-276.
- Huang, A.H. (1992) Oil Bodies and Oleosins in Seeds. Annual Review of Plant Biology, 43, 177-200. http://dx.doi.org/10.1146/annurev.pp.43.060192.001141
- Murphy, D. (1990) Storage Lipid Bodies in Plants and Other Organisms. Progress in Lipid Research, 29, 299-324.
- Hu, Z., Wang, X., Zhan, G., Liu, G., Hua, W. and Wang, H. (2009) Unusually Large Oilbodies Are Highly Correlated with Lower Oil Content in Brassica Napus. Plant Cell Reports, 28, 541-549. http://dx.doi.org/10.1007/s00299-008-0654-2
- Itabe, H. (2010) Intracellular Lipid Droplet-Associated Proteins: Unique Members and Their Biological Functions
Foreword. Biological and Pharmaceutical Bulletin, 33, 341-341. http://dx.doi.org/10.1248/bpb.33.341 - Shimada, T.L. and Hara-Nishimura, I. (2010) Oil-Body-Membrane Proteins and Their Physiological Functions in Plants. Biological and Pharmaceutical Bulletin, 33, 360-363. http://dx.doi.org/10.1248/bpb.33.360
- Nikiforidis, C.V., Kiosseoglou, V. and Scholten, E. (2013) Oil Bodies: An Insight on Their Microstructure—Maize Germ Vs Sunflower Seed. Food Research International, 52, 136-141. http://dx.doi.org/10.1016/j.foodres.2013.02.052
- Teh, H.F., Neoh, B.K., Hong, M.P.L., Low, J.Y.S., Ng, T.L.M., Ithnin, N., Thang, Y.M., Mohamed, M., Chew, F.T. and Yusof, H.M. (2013) Differential Metabolite Profiles During Fruit Development in High-Yielding Oil Palm Mesocarp. PloS ONE, 8, e61344. http://dx.doi.org/10.1371/journal.pone.0061344
- Ross, J.H., Sanchez, J., Millan, F. and Murphy, D.J. (1993) Differential Presence of Oleosins in Oleogenic Seed and Mesocarp Tissues in Olive (Olea europaea) and Avocado (Persea americana). Plant Science, 93, 203-210. http://dx.doi.org/10.1016/0168-9452(93)90050-A
- Platt-Aloia, K.A. and Thomson, W.W. (1981) Ultrastructure of the Mesocarp of Mature Avocado Fruit and Changes Associated with Ripening. Annals of Botany, 48, 451-466.
- Platt, K. and Thomson, W. (1992) Idioblast Oil Cells of Avocado: Distribution, Isolation, Ultrastructure, Histochemistry, and Biochemistry. International Journal of Plant Sciences, 153, 301-310. http://dx.doi.org/10.1086/297033
- Mohankumar, C. and Arumughan, C. (1990) Histological Localization of Oil Palm Fruit Lipase. Journal of the American Oil Chemists’ Society, 67, 665-669.
- Tranbarger, T.J., Dussert, S., Joët, T., Argout, X., Summo, M., Champion, A., Cros, D., Omore, A., Nouy, B. and Morcillo, F. (2011) Regulatory Mechanisms Underlying Oil Palm Fruit Mesocarp Maturation, Ripening, and Functional Specialization in Lipid and Carotenoid Metabolism. Plant Physiology, 156, 564-584. http://dx.doi.org/10.1104/pp.111.175141
- Shimada, T.L., Shimada, T., Takahashi, H., Fukao, Y. and Hara-Nishimura, I. (2008) A Novel Role for Oleosins in Freezing Tolerance of Oilseeds in Arabidopsis Thaliana. The Plant Journal, 55, 798-809. http://dx.doi.org/10.1111/j.1365-313X.2008.03553.x
- Ting, J.T., Lee, K., Ratnayake, C., Platt, K.A., Balsamo, R.A., and Huang, A.H. (1996) Oleosin Genes in Maize Kernels Having Diverse Oil Contents Are Constitutively Expressed Independent of Oil Contents. Planta, 199, 158-165. http://dx.doi.org/10.1007/BF00196892
- Siloto, R.M., Findlay, K., Lopez-Villalobos, A., Yeung, E.C., Nykiforuk, C.L. and Moloney, M.M. (2006) The Accumulation of Oleosins Determines the Size of Seed Oilbodies in Arabidopsis. The Plant Cell Online, 18, 1961-1974. http://dx.doi.org/10.1105/tpc.106.041269
- Liu, W.X., Liu, H.L. and Qu, L.Q. (2013) Embryo-Specific Expression of Soybean Oleosin Altered Oil Body Morphogenesis and Increased Lipid Content in Transgenic Rice Seeds. Theoretical and Applied Genetics, 126, 2289-2297. http://dx.doi.org/10.1007/s00122-013-2135-4
- Jamek, S. (2008) Isolation and Characterization of Full Length Oleosin Cdna Clone from Oil Palm (Elaies Guineensis Jacq.) Kernel, Masters Thesis, Universiti Putra Malaysia, Serdang.
- van der Schoot, C., Paul, L.K., Paul, S.B. and Rinne, P.L. (2011) Plant Lipid Bodies and Cell-Cell Signaling: A New Role for an Old Organelle? Plant Signaling & Behavior, 6, 1732-1738. http://dx.doi.org/10.4161/psb.6.11.17639
List of Abbreviations
µm: Micrometer
µm2: Micrometer Square CLSM: Confocal Laser Scanning Microscope CW: Cell Wall FFB: Fresh Fruit Bunch Hy: High Oil-Yielding ICJ: Intercellular Junction LM: Light Microscope Ly: Low Oil-Yielding PB: Protein Body SV: Storage Vacuole TAG: Triacylglycerol TEM: Transmission Electrone Microscope V: Vacuole WWA: Week after Anthesis


