American Journal of Plant Sciences
Vol.4 No.2(2013), Article ID:27754,9 pages DOI:10.4236/ajps.2013.42029
Evaluation of Antioxidant Capacity of Two Ocimum Species Consumed Locally as Spices in Nigeria as a Justification for Increased Domestication
![]()
Department of Genetics and Biotechnology, University of Calabar, Calabar, Nigeria.
Email: *gen_uyoh @yahoo.com
Received December 19th, 2012; revised January 21st, 2013; accepted January 28th, 2013
Keywords: Antioxidant Activity; Scavenging Activity; Reducing Power; Ocimum spp.; Spices; Domestication
ABSTRACT
The antioxidant activities of Ocimum basilicum and Ocimum gratissimum consumed as spices in Nigeria were evaluated in a bid to provide better scientific basis for increased domestication efforts on them. Total phenol and flavonoid contents of the spice extracts ranged from 9.09 - 27.41 µg GAE/mg and 5.38 - 22.88 µg RE/mg respectively. The DPPH and hydroxyl radical scavenging activities of the spice extracts ranged from 58.43% - 92.37% and 6.27% - 16.67% respectively. The total antioxidant capacities and reducing powers of the extracts (measured as absorbance values) ranged from 0.137 - 0.160 and 0.130 - 0.158 respectively. Generally, Ocimum basilicum maintained superior antioxidant activities to O. gratissimum in all the test assays, and all the extracts showed dose-dependent antioxidant activities. Ascorbic acid, Gallic acid and Rutin used as reference compounds generally showed higher antioxidant activities to the spice extracts except in the hydroxyl radical scavenging assay. Put together, these results confirm that Ocimum basilicum and O. gratissimum extracts possess appreciable natural antioxidant potentials, thereby providing good justification for their increased domestication and consumption.
1. Introduction
Spice plants have important uses in food and medicine and interestingly, the Nigerian ecology has been adjudged as favourable for the production of a wide range of these spices [1]. Many of them, however, are in the wild where a lot of factors including forest fires, over exploitation, lack of conservation programmes, land clearing for roads and development, none-recognition of valuable species by incidental farmers and uncontrolled deforestation threaten their production. Significant among the factors limiting optimal production and domestication of some of these spices is general lack of knowledge on the full range of benefits, especially medicinal, that these plants possess.
Ocimum basilicum L. and Ocimum gratissimum L. are two popular spices used widely in many Nigerian cuisines. Commonly known as scent leaf in Nigeria, Ocimum gratissimum L. is also used in traditional medicine for treatment of several ailments such as urinary tract and gastro intestinal infections [2]. The leaves are used as a general tonic and possess anti-diarrhoeal [3] and antidiabetic properties [4]. Essential oils of O. gratissimum contain eugenol and show some evidence of anti-bacterial activity [5]. Ocimum basilicum is used in folk medicine due to its stimulant, carminative and anti-spasmodic properties. It has important uses in pharmaceutical and cosmetic industries [6]. Its green aromatic leaves are used fresh or dried as flavourings or spices while the essential oil is an important part of toiletry products like mouth washes and dental creams.
Among the medicinal benefits of plants, antioxidant properties have received increasing attention due to their role in preventing or down regulating myriads of oxidative damages caused by free radicals in the body [7]. Oxidative stress is initiated by free radicals, which seek stability through electron pairing with biological macromolecules such as proteins, lipids and DNA in healthy human cells and cause protein and DNA damage along with lipid peroxidation. These changes contribute to cancer, atherosclerosis, cardiovascular diseases, ageing and inflammatory diseases [8,9].
Spices are harmless sources for natural antioxidants [10], which are generally preferred to synthetic counterparts in combating free radical damage due to the increased risk of carcinogenesis associated with the use of synthetic products [11]. The antioxidant capacities of Ocimum basilicum and Ocimum gratissimum consumed locally in Nigeria have not been clearly presented. Such information, if provided, will not only possibly introduce the spice plants as cost effective and accessible sources of natural antioxidants but also justify the need for renewed domestication efforts on them. Against this backdrop, this study is aimed at evaluating the antioxidant activities of Ocimum basilicum and Ocimum gratissimum in selected in vitro assay systems.
2. Materials and Methods
2.1. Collection and Extraction of Plant Material
Fresh leaves of Ocimum basilicum and Ocimum gratissimum (Plate 1) were procured from Watt market in Calabar (located at 4˚59'36''N, 8˚19'05''E), Cross River State, Nigeria and authenticated by the plant taxonomist in Botany Department, University of Calabar. The plant materials were freed from debris, dried at room temperature for one week and milled separately into fine powder using a blender (5 speed kitchen-aids 5KSB655CCS0). Ten (10) grams of each milled sample were extracted by soaking in 100mls of 90% methanol for 72 h at room temperature with intermittent shaking. The samples were subsequently filtered using Whatman No. 1 filter paper at the end of the extraction period and concentrated under vacuum in a rotary evaporator at 45˚C for complete solvent removal. A sample stock solution of 3000 µg/ml was prepared for each extract by accurately weighing 0.3 g of concentrated crude extract and dissolving in 100 ml of distilled water. Working solutions of each extract were

Plate 1. Shoots of Ocimum gratissimum (a) and O. basilicum (b).
prepared as desired by appropriate dilutions of the stock solutions.
2.2. Chemicals
DPPH (1,1-diphenyl-2-picryl hydrazyl) radical and Rutin were purchased from Sigma Aldrich Chemical Company, USA; Folin and Ciocalteau’s Phenol reagent and Trichloroacetic acid (TCA) from Qualikems Fine Chemical Pvt. Ltd., New Delhi, India; Gallic acid monohydrate from Kem Light Laboratories Pvt. Ltd., Mumbai, India. Solvents and other chemicals used for this study were of analytical grade, while water was glass distilled.
2.3. Determination of Total Phenol Content (TPC)
The total phenol contents of Ocimum basilicum and Ocimum gratissimum leaf extracts were determined by the Folin-Ciocalteau method according to [12]. To 500 µl of the different extract solutions were added 100 µl of Folin Ciocalteau reagent plus 6 ml of distilled water and shaken for one (1) min. Thereafter, 2 ml of 15% sodium carbonate was added to the mixture and shaken once again for 30 sec. Finally, the solution was brought up to 10 ml by adding distilled water. After 1.5 h incubation at room temperature, the absorbance at 750 nm was evaluated using a spectrophotometer (LABTECH UV/VIS Spectrophotometer, India). Gallic acid monohydrate, a standard phenol, in the range of 0 - 240 µg/ml was used to prepare a standard reference curve. The total phenol contents (TPC) of the extracts were expressed as Gallic Acid Equivalents (GAE) from the linear regression curve of Gallic acid.
2.4. Determination of Total Flavonoid Content (TFC)
The total flavonoid contents of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were determined using the aluminium chloride colorimetric method according to [13]. The different extract solutions (1 ml containing 100 µg/ml) were diluted with 4 ml of distilled water in a 10 ml volumetric flask. Thereafter, 0.3 ml of 5% sodium nitrite solution was added to each volumetric flask; 5 min later, 10% aluminium chloride (0.3 ml) was added; 1 min later, 2 ml of 1.0 M sodium hydroxide was added and finally, 2.4 ml of distilled water was then added to the reaction flask and mixed well. Absorbance of the reaction mixture was read at 510 nm. Rutin, a standard flavonoid, in the range of 0 - 135 µg/ml was used to prepare the standard reference curve. Total flavonoid contents (TFC) of the extracts were expressed as Rutin Equivalents (RE) from the linear regression curve of rutin.
2.5. DPPH (1, 1-Diphenyl-2-Picryl Hydrazyl) Radical Scavenging Assay
Antioxidant activity of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were measured in this assay as ability to scavenge stable DPPH radicals according to [14]. Four (4) different concentrations (30, 60, 90 and 120 µg/ml) of the test extracts were prepared in methanol. To 2.5 ml solution of each extract concentration was added 1 ml of 0.3 mM of freshly prepared DPPH solution in methanol and allowed to react in the dark at room temperature for 30 min. Absorbance of the resulting solution was measured at 518 nm. Methanol (1 ml) added to 2.5 ml of each extract concentration was used as blank, while 1 ml of 0.3 mM DPPH solution added to 2.5 ml of methanol served as a negative control. Ascorbic acid and gallic acid, prepared in the same concentrations as the test extracts, were used as reference standards (positive controls) for comparison.
Percentage DPPH scavenging activities of the extracts and reference standards were determined using the formula:
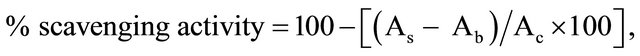
where As = Absorbance of sample (extract or reference standard), Ab = Absorbance of blank and Ac = Absorbance of negative control [14].
2.6. Total Antioxidant Capacity (TAC) Assay
The total antioxidant capacity (TAC) of the Ocimum basilicum and Ocimum gratissimum leaf extracts were determined by the phosphomolybdate method according to [15]. An aliquot (300 µl) of different concentrations (30, 60, 90 and 120 µg/ml) of the test extracts were mixed with 3 ml of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, 4 mM ammonium molybdate) taken in test tubes. The tubes were capped with aluminium foil and incubated in a boiling water bath at 95˚C for 90 min. The reaction mixture was allowed to cool to room temperature and the absorbance of the solution was measured at 695 nm against a blank containing 3 ml of reagent solution and the appropriate volume of the dissolving solvent. The blank was incubated under the same conditions as the test samples. Ascorbic acid and rutin were used as standard reference compounds to compare the activities of the extracts. Results were expressed in absorbance values at 695 nm.
2.7. Hydroxyl (OH) Radical Scavenging Assay
The hydroxyl radical scavenging activity of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were determined by the Fenton reaction using the orthophenanthroline method according to the modified procedure of [16]. Four (4) ml of sodium phosphate buffer (0.2 M, pH 7.4), 1.5 ml of 5 mM orthophenanthroline (1, 10 phenanthroline) in ethanol and I ml of 7.5 mM Iron (II) sulphate were mixed simultaneously. Then, 1 ml of different concentrations (1.5 - 3.0 mg/ml) of each extract, 1.5 ml of distilled water and 1 ml of 1% hydrogen peroxide were added to the mixture solution in sequence. After incubating at 37˚C for 60 min, the change of reaction mixture in absorbance, caused by the colour change of Fe-orthophenanthroline was measured at 510 nm. A damage control (control in the hydroxyl radical generation system) was constituted with distilled water in place of extracts and references. A blank was constituted with distilled water, without sample and hydrogen peroxide. Ascorbic acid and rutin were used as standard antioxidant compounds for comparison of the extracts’ activities. Hydroxyl radicals scavenging activity was evaluated as:
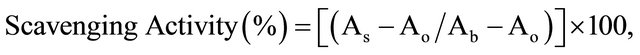
where As = Absorbance of reaction mixture with sample or standard, Ao = Absorbance of damage control and Ab = Absorbance of blank.
2.8. Reducing Power Assay
Antioxidant activity of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were determined in this assay as their Fe3+ reducing ability according to the method of [17]. Different concentrations (30, 60, 90 and 120 µg/ml) of each extract were prepared and 1 ml of each concentration was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.8) and 2.5 ml of potassium ferricyanide. The mixture was incubated in a water bath at 50˚C for 20 min. To this mixture, 2.5 ml of 10% trichloroacetic acid was added and then centrifuged (Rotofix 32, Germany) at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride was added. Absorbance of the Pert Prussian blue solution formed was measured at 700 nm using a spectrophotometer. Ascorbic acid and rutin were used as reference standards for comparison and were prepared in same concentrations as the extracts. Results were expressed as absorbance values at 700 nm (Increasing absorbance value indicated increaseing reducing power).
2.9. Statistical Analysis
The experiment was laid out in a Completely Randomized Design (4 × 4 factorial arrangement) and data were analyzed using a one-way analysis of variance (ANOVA), except results for total phenol and flavonoid contents of the extracts that were analyzed using the student t-test and presented as means ± standard error. All measurements were replicated five times. Significant means were separated using the Least Significant Difference (LSD) analysis.
3. Results
3.1. Total Phenol Content (TPC)
The total phenol contents of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were estimated quantitatively from a linear regression curve (y = 0.4982 + 0.0101x, r2 = 0.948) of Gallic acid, a standard phenol, and expressed in micrograms Gallic acid equivalents per milligram of sample (µg GAE/mg sample). The results, as presented in Table 1, showed that Ocimum basilicum leaf extract had a mean total phenol content of 27.41 µg GAE/mg which was significantly higher (p < 0.01) than that of Ocimum gratissimum with a mean value of 9.09 µg GAE/mg.
3.2. Total Flavonoid Content (TFC)
The total flavonoid contents of the leaf extracts of Ocimum basilicum and Ocimum gratissimum were estimated quantitatively from a linear regression curve (y = 0.0937 + 0.0008x, r2 = 0.954) of Rutin, a standard flavonoid, and presented in microgram Rutin equivalents per milligram of sample (µg RE/mg sample). The results, as presented in Table 1, showed that the leaf extract of Ocimum basilicum had a mean total flavonoid content of 22.88 µg RE/mg of sample which was significantly higher (p < 0.01) than that of Ocimum gratissimum with a mean value of 5.38 µg RE/mg.
3.3. DPPH Radical Scavenging Assay
In this assay, the ability of the investigated extracts to act as donors of hydrogen atoms or electrons in transformation of DPPH radical into its reduced form was investigated. The results indicated that Ocimum basilicum extract had a mean scavenging activity of 92.37% which was significantly higher (p < 0.001) than that of Ocimum gratissimum with a mean scavenging activity of 58.43% (Table 2). The scavenging activities of the tested extracts were, however, lower than those of ascorbic acid (96.99%)
Table 1. Total phenol and flavonoid contents (TPC and TFC) of the leaf extracts of Ocimum basilicum and Ocimum gratissimum.
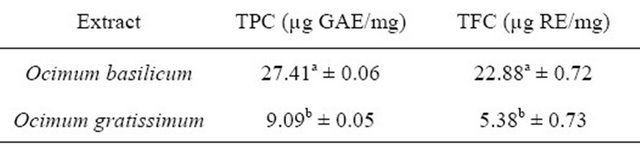
aValues are means of triplicate determinations; bMeans with different superscripts within each column differ significantly (p < 0.01) from each other.
and gallic acid (95.01%) used as reference standards. DPPH scavenging activities of all the tested samples were observed to be dose-dependent, with higher concentrations of each sample showing higher scavenging activities (Table 3 and Figure 1).
3.4. Total Antioxidant Capacity (TAC)
The ability of the constituent antioxidant compounds in Ocimum basilicum and Ocimum gratissimum extracts to reduce Mo (VI) ions to Mo (V) was taken as an indication of total antioxidant capacity and results were reported as absorbance values at 695 nm. Ocimum basilicum extract had a mean absorbance value of 0.160 which was significantly higher (p < 0.001) than that of Ocimum gratissimum with a mean value of 0.137 (Table 2).
Table 2. DPPH radical scavenging activity, Hydroxyl radical scavenging activity, Reducing power and Total antioxidant capacity (TAC) of Ocimum basilicum and Ocimum gratissimum leaf extracts compared with reference compounds.
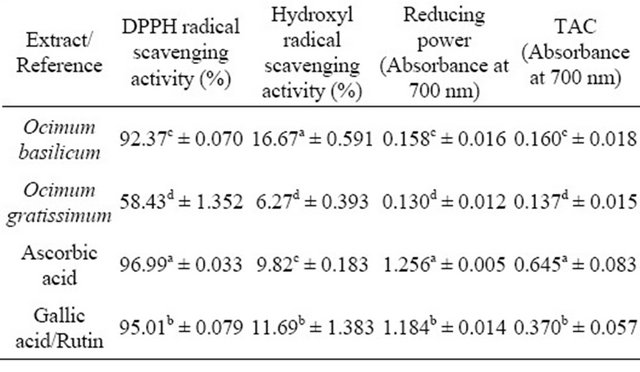
aMeans with different superscripts within each column differ significantly (p < 0.001) from one another; bGallic acid was used as a second reference compound for only DPPH assay, while Rutin was used for the remaining assays.
Table 3. Pooled DPPH radical scavenging activity, Hydroxyl radical scavenging activity, Reducing power and Total antioxidant capacity (TAC) of the different tested concentrations of Ocimum extracts and reference compounds.
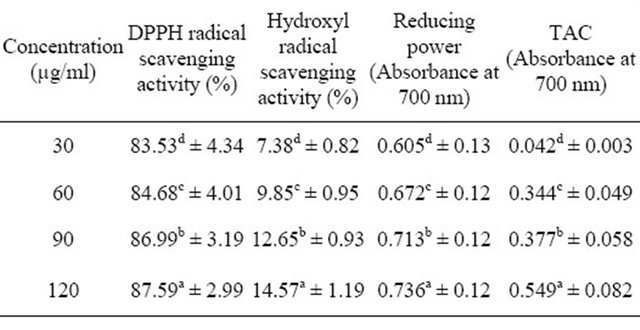
aMeans with different superscripts within each column differ significantly (p < 0.001) from one another; bHydroxyl radical assay was tested at concentrations of 1500, 2000, 2500 and 3000 µg/ml.
Ascorbic acid and rutin used as reference compounds were superior to the extracts in this assay (0.645 and 0.370 respectively). Generally, total antioxidant capacities of all the test samples were dependent on concentration. Higher concentrations of each sample showed significantly higher activity as evident from Table 3 and Figure 2.
3.5. Hydroxyl Radical Scavenging Assay
The hydroxyl radical scavenging abilities of Ocimum basilicum and Ocimum gratissimum extracts, compared with ascorbic acid and rutin are presented in Table 2. Significant differences were observed in the scavenging activities of the samples. Ocimum basilicum had the highest scavenging activity (16.67%), followed by rutin (11.69%) and ascorbic acid (9.82%) while Ocimum gratissimum had the least (6.27%). Increasing concentrations of all the samples resulted in increased scavenging activities (Table 3), indicating that the antioxidant activity in this assay is dose-dependent (Figure 3).
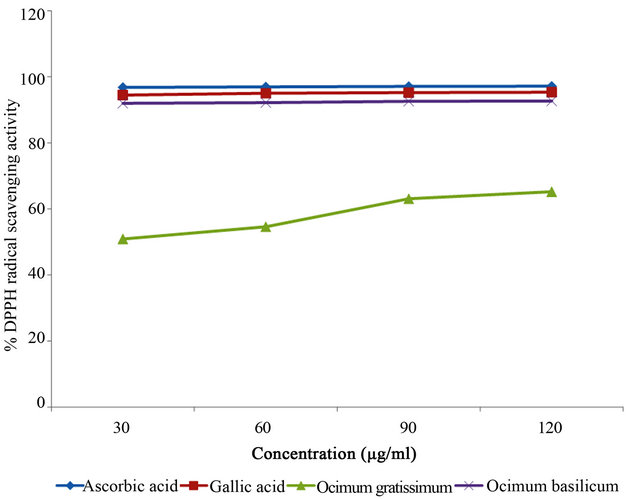
Figure 1. DPPH radical scavenging activities of Ocimum basilicum and Ocimum gratissimum leaf extracts and reference compounds at different concentrations.
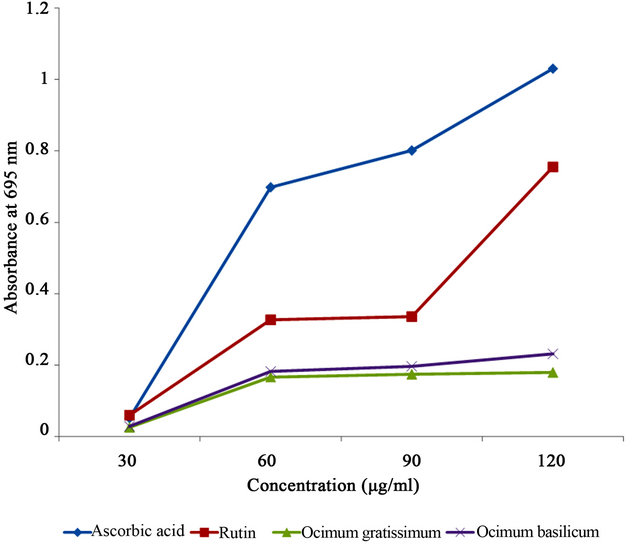
Figure 2. Total antioxidant capacity of Ocimum basilicum and Ocimum gratissimum leaf extracts, and reference compounds at different concentrations.
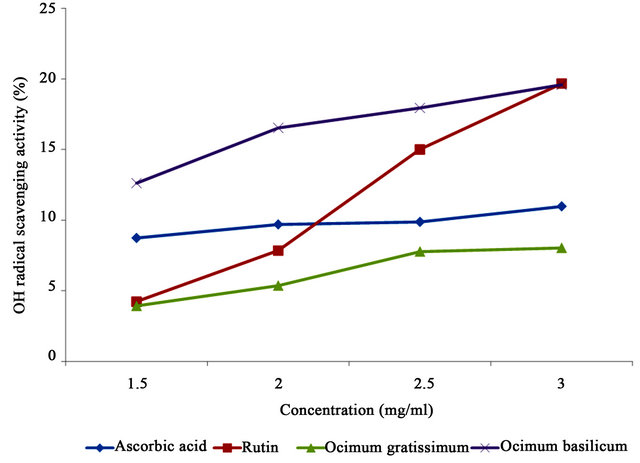
Figure 3. Hydroxyl radical scavenging activities of Ocimum basilicum and Ocimum gratissimum leaf extracts and reference compounds at different concentrations.
3.6. Reducing Power
The reducing abilities of Ocimum basilicum and Ocimum gratissimum extracts, compared with ascorbic acid and rutin are presented as absorbance values in Table 2. Higher absorbance values indicate higher reducing power. Ocimum basilicum had a mean absorbance value of 0.158 which was significantly higher than that of Ocimum gratissimum (0.130). Reducing powers of the extracts were however significantly lower than those of ascorbic acid (1.256) and rutin (1.184). Reducing powers of all the samples responded to concentrations, with higher concentrations resulting in higher reducing powers (Table 3 and Figure 4).
4. Discussion
Spices and herbs have been mentioned as one of the most important targets to search for natural antioxidants from the point of view of safety [10,18]. The abundant locally consumed spice plants in Nigeria may therefore be potential rich reservoirs of antioxidants to be harnessed if studied and established. The activities of antioxidants have been attributed to various mechanisms including prevention of chain initiation, decomposition of peroxides, radical scavenging and reducing capacity [19]. Consequently, these activities vary with assay methods and a single assay may be inadequate [20]. It is for this reason that antioxidant activities of the spice extracts in this study were evaluated in various assays based on different mechanisms.
Phenols and flavonoids represent two phytochemicals whose relative abundance in plant extracts has been profusely linked to antioxidant activities. Phenol compounds constitute one of the most diverse and widespread groups of natural compounds, possessing a broad spectrum of biological activities including antioxidant and radical scavenging properties [21]. The antioxidant potential of phenols is believed to be conferred on them by their hydroxyl group (-OH), which is bonded directly to an aromatic hydrocarbon (phenyl) ring. This makes them donate electrons easily to electron-seeking free radicals, thus down-regulating their menace in living cells. Flavonoids represent the most common and widely distributed groups of plant phenolics. They are potent watersoluble super antioxidants that function in scavenging free radicals, inhibition of peroxidation and chelating transition metals [22]. The high amounts of phenols and flavonoids in extracts may explain their high antioxidant activities [19]. The amounts of total phenol and flavonoids reported in the present study for the two Ocimum species are higher than the range of 0.125 - 1.718 µg GAE/mg reported for ten edible spices consumed in Egypt [18]. This suggests that among spices, Ocimum species rank high in constitution of antioxidant phytochemicals, even though the methods used in the two situations were not exactly the same.
DPPH radical scavenging assay provides an easy, rapid, and convenient method to evaluate antioxidants and radical scavengers [23]. It is based on the ability of 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), a stable free radical, to decolorize in the presence of antioxidants. The DPPH radical contains an odd electron, which is responsible for the absorbance at 515nm and also for the visible deep purple colour. When DPPH accepts an electron do-
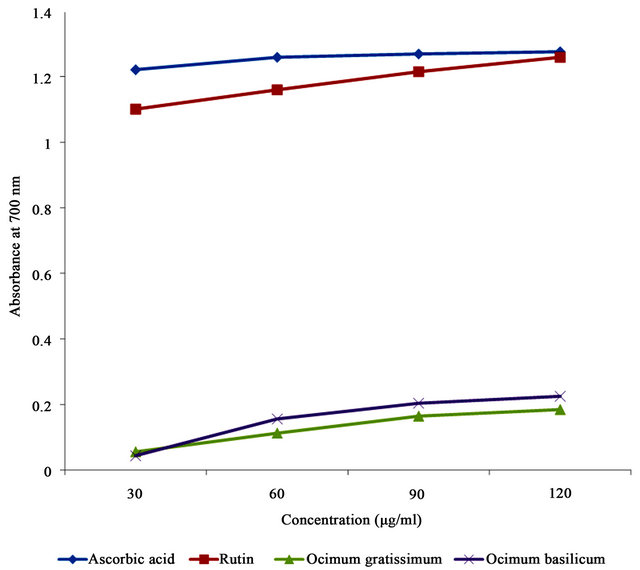
Figure 4. Reducing powers of Ocimum basilicum and Ocimum gratissimum leaf extracts, and reference compounds at different concentrations.
nated by an antioxidant compound, the DPPH is decolorized, and this effect can be quantitatively measured from the changes in absorbance [24]. The Ocimum species tested in this study showed appreciably high DPPH radical scavenging activities with Ocimum basilicum scavenging at a very close range with the reference compounds. This finding validates the evaluated spices as potent radical scavengers which could find application as good natural antioxidants.
Reduction of metal ions is an important mechanism of antioxidant action and a potent antioxidant often acts as a potent reductant [25]. The ability of the Ocimum species to reduce Mo (VI) to Mo (V) complex, though not comparable with the activities of the reference compounds, however confirms the presence of reductants in them and proves that they could serve as electron donors, terminating the free radical chain reactions [26].
The hydroxyl radical scavenging activities of both spice extracts evaluated in this study and those of the well-studied antioxidant compounds used for comparison were generally low at the concentrations tested. However, it is remarkable that Ocimum basilicum extracts scavenged more of the hydroxyl radicals than the rest of the samples. This may suggest that the extracts may also prevent bio membranes and bio molecules from being attacked by free radicals [27].
In the reducing power assay, the presence of antioxidants in the samples would result in the reduction of Fe3+ to its lower valence state, Fe2+, by donating an electron. Amount of Fe2+ complex can then be monitored by measuring the formation of Perl’s Prussian blue at 700 nm. Increasing absorbance at 700 nm indicates an increase in reductive ability [26,28]. The reducing properties are generally associated with the presence of reducetones which have been shown to exert antioxidant action by breaking the free radical chain through electron donation [29]. The absorbance values obtained with the spice extracts studied, though not comparable to reference compounds, confirm that Fe3+-Fe2+ transformation occurred in the presence of the extracts, thereby confirming their antioxidant potentials.
5. Conclusion
The results of the present study indicate that the spice plants—Ocimum basilicum and Ocimum gratissimum consumed locally in Nigeria possess significantly different but appreciably potent antioxidant activities that cannot be neglected. The study therefore not only reveals the spices as accessible reservoirs of natural antioxidants to be utilized nutritionally and pharmaceutically, but very importantly, provides good scientific justification for increased domestication of these plants.
6. Acknowledgements
The authors are grateful to Science Technology Education Post Basic (STEP-B) in Nigeria for the provision of some of the equipment used in this research.
REFERENCES
- B. A. Adelaja and I. O. Fasidi, “Survey and Collection of Indigenous Spice Germplasm for Conservation and Genetic Improvement in Nigeria,” Biodiversity International, Vol. 153, 2008, pp. 67-71.
- S. V. F. Madeira, F. J. A. Mantos, J. H. Leal-Cardoso and D. N. Criddle, “Relaxant Effects of the Essential Oil of Ocimum gratissimum on Isolated Ileum of the Guinea Pig,” Journal of Ethnopharmacology, Vol. 81, No. 1, 2002, pp. 1-4. doi:10.1016/S0378-8741(02)00049-1
- V. Offiah and U. Chikwendu, “Antidiarrhoeal Effects of Ocimum gratissimum Leaf Extract in Experimental Animals,” Journal of Ethnopharmacology, Vol. 68, No. 1-3, 1999, pp. 327-330. doi:10.1016/S0378-8741(99)00100-2
- A. Mohammed, Y. Tanko, M. A. Okasha, R. A. Magaji and A. H.Yaro, “Effects of Aqueous Leaf Extracts of Ocimum gratissimum on Blood Glucose Levels of Streptozocin-Induced Diabetic Wistar Rats,” African Journal of Biotechnology, Vol. 6, No. 18, 2007, pp. 2087-2090.
- F. O. J. Oboh, H. I. Madsodje and S. A. Enabulele, “Nutritional and Antimicrobial Properties of Ocimum gratissimum,” Journal of Biological Sciences, Vol. 9, No. 4, 2009, pp. 377-380. doi:10.3923/jbs.2009.377.380
- B. Bozin, N. Mimica-Dukic, N. Simin and G. Anackov, “Characterization of the Volatile Composition of Essential Oil of Some Lamiaceae Species and the Antimicrobial and Antioxidant Activities of the Entire Oils,” Journal of Agriculture and Food Chemistry, Vol. 54, No. 5, 2006, pp. 1822-1828. doi:10.1021/jf051922u
- Y. Abiy, “Antimicrobial Flavonoids from the Stem Bark of Erythrina burtii,” Fitoterapia, Vol. 96, 2005, pp. 496- 499.
- A. Braca, C. Sortino, M. Politi, J. Morelli and J. Mendez, “Antioxidant Activity of Flavonoids from Licania licaniaeflor,” Journal of Ethnopharmacology, Vol. 79, No. 3, 2002, pp. 379-381. doi:10.1016/S0378-8741(01)00413-5
- U. Ozgen, A. Mavi, Z. Terzi, A. Yildrim, M. Coskun and P. J. Houghton, “Antioxidant Properties of Some Medicinal Laminaceae species,” Pharmaceutical Biology, Vol. 44, No. 2, 2006, pp. 107-112. doi:10.1080/13880200600592061
- D. E. Okwu, “Phytochemicals and Vitamin Content of Indigenous Spices of South Eastern Nigeria,” Journal of Sustainable Agriculture and Environment, Vol. 6, 2004, pp. 30-34.
- N. S. Hettiarachchy, K. C. Glen, R. Gnanaesbandam and M. G. Johnson, “Natural Antioxidant Extract from Fenugreek (Trigonella foenumgraecum) for Ground Beef Patties,” Journal of Food Science, Vol. 61, No. 3, 1996, pp. 516-519. doi:10.1111/j.1365-2621.1996.tb13146.x
- J. M. Duarte-Almeida, A. V. Novoa, A. F. Linares, F. M. Lajolo and M. I. Genovese, “Antioxidant Activity of Phenolic Compounds from Sugar Cane (Saccharum officinarum L.) Juice,” Plant Foods For Human Nutrition, Vol. 61, No. 4, 2006, pp. 187-192. doi:10.1007/s11130-006-0032-6
- V. Dewanto, X. Wu, K. K. Adom and R. H. Liu, “Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 10, 2002, pp. 3010-3014. doi:10.1021/jf0115589
- L. L. Mensor, F. S. Menezes, G. G. Leitao, A. S. Reis, T. C. dos Santos, C.S . Coube and S. G. Leitao, “Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method,” Phytotherapy Research, Vol. 15, No. 2, 2001, pp. 127-130. doi:10.1002/ptr.687
- G. K. Jayaprakasha, B. S. Jena, P. S. Negi and K. K. Sakariah, “Evaluation of Antioxidant Activities and Mutagenicity of Turmeric Oil: A Byproduct from Curcumin Production,” Zeitschrift für Naturforschung, Vol. 57, 2002, pp. 828-835.
- K. R. Nagulendran, S. Velavan, R. Mahesh and B. V. Hazeena, “In Vitro Antioxidant Activity and Total Polyphenolic Content of Cyperus rotundus Rhizomes,” European Journal of Chemistry, Vol. 4, 2007, pp. 440-449.
- A. Sheetal, S. B. Millind and H. Srinivasa, “Antioxidant Activity of Stem Bark of Tespesia populnea,” Journal of Natural Remedies, Vol. 7, No. 1, 2007, pp. 135-141.
- M. A. Hala, “Comparative Antioxidant Activity Study of Some Edible Plants Used as Spices in Egypt,” Journal of American Science, Vol. 7, No. 1, 2011, pp. 1118-1122.
- N. C. Cook and S. Samman, “Flavonoids-Chemistry, Metabolism, Cardioprotective Effects and Dietary Sources,” Journal of Nutritional Biochemistry, Vol. 7, 1996, pp. 66- 76. doi:10.1016/0955-2863(95)00168-9
- G. C. Yen, P. D. Duh and H. J. Su, “Antioxidant Properties of Lotus Seed and Its Effect on DNA Damage in Human Lymphocytes,” Food Chemistry, Vol. 89, No. 3, 2005, pp. 379-385. doi:10.1016/j.foodchem.2004.02.045
- E. A. Melo, J. M. Filho and N.B. Guerra, “Characterization of Antioxidant Compounds in Aqueous Coriander Extracts (Coriander sativum L.),” Lebensmittel-Wissenschaft and Technologie, Vol. 38, 2005, pp. 15-19.
- B. Nickavar, M. Kamalinejad and H. Izadpanah, “In Vitro Free Radica Scavenging Activity of Five Salvia species,” Pakistan Journal of Pharmaceutical Science, Vol. 20, No. 4, 2007, pp. 291-294.
- V. Roginsky and E. A. Lissi, “Review of Methods to Determine Chain-Breaking Antioxidant Activity in Food,” Food Chemistry, Vol. 92, No. 2, 2005, pp. 235-254. doi:10.1016/j.foodchem.2004.08.004
- M. R. Saha, S. M. R. Hasan, R. Akter, M. M. Hossain, M. S. Alam, M. A. Alam and M. E. H. Mazunder, “In Vitro Free Radical Scavenging Activity of Methanol Extract of the Leaves of Mimusops elengi Linn,” Bangladesh Journal of Veterinary Medicine, Vol. 6, No. 2, 2008, pp. 197- 202.
- E. Niki, “Assessment of Antioxidant Capacity in Vitro and in Vivo,” Free Radical Biology and Medicine, Vol. 49, No. 4, 2010, pp. 503-515. doi:10.1016/j.freeradbiomed.2010.04.016
- M. A. Ebrahimzadeh, S. F. Nabavi, S. M. Nabavi, B. Eslami and H. Asgarirad, “In Vitro Antioxidant and Free Radical Scavenging Activity of Leonurus cardiac subsp. Persicus, Grammosciadium platycarpum and Onosma demawendicum,” African Journal of Biotechnology, Vol. 9, No. 51, 2010, pp. 8865-8871.
- E. O. Farombi, T. O. Akuru and M. C. Alabi, “Mechanisms of the Hepatoprotective Action of Kolaviron: Studies on Hepatic Enzymes, Microsomal Lipids and Lipid Peroxidation in Carbon Tetrachloride-Treated Rats,” Pharmacology Research, Vol. 42, No. 1, 2000, pp. 75-80. doi:10.1006/phrs.1999.0648
- K. N. V. Rao, R. Aradhana, D. Banjii, R. S. N. Chaitanya, and A. Anil Kumar, “In Vitro Anti-Oxidant and Free Radical Scavenging Activity of Various Extracts of Tectona grandis Linn Leaves,” Journal of Pharmacy Research, Vol. 4, No. 2, 2011, pp. 440-442.
- P. D. Duh, Y. Y. Tu and G. C. Yen, “Antioxidant Activity of the Aqueous Extract of Harn Jyur (Chrysanthemum morifolium Ramat),” Lebensmittel-Wissenschaft and Technologie, Vol. 32, 1999, pp. 269-277.
NOTES
*Corresponding author.

