International Journal of Nonferrous Metallurgy
Vol.1 No.3(2012), Article ID:24216,6 pages DOI:10.4236/ijnm.2012.13008
Aminododecyldiphosphonic Acid for Solvent Extraction of Bismuth Ions
1Laboratory of Separation and Purification Technology, Department of Chemistry, Faculty of Sciences, Tlemcen University, Tlemcen, Algeria
2Laboratoire de Chimie Moléculaire et Thioorganique, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ENSICAEN, Caen, France
Email: *madidi13@yahoo.fr
Received July 29, 2012; revised September 8, 2012; accepted September 20, 2012
Keywords: Bismuth(III); Aminododecyldimethylenediphosphonic Acid; Solvent Extraction
ABSTRACT
The extraction of Bi(III) in nitrate media has been investigated using aminododecyldimethylenediphosphonic acid, ADDMDPA, which was previously synthesized and characterized. The extraction of the cation was carried out in different media with the addition of CH3COONa, KNO3 and HNO3. The maximum extraction yield for Bismuth is 70% after addition of 0.01 M of potassium nitrate at pHi = 2.9, in one step.
1. Introduction
Bismuth is used in the cosmetics industry for the preparation of creams and hair dyes, while some of its colloidal salts (subcitrate and subgallate), due to their antiseptic, astringent and diuretic properties, have important applications in pharmaceutical preparations and are employed as anti-ulcer, antibacterial, anti-HIV and radiotherapeutic agents [1].
World reserves of bismuth are usually obtained as a sub-product in lead, copper, tin and gold ores [2-4]. During the industrial metallurgical process of these ores, leaching stages with H2SO4, HCl and HNO3 are involved, and highly acidic solutions with base metals and bismuth are obtained [5,6].
Bismuth is a curious metal and could be toxic in an unsuitable form [2,7]. Its metal extraction is a major challenge in the metal addressing the environmental pollution, its mode of physiopathological action was little studied and it is not yet understood [6,7].
The synthesis of new organophosphorus extractants which form stable complexes with metallic species is of great importance for improving existing hydrometallurgycal processes for their recovery [8,9].
In fact, we have synthesized aminododecyldimethylenediphosphonic acid for such a purpose. The characterization of this product was achieved using various spectroscopic methods including (1H NMR, 31P NMR, 13C NMR, FTIR and the like).
We have studied this acid in the recovery of metal species Bi+3 under the optimal conditions in different media, in one step.
2. Experimental
2.1. Reagents and Solutions
The reagents used in this work were dodecan, phosphorus acid (35%) from (Aldrich). HCHO and Bismuth nitrate was purchased from Merck. The aqueous solutions concentrations of Bi(III) in nitrate medium taken as 0.5 mmol·L−1 and the organic (ADDMDPA) solution concentration was taken from 0.5 to 10 mmol·L−1.
2.2. Instrumentation
13C {-1H}, 31P {-1H} and 1H NMR spectra were measured on a Bruker AC 250 working at 250 MHz in a CDCl3 solution. Infrared spectra were measured on a Perkin Elmer 16 PC-FTIR equipped with a thermostat to maintain the temperature of the sample at 25.0˚C ± 0.1˚C. A Phywe WTM 320 with combined glass electrode was used to measure the pH of the aqueous solution before and after extraction. In a water-acetone mixture (15:5), a known mass of the ADDMDPA was titrated with a solution of NaOH (5 mmol·L−1). Metal ions were determined using the electrothermal atomic absorption GFAAS, system GBC 932, and system 3000 automated graphite furnace (GBC Scientific Equipment, Dandenong, Australia). Background correction was made with a deuterium lamp and pyrolytic graphite tubes were used. Settings such as lamp current, wavelength, temperature programs, and slit width were those recommended in the operating manual.
2.2.1. Synthesis of the Extractant and Characterization
ADDMDPA was synthesized following a method first described by Largman et al. [6] with an original modification developed in our laboratory [7]. The product presented the following properties, and its formula is shown in Figure 1. Formula: CH3-(CH2)11 N(CH2 P(O)(OH)2)2 IR u (cm−1): 2750(νas PO), 1120 (νs PO), 1015 (νas P-OH), 966 s P-OH); 1H NMR (D2O, Na2CO3) δ/TMS (ppm): 1.35 (s, 4H, CH2), 1.75 (m, 12H, CH2), 3.125 (d, 2JHP = 8.92 NCH2-P), 3.35 (m, 2H, N-CH2); 31P NMR(D2O, Na2CO3): δ/H3PO4 (ppm) 8.78; 13C NMR (D2O, Na2CO3) (ppm):13.5 (s, C1), 17.14(s, C2), 30 (s, C3), 50 (d, 2JCP = 138.7, NCH2-P).
The obtained pKai: 3.25, 8.4 and 9.3 indicated that in water-acetone medium, the fourth acidity is not attained corresponding to very weak acidity.
The presence of P = O wide band indicates hydrogen intermolecular bonds P = O·H-OP and N|·> H-O-P.
The ADDMDPA is soluble in most organic solvents, and shows intermolecular hydrogen bonding, forming polymers depending on the solvent polarity [10].
Thus, in chloroform that we used in our study, the ADDMDPA is generally present in a dimeric form, as shown in Figure 1 [11,12].
2.2.2. Extraction Experiments
The extraction experiments were carried out with ADDMDPA dissolved in chloroform. After various preliminary tests with different solvents, chloroform has been chosen since it dissolves the extractant without trouble or emulsion.
The appropriate volume of the aqueous solution (10 mL) containing the metal ion and the ADDMDPA (aqueous/organic volume ratio of 1:1) were mixed in glass flasks.
The mixtures were shaken in a moderate way, for at least 6 min for Bi(III). All the experiments were carried out at 20˚C.
3. Results and Discussion
The results of the extraction experiments will be dis-
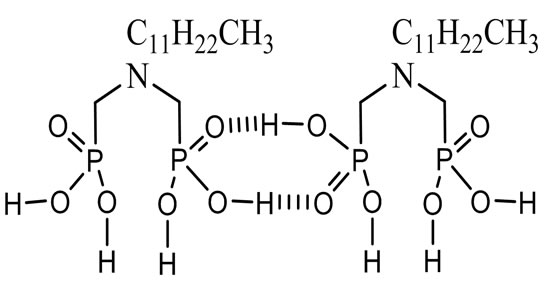
Figure 1. Dimeric form of ADDMDPA.
cussed in terms of the distribution coefficient, D, and the extraction yield, Y [13].
 (1)
(1)
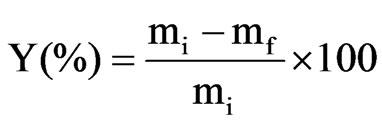 (2)
(2)
with the variables being as follows: mi: initial mass of metal ion in the aqueous phase; mf: mass of metal ion in the aqueous phase after extraction; Vorg: volume of the organic phase; Vaq: volume of the aqueous phase.
The molar ratio Q is defined as the ratio of the number of moles of the ligand in the organic phase versus the number of moles of metal in the aqueous phase before extraction.
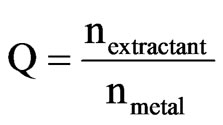 (3)
(3)
3.1. Extraction Kinetics
The equilibrium times were 6 min for Bi(III). The results are shown in Figure 2.
Figure 2 shows that the maximum extraction yield was obtained after 6 min of moderate shaking
3.2. Effect of the Molar Ratio
Figure 3 shows that the optimal extractant concentration that gives the maximum yield (52%) was 2 that correspond to extactant concentration equal to 1 mmol.
The stoichiometric coefficients obtained from the plots ln D vs ln [AADDMDP] and ln D vs pHeq, shown in Figures 4 and 5 respectively, may suggest the reaction mechanism in neutral media. This result led us to propose the following extraction equilibrium.
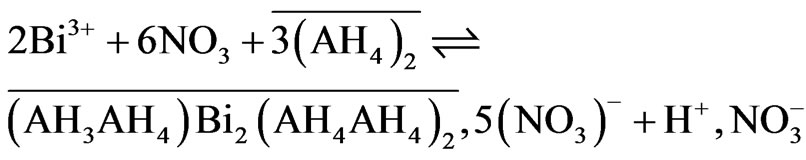 (4)
(4)
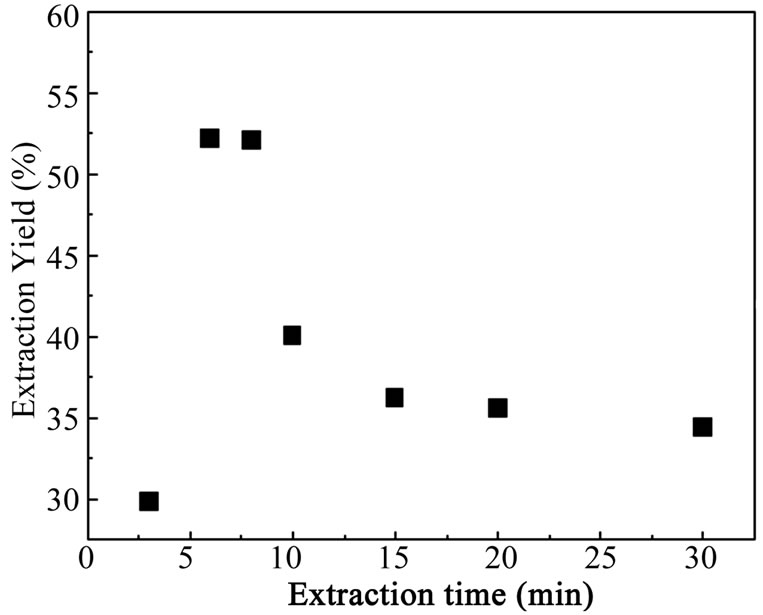
Figure 2. Extraction kinetics of Bi(III); [Bi+3]0 = 5 × 10−4 M, [ADDMDPA]0 = 10−3 M, Vaq/Vorg = 1, T = 20˚C, pHi = 2.9.

Figure 3. Effect of the molar ratio on the extraction yield of Bi (III) in nitrate medium; [Bi3+]0 = 5 × 10−4 M, Vaq/Vorg = 1, t = 6 min, T = 20˚C.

Figure 4. Effect of extractant concentration on the distribution ratio of Bi(III). T = 20˚C, Vaq/Vorg = 1.
The AADDMDP extracts in cationic exchange mode with substantial yields because the extractions are done to only one cycle.
3.3. Influence of the Ionic Strength on the Extraction of Bi3+
The influence of the ionic strength on the extraction yields of Bi(III) with AADDMDP diluted in chloroform, was studied by adding potassium nitrate and sodium acetate to the aqueous phase at the same concentration 0.01 M.
The yield of extraction of Bi(III) decreases with the increase of concentration of the sodium acetate in aqueous phase.
According to these results, it is noticed that the addition of CH3COONa decreases the extraction yield, on the other hand the addition of KNO3 increased it to 70%. This is due probably to the basic medium imposed by the sodium acetate; consequently we were interested to the study of the evolution of the yield according to the variation of the concentration of KNO3 in the aqueous phase.
3.4. Effect of the Addition of KNO3
In order to study the influence of the ionic force on the
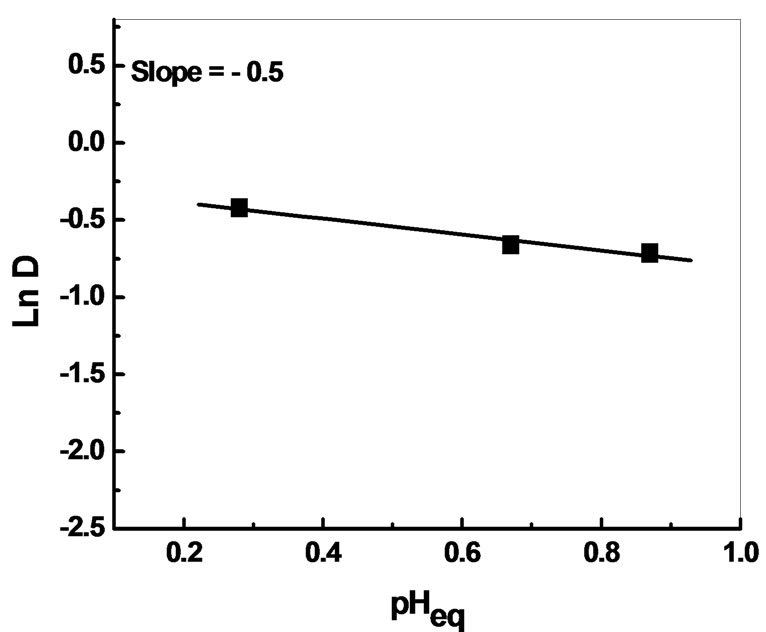
Figure 5. Effect of equilibrium pH on the distribution ratio of Bi(III).
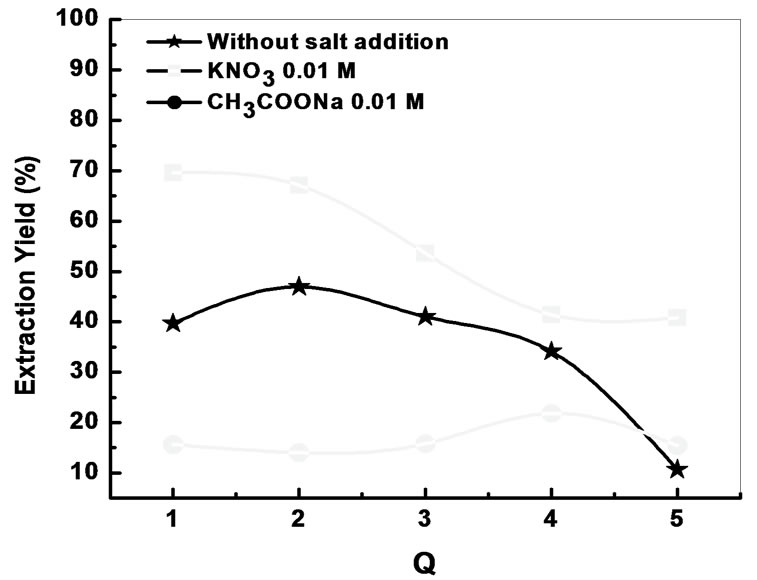
Figure 6. Effects of molar ratio on the extraction yield, with and without salt addition.
extraction of Bi3+, three quantities of KNO3 were added to the aqueous phase before extraction.
According to these results, after the addition of KNO3 0.01 M, the yield is 70% with Q = 1. In the interval of 0.1 - 1 M, the increase of the concentration of KNO3 will have a negative effect on the extraction yield, because of the competition between the two cations (K+, Bi3+).
The stoichiometric coefficients obtained from the plots Log E vs. Log [AADDMDP] and Log E vs. pHeq, shown in Figures 8 and 9 respectively may suggest the reaction mechanism of bismuth extraction.
The slopes are respectively equal to (0.75) near to 1 for extractant and 1 for the pH.
The equilibrium equation is written as follows:
 (5)
(5)
3.5. Effect of Nitric Acid on the Extraction of Bi (III)
We note that in acidic media, the extraction yield decreases drastically reaching a minimum of 5%.
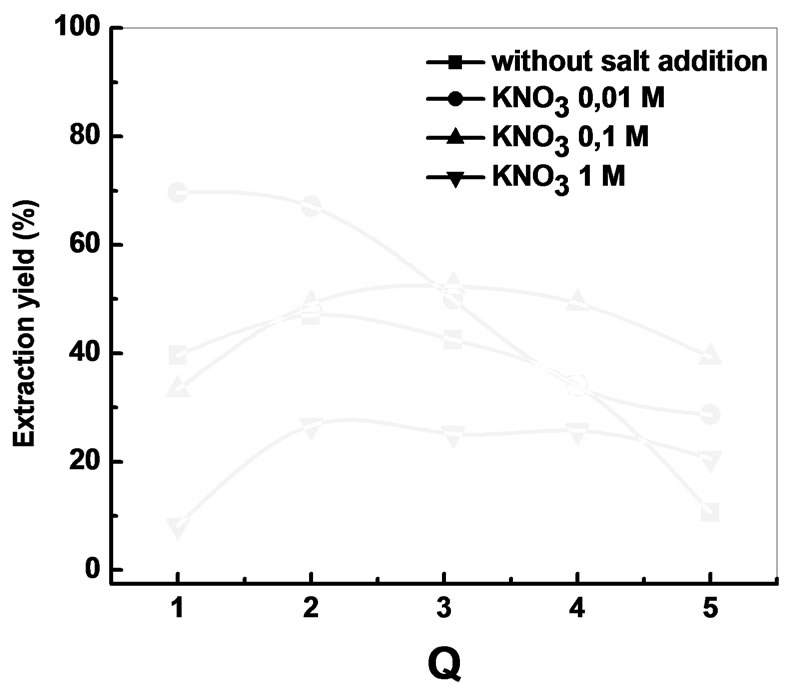
Figure 7. Effects of molar ratio on the extraction yield after the addition of potassium nitrate [Bi3+ ] = 5 ´ 10−4 M, Vaq/ Vorg = 1, t = 6 min, T = 20˚C.
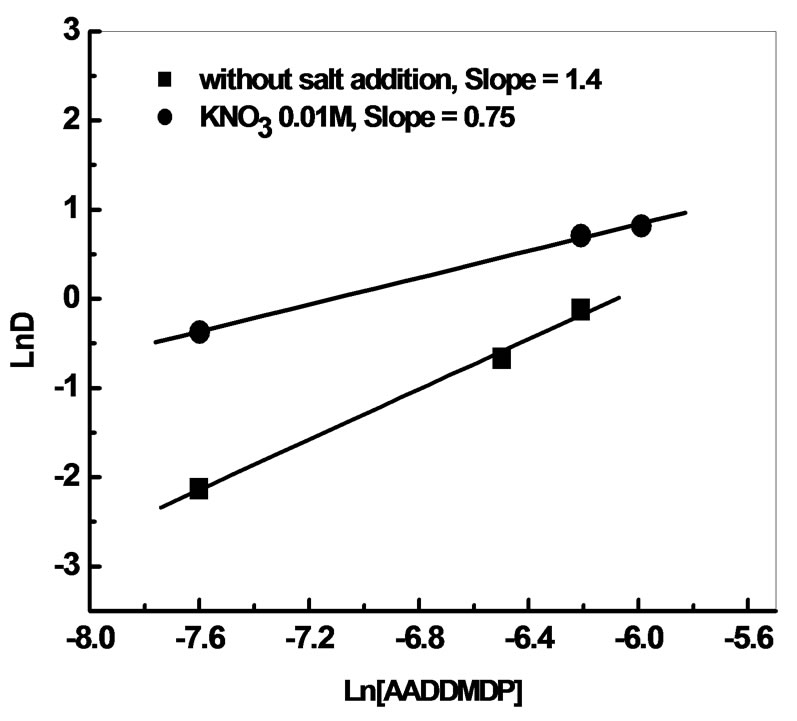
Figure 8. Effect of extractant concentration on the distribution ratio of Bi(III) before and after addition of potassium nitrate. [Bi3+] = 5.10−4M, Vaq/Vorg = 1, t = 6 min, T = 20˚C.
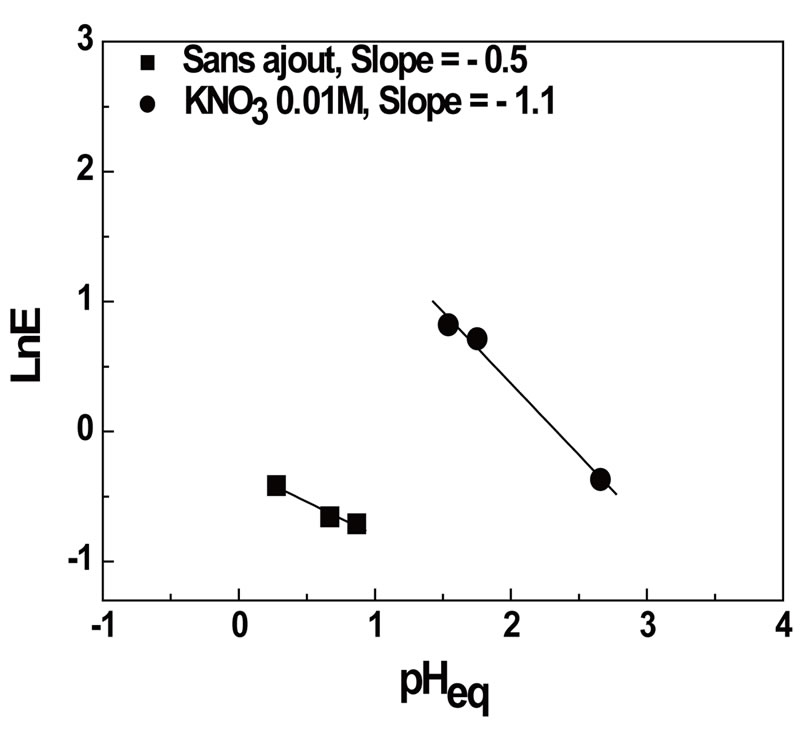
Figure 9. Effect of equilibrium pH on the distribution ratio of Bi(III) before and after addition of potassium nitrate. [Bi3+]0 = 5 × 10−4M, Vaq/Vorg = 1, t = 6 min, T = 20˚C.
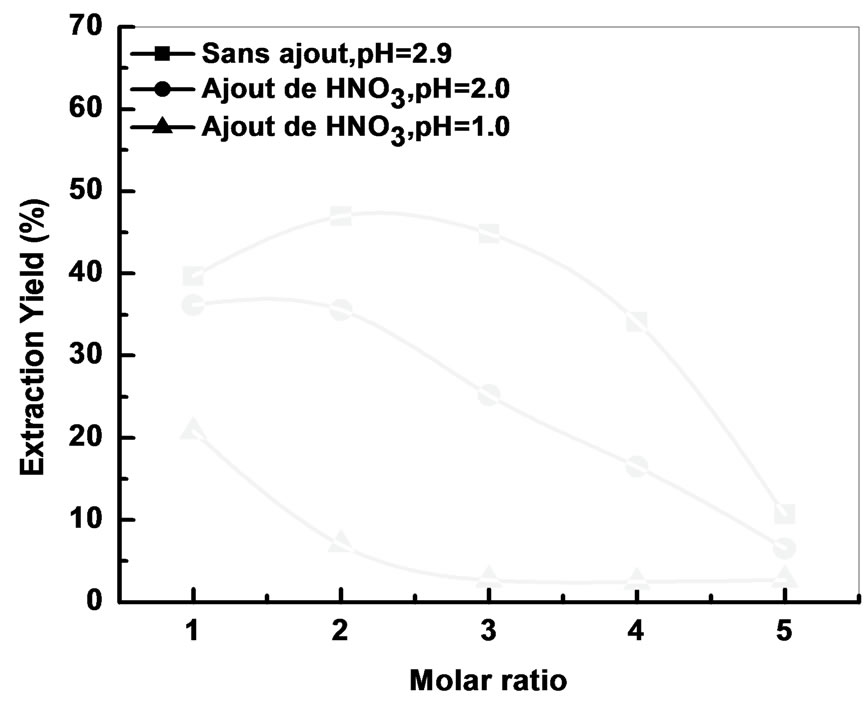
Figure 10. Effect of pH on the extraction yield [Bi3+] = 5 × 10−4 M, Vaq/Vorg = 1, t = 6 min, T = 25˚C.
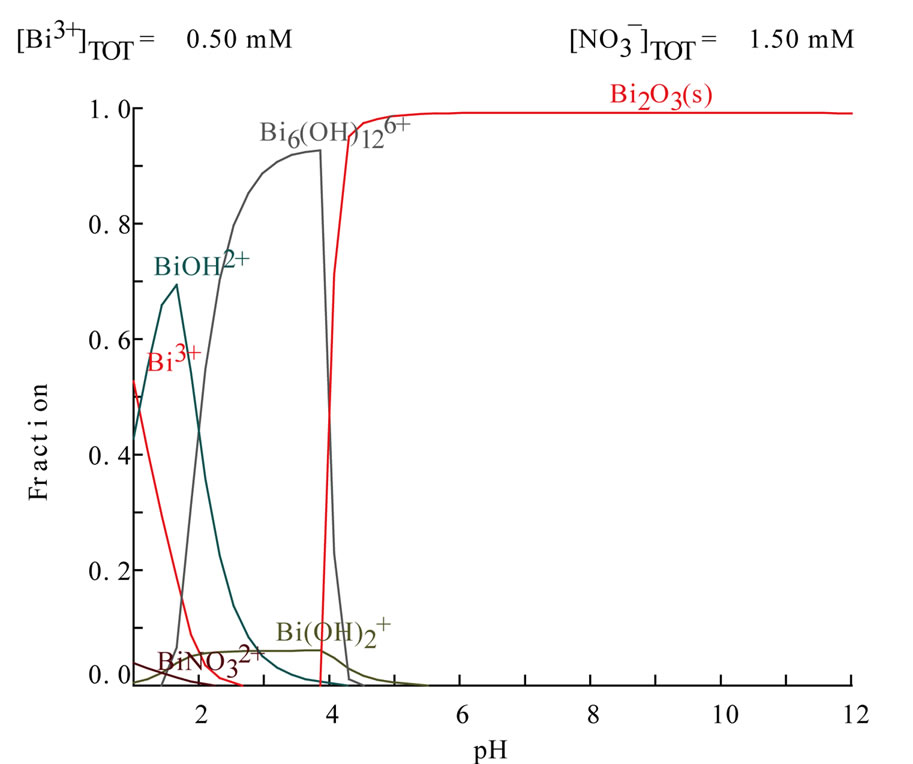
Figure 11. Distribution diagrams of bismuth ion (0.5 mmol·L−1) in nitrate media using Medusa and Hydra programs [14].
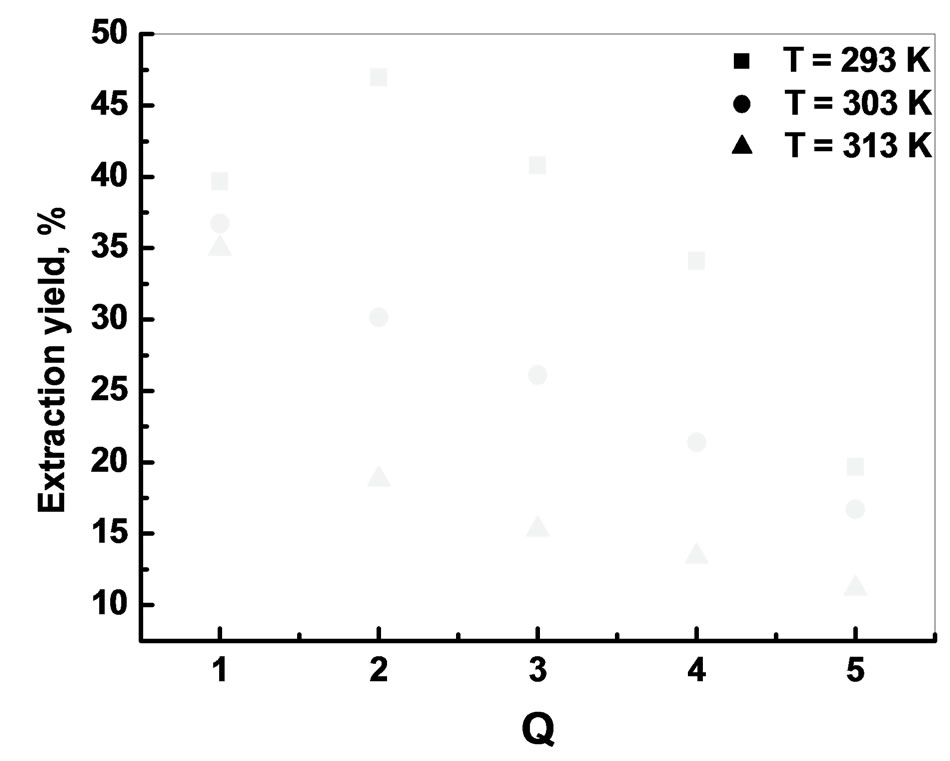
Figure 12. Effet of temperature on the extraction yield [Bi3+]0 = 5 × 10−4 M, Vaq/Vorg = 1, t = 6 min, T = 20˚C.
3.6. Effect of Temperature
The effect of temperature on the extraction of bismuth (III) ions was studied under optimum conditions.
Different thermodynamic parameters were computed using Van’t Hoff equation in the form.
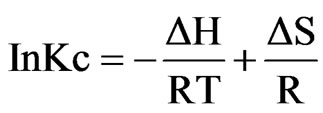 (6)
(6)
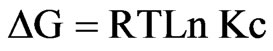 (7)
(7)
Where ∆H, ∆S, ∆G, and T are the enthalpy, entropy, Gibbs free energy, and temperature in Kelvin, respectively. The values of equilibrium ratio (Kc), were calculated at each temperature using the relationship.
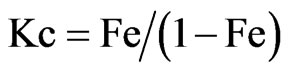 (8)
(8)
Where Fe is the fraction of Bi(III) ions extracted at equilibrium.
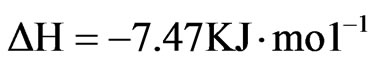
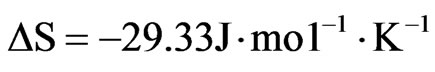
The plot of log Kc vs 1/T is a straight line as shown in Figure 13 with correlation coefficient r = 0.9947. The numerical values of ∆H, ∆S are computed from the slope. The negative value of Gibbs free energy as shown in Table 1 indicates the spontaneous nature of extraction, while negative value of ∆H reflects the exothermic extraction behavior. The negative value of ∆S indicates the complex stability
4. Conclusions
The solvent extraction of the species Bi(III) with ADDMDPA dissolved in chloroform was explained taking into account the formation of different complexes.
In nitrate medium, the extraction kinetic is very fast. The optimal extraction parameters for metal ions concentration of 0.5 mmol·L−1 in the aqueous phase and 1

Figure 13. Variation of log Kc with 1/T for the extraction of bismuth(III).
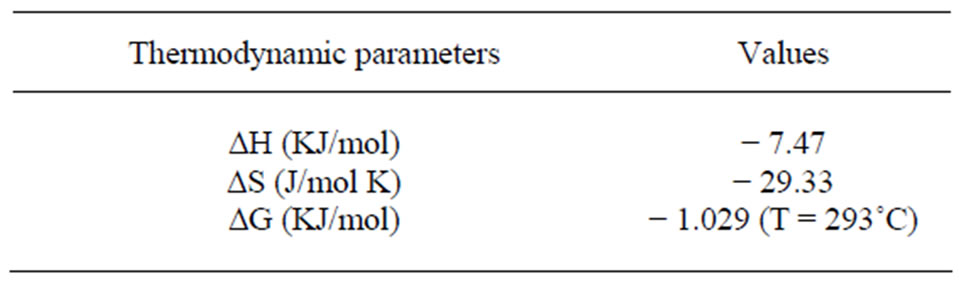
Table 1. Thermodynamic constants of the extraction of bismuth (III)) ions
mmol·L−1 in the organic phase, are Q = 2, Vaq/Vorg = 1, T = 25˚C.
The addition of CH3COONa decreased the yield of extraction much while the addition of KNO3 to the same concentration of 0.01M with increased the extrability of our cation up to 70% with Q = 1.
The increase in the concentration of H+ of the aqueous phase had an antagonistic effect on the yield of extraction
5. Acknowledgements
We gratefully acknowledge the “Ministère de la Recherche et des Nouvelles Technologies”, CNRS (Centre National de la Recherche Scientifique) and the Program TASSILI 10MDU799 for their financial support.
REFERENCES
- M. Burguera, J. L. Burguera, C. Rondon and M. I. Garcia, “Determination of Bismuth in Biological Tissues by Electrothermal Atomic Absorption Spectrometry Using Platinum and Tartaric Acid as Chemical Modifier,” Journal of Analytical Atomic Spectrometry, Vol. 16, No. 10, 2001, pp. 1190-1195.
- F. Habashi, “Arsenic, Antimony, and Bismuth Production,” Encyclopedia of Materials: Science and Technology, 2008, pp. 332-336.
- J. D. Jorgenson, “US Geological Survey,” Mineral Commodity Summaries, Eastern Region, Reston, 2003, p. 37.
- J. A. Reyes-Aguilera, M. P. Gonzalez, R. Navarro, T. I. Saucedo and M. Avila-Rodriguez, “Supported Liquid Membranes (SLM) for Recovery of Bismuth from Aqueous Solutions,” Journal of Membrane Science, Vol. 310, No. 1-2, 2008, pp. 13-19. HUdoi:10.1016/j.memsci.2007.10.020U
- E. M. Donaldson and M. Wang, “Determination of Silver, Antimony, Bismuth, Copper, Cadmium and Indium in Ores, Concentrates and Related Materials by Atomicabsorption Spectrophotometry after Methyl Isobutyl Ketone Extraction as Iodides,” Talanta, Vol. 33. No. 3, 1986, pp. 233-242. HUdoi:10.1016/0039-9140(86)80057-1U
- J. G. Yang, J. Y. Yang, M. T. Tang, C. B. Tang and W. Liu, “The Solvent Extraction Separation of Bismuth and Molybdenum from a Low Grade Bismuth Glance Flotation Concentrate,” Hydrometallurgy, Vol. 96, No. 4, 2009, pp. 342-348. HUdoi:10.1016/j.hydromet.2008.12.006U
- K. Campos, R. Domingo, T. Vincent, M. Ruiz and A. M. S. Guibal, “Bismuth Recovery from Acidic Solutions Using Cyphos IL-101 Immobilized in a Composite Biopolymer Matrix,” Water Research, Vol. 42, No. 14, 2008, pp. 4019-4031. HUdoi:10.1016/j.watres.2008.07.024U
- E. P. Horwitz, H. Diamond, K. A. Martin and R. Chiarizia, “Extraction of Americium (III) from Chloride Media by Octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine Oxide,” Solvent Extraction and Ion Exchange, Vol. 5, No. 3, 1987, pp. 419-446. HUdoi:10.1080/07366298708918575U
- D. Villemin, B. Moreau, A. Elbilali, M. A. Didi, M. Kaid and P. A. Jaffres, “Green Synthesis of Poly(aminomethylenephosphonic) Acids,” Phosphorus, Sulfur and Silicon, Vol. 185, No. 12, 2010, pp. 2511-2519. HUdoi:10.1080/10426501003724897U
- A. Buch, M. Stambouli, D. Pareau and G. Durang, “Solvent Extraction of Nickel (II) by Mixture of 2-Ethylhexanal Oxime and Bis(2-ethylhexyl) Phosphoric Acid,” Solvent Extraction and Ion Exchange, Vol. 20, No. 1, 2002, pp. 49-66. HUdoi:10.1081/SEI-100108824U
- M. A. Didi, A. Elias and D. Villemin, “Effect of Chain Length of Alkane-1-hydroxy-1,1’-methyl Diphosphonics Acids on the Iron (III) Liquid-Liquid Extraction,” Solvent Extraction and Ion Exchange, Vol. 20, No. 4-5, 2002, pp. 407-415. HUdoi:10.1081/SEI-120004813U
- J. R. Ferraro, A. W. Herlinger and R. Chiarizia, “Correlation of the Asymmetric and Symmetric POO Frequencies with the Ionic Potential of the Metal Ion in Compounds of Organophosphorus Acid Extractants: A Short Review,” Solvent Extraction and Ion Exchange, Vol. 16, No. 3, 1998, pp. 775-794. HUdoi:10.1080/07366299808934552U
- P. E. Body, P. R. Dolan and D. E. Mulcahy, “Environmental Lead: A Review,” Critical Reviews in Environmental Control, Vol. 20, No. 5-6, 1991, pp. 299-310. HUdoi:10.1080/10643389109388403U
- I. Puigdomenech, “HYDRA (Hydrochemical EquilibriumConstant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs,” Royal Institute of Technology, Sweden. http://www.kemi.kth.se/medusa
NOTES
*Corresponding author.

