Computational Water, Energy, and Environmental Engineering
Vol.06 No.03(2017), Article ID:77938,23 pages
10.4236/cweee.2017.63019
Carbon Footprint Analyses of Wastewater Treatment Systems in Puducherry
G. Vijayan1*, R. Saravanane2, T. Sundararajan2
1Public Works Department, Puducherry, India
2Department of Civil Engineering, Pondicherry Engineering College, Puducherry, India

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 21, 2017; Accepted: July 24, 2017; Published: July 27, 2017
ABSTRACT
Carbon footprint analysis is a method to quantify the life cycle Greenhouse Gases (GHGs) emissions and identify the measure to reduce climate change impacts. The Intergovernmental Panel on Climate Change (IPCC) has identified that the global warming and climate change which is one of the most important issues in the domain of environment are caused by the excessive emission of Greenhouse Gases (GHG) mainly constituting Carbon dioxide (CO2), Methane (CH4) and Nitrous oxide (N2O). The municipal wastewater treatment plant receives wastewater for treatment and finally discharges the treated effluent. The emissions of GHG during the treatment of wastewater as well as during the treatment process of sludge and also for energy generation are known to be on-site GHG emissions. Off-site GHG emissions are generated due to transportation and disposal of sludge, off-site energy and chemical production. In Puducherry, the municipal wastewater is being treated using oxidation ponds, Upflow Anaerobic Sludge Blanket (UASB) and Sequencing Batch Reactor (SBR). Wastewater treatment using Sequencing Batch Reactor (SBR) technology is one of the state-of-the art wastewater management systems. In this technology equalization, biological treatment and secondary clarification are performed in a single reactor in a time control sequence. The emissions of GHG from the Oxidation ponds of 12.5 MLD, UASB reactor of 2.5 MLD and SBR of 17 MLD were assessed based on the IPCC guidelines and the total emissions of GHG in terms of equivalent of CO2 were compared. The performance of the SBR is more efficient and the emissions of GHG are less than the emissions in the UASB as well as in oxidation ponds. The emission of GHG in SBR is about 60% of the existing treatment systems of oxidation ponds and UASB thus a reduction of 40% GHG emission could be achieved.
Keywords:
Greenhouse Gas (GHG), Intergovernmental Panel on Climate Change (IPCC), Global Warming Potential (GWP), Sequencing Batch Reactor (SBR), Upflow Anaerobic Sludge Blanket (UASB)

1. Introduction
1.1. Definition of Carbon Footprint
Carbon footprint is defined as the total set of greenhouse gas emissions caused by an activity or product expressed as carbon dioxide equivalent. It is a measure of the total amount of carbon dioxide (CO2), methane (CH4) and Nitrous Oxide (N2O) emissions of a defined system or activity, considering all relevant sources and sinks within the system or activity. It is calculated as carbon dioxide equivalent using the relevant 100-year global warming potential (GWP100). The main constituents of the GHG are carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O). The accounted GHG emissions are indexed in terms of global warming potential (GWP) by converting in terms of CO2 with a base value over a period of 100 years namely, 1 for CO2, 21 for CH4 and 310 for N2O [1] [2] . Even though CO2 emissions from biological wastewater treatment are not normally considered, some studies have pointed out that about 20% of the carbon present in the wastewaters can be of fossil origin and the emissions of fossil CO2 from wastewater treatment were underestimated. The sources of GHG may be either natural or anthropogenic. Further the identification and quantification of all sources are essential for developing the strategy to control and reduce the rate of increase of the emissions of GHG. The wastewater treatment plants (WWTP) are considered as source of GHG emissions because of the generation of CO2, CH4 and N2O during the process of treatment and energy demand.
In a defined system boundary, the emissions of GHG from different scenarios can be estimated and evaluated. The GHG emissions include 1) direct emission of GHG from wastewater treatment comprising emission of CO2 due to degradation of organic matters, emission of N2O during the process of nitrification and denitrification and emissions of CH4 and N2O from anaerobic digestion during sludge treatment and 2) indirect emissions of GHG from sludge treatment, usage of electrical power and chemicals during the operation and maintenance of the treatment plant and disposal of sludge. Apart from these, the production and transportation of construction materials also cause indirect emissions of GHG [3] .
1.2. Carbon Credit Scheme in Wastewater Treatment
Global warming and climatic change is being viewed as an international problem and several studies establish the significance of Greenhouse Gas (GHG) emissions from the wastewater process and the impact on the ecosystem. Wastewater treatment plants (WWTPs) are some of the sources of GHG emission. The wastewater treatment processes use biological and physio-chemical processes for the removal of contaminants and produce the three primary GHGs, i.e. carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) during the treatment operation and energy generation processes.
Aerobic treatment systems produce primarily CO2, whereas anaerobic systems produce a mixture of CH4 and CO2. When the sludge generated from the wastewater treatment unit is digested on-site, then, there will be additional CO2 and CH4 emissions. N2O is produced during the processes denitrification, nitrification and during chemical reactions that take place in the WWTP. There is considerable interest to determine carbon footprints of Wastewater Treatment Plants (WWTPs) with respect to Greenhouse Gas (GHG) emissions, energy usage, energy production, and carbon credits.
The Global Warming Potential (GWP) of a GHG is the ratio of heat trapped by one unit mass of the gas compared to one unit mass of CO2 over a specified time period (typically 100 years). The GWP of N2O is 310 kg equivalent CO2 and for CH4 it is 21. To model the GHGs of a WWTP it is therefore important to take the N2O emissions into account. The GWP varies significantly, depending on the type of GHG. Therefore, a small quantity of GHG emitted with a high GWP has a greater effect on the atmosphere than a GHG with low GWP.
1.3. The Global Greenhouse Gas Emissions
The IPPC has developed the concept of Global warming potential (GWP) for comparing the ability of each of the GHG to trap heat in the atmosphere relative to another gas. The GWP of a GHG is the ratio of heat trapped by one unit mass of the gas compared to one unit of CO2 over a time period of 100 years [4] . The key greenhouse gases emitted due to anthropogenic activities are CO2, CH4 and N2O. As per IPCC (2014) based on global emissions from 2010, out of the total global GHG emissions, CO2 alone constitutes about 76% from fossil fuel, industries, forestry and land use. The CH4 constitute about 16%, N2O constitute 6% and F-gases constitute about 2% as shown in Figure 1.
The global greenhouse gas emissions by economic sector various sectors as per IPCC 2014 based on global emissions from 2010 are indicated in Figure 2. Out of the total GHG emissions, 25% is from Electricity & heat production, 24% is from agriculture, forestry & other land use, 6% from buildings, 14% is from transportation, 21% from industrial process and 10% from other energy. Generally, the CO2 emission from biological wastewater treatment is not considered while accounting the GHG emissions since it is biogenic origin. Out of the total emissions of CH4 and N2O from various sources about 18.1% is from waste disposal
Figure 1. Global greenhouse gas emissions by gas. Source: IPCC (2014) based on global emissions from 2010.
Figure 2. Global GHG emissions by economic sector.
and treatment. Similarly, out of the total emissions of N2O, 2.3% is from waste disposal and treatment.
1.4. Trends in Global CO2 Emissions: 2014 Report
Among the six largest CO2 emitting countries, remarkable trends were reported in the top 3 CO2 emitting countries, which account for 55% of total global CO2 emissions in 2011 [5] . China is the largest CO2 emitting country, sharing about 28% of the total emissions of CO2 in 2011 which was much larger than the second-largest, the United States, with 16%, the European Union with 10%, India with 6%, The Russian Federation with 6% and Japan with 4% as shown in Figure 3. India is the fourth largest CO2 emissions country in 2011 with a CO2 emission of about 2.1 billion tonnes. The increase in emission level is partly because of increase in population and economy. The per capita CO2 emission of India is 1.7 tonnes of CO2, which is much lower than those of most developed countries and China. The output of domestic wastewater in urban areas is influenced by multiple factors. The gross domestic product (GDP) is an important comprehensive indicator reflecting the economic development of a country. Economic development and improvement of people’s living conditions promote an increase in domestic wastewater and COD discharge and removal. A study on the characteristic data and GDP data on domestic wastewater treatment showed that the quantity of domestic wastewater effluent grows annually with a stable GDP growth. The statistical calculation model for the quantity of domestic wastewater effluent showed a linear relationsahip with GDP. The relevant data of GDP and the characteristics of wastewater can be used to analyze the relationship between GDP and domestic wastewater discharge, and COD discharge and removal. The trends in share of national GDP and the CO2 emissions are shown in Table 1.
1.5. Greenhouse Gas Emissions in India
Internationally, the Indian Government has voluntarily agreed to reduce the
Figure 3. National CO2 emissions. Source: IPCC (2014) based on global emissions from 2010 and 2011.
Table 1. Trends in national GDP (%) and CO2 emissions in during 2013.
Source: Trends in global CO2 emissions: 2014, PBL Netherlands Environmental Assessment Agency.
emissions intensity of its gross domestic product (GDP) by 20 - 25 percent from 2005 levels by 2020. Indian and international studies suggest that India is likely to meet or even exceed this pledge based on its existing policy package and macroeconomic trends. Nevertheless, significant uncertainty surrounds the effective implementation of these policies and changes in the GDP composition [6] .
The emissions of GHG from wastewater are from domestic and disposal from the industries. The total CO2 equivalent emissions from waste water generating sources in India in 2007 was 45 million tons, which is 82% of the total CO2 equivalent emissions from the waste sector [7] . The total methane emitted in 2007 was 1.9 million tons and N2O emitted was 15.8 thousand tons as shown in Table 2.
In India, domestic wastewater has been categorized as urban high, urban low & rural, since the characteristics of the municipal wastewater vary from place to place depending on several factors such as economic status, food habits of the community, water supply status and climatic conditions of the area. In India, the wastewater treatment is provided only in Class I and II cities. Sewage contributes to 60% of the total pollution load in terms of biological oxygen demand which is beneficial if recovered through the anaerobic treatment process.
Table 2. GHG emission from waste water sector in India (Thousand tonnes).
1.6. The GWP Values for the GHG
Determination of carbon footprints of Wastewater Treatment Plants (WWTPs) with respect to greenhouse gas emissions, energy usage, energy production, and carbon credits are gaining interest. For the estimation of GHG emissions in a WWTP, the inventory of all GHGs emitted has to be considered and the appropriate global warming potential (GWP) for each gas has to be determined. The Global Warming Potential (GWP) of a GHG is the ratio of heat trapped by one unit mass of the gas compared to one unit mass of CO2 over a specified time period (typically 100 years). The GWP varies significantly based on the type of constituent GHG. A small quantity of gas emitted with a high GWP will have the same heat trapping potential as that of large quantity of gas emitted with low GWP [8] .
The GWP varies significantly, depending on the type of gas. Therefore, a small quantity of gas emitted with a high GWP has a greater effect on the atmosphere than a gas with low GWP. For example one kilogram (kg) of N2O emitted will have the same heat trapping potential as 310 kg of CO2. The Global Warming Potential of GHGs produced in WWTPs as per IPCC, 2001, Research Triangle Institute, 2010 (USEPA) are shown in Table 3.
The emissions of GHG in WWTPs are associated with both on-site and off-site sources. The on-site sources of GHG emission include liquid and solid treatment processes as well as the combustion of fuels and biogas for energy generation. The off-site sources include the production and transmission of electricity, fuel and chemicals for off-site use, as well as solid waste transportation and disposal such as landfill, composting and degradation of remaining constituents in liquid effluent. The GHG emission by on-site and off-site processes in aerobic, anaerobic and hybrid system of biological wastewater treatment plants is shown in Figure 4.
Table 3. Global warming potential (GWP) of the GHGs.
Source: RTI (USEPA)-Research Triangle Institutes International, United States Environmental Protection Agency.
1.7. Objective of the Study
The objective of this study is to examine the emission of the greenhouse gases and the global warming potential due to the treatment of wastewater in Puducherry, India and to assess the possible reduction potential of GHG emissions to obtain carbon credit.
2. Study Area and Present Scenario
Puducherry, the erstwhile French Colony is a coastal city, located at 11˚58’12"N, 79˚48’40"E and it is located at 162 km south of Chennai, India. The urban population of Puducherry as per 2011 census is 6.54 lakhs. Puducherry town has been provided with underground sewerage facilities partially as early as 1980 and the municipal wastewater has been treated with an oxidation pond of 2.9 MLD capacity at Karuvadikuppam, in the north western part of the Puducherry. Later on, three more oxidation ponds with treatment capacities of 2.9, 2.2, 4.8 MLD and two Upflow Anaerobic Sludge Blanket (UASB) reactors each having a capacity of 2.5 MLD were added. The installed capacity of all the existing STPs is 17.8 MLD. The BOD removal efficiency of the oxidation ponds and UASBs are about 60% only. Rapid urbanization and limitation of available space have necessitated adopting modern treatment methods. Accordingly, three Sequencing Batch Reactors (SBRs), each having a capacity of 17 MLD have been provided at
Figure 4. GHG emission by on-site and off-site processes in the biological wastewater treatment plants (NBR = no biogas recovery, WBR = with biogas recovery). Source: Water Science & Technology 2013, 67.5, p. 1163.
Karuvadikuppam, Dubrayapet and Kanakaneri. All these sewage treatment plants are small scale WWTPs with low carbon footprint. The sludge treatment and disposal not only concern environmental pollution problems, but also play a critical role in reducing the carbon footprint of the whole process. In other words, the energy content in organic matters in the wastewater is either converted to CO2 (or CH4) or wasted as sludge through the biological process. The sludge from all these processes are treated by aerobic composting in sludge drying yard and the digested sludge are used for land application. The location of the study area and the STP sites is shown in Figure 5.
3. Methodology
3.1. GHG Emissions
Both aerobic and anaerobic wastewater treatment processes remove BOD5 and the bacteria in both processes also generate CO2, CH4 and N2O [9] . The methodology used to estimate GHG emissions is based on the Intergovernmental Panel on Climate Change (IPCC, 1996) Revised Guidelines for National Greenhouse Gases Inventory and the IPCC Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories.
Aerobic: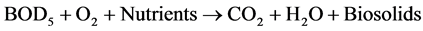
Figure 5. Location map of the study area.
The anaerobic process is actually performed by heterotrophic bacteria in a 2-step process as follows:
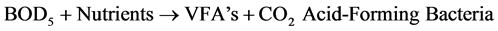
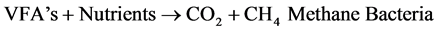
During aerobic treatment, ammonia (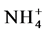 ) or organic nitrogen is biologically oxidized to nitrites (
) or organic nitrogen is biologically oxidized to nitrites (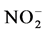 ) and nitrates (
) and nitrates ( ) by autotrophic bacteria through a process called nitrification.
) by autotrophic bacteria through a process called nitrification. 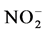 and
and 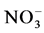 can then be converted to nitrogen gas (N2) under anoxic conditions (i.e., where dissolved oxygen is absent) by heterotrophic bacteria through a process called denitrification. N2O is a by-product of the nitrification process and an intermediate product of the denitrification process.
can then be converted to nitrogen gas (N2) under anoxic conditions (i.e., where dissolved oxygen is absent) by heterotrophic bacteria through a process called denitrification. N2O is a by-product of the nitrification process and an intermediate product of the denitrification process.
Nitrification: 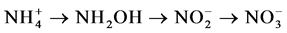
Denitrification: 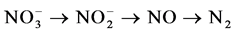
3.1.1. Aerobic Treatment
The aerobic treatment is the process in which the complex organic matter present in the wastewater is synthesized by the aerobic bacteria and converted into CO2. In aerobic treatment aeration is carried out either by diffused or submerged aerators in order to maintain adequate quantity of microorganism to oxidize the organic matters. Equalization, biochemical reactions and flocculation take place during aeration. The mixed liquor suspended solids shall be controlled to have sufficient concentration of aerobic bacteria or biomass. The aerated and well mixed liquor is subjected for sedimentation in the settling tank or clarifier. The excess sludge is removed from the setting tank. The activated sludge process is one of the most commonly used biological wastewater treatment system for treatment of both municipal and industrial wastewater. Sequencing batch Reactor system is known to be the pioneer of all activated sludge system and the SBR system operates in time rather than in space [10] - [15] .
3.1.2. Anaerobic Treatment
The organic matters are metabolized by methanogenic bacteria and synthesized in to new biomass or converted to CO2 or CH4 under anaerobic environment. Even though the growth of anaerobic bacteria is slower than the aerobic bacteria, the degradation of certain types of wastes by the anaerobic bacteria is more rapid than by the aerobic bacteria. During anaerobic digestion the acid- and CH4- forming (methanogenic) bacteria convert organic matter into a biogas consisting of approximately 60% - 70% CH4, 30% - 40% CO2, and trace amounts of N2, hydrogen (H2), hydrogen sulphide (H2S), and O2 [2] . The Upflow Anaerobic Sludge Blanket (UASB) is one of the efficient anaerobic treatment processes for treating both domestic and industrial wastewater.
Anaerobic wastewater treatment system is a more advantageous than the aerobic processes because of its low energy consumption and sludge production [16] . When methane is used as an energy source, GHGs emissions in anaerobic treatment are lower than that of the aerobic technologies and this process has the advantages of energy saving, biogas recovery and lower sludge production. Earlier it was viewed that anaerobic process was suitable for high-strength, high temperature wastewaters only, and that it required rigorous pH control and protection from toxic shocks. But recent researches have shown that anaerobic processes could also be applied to low strength wastewaters at low temperatures successfully.
3.1.3. Oxidation Ponds
Oxidation ponds or lagoons may be aerobic (shallow), anaerobic (deep) or facultative (medium depth) depending on the depth of the lagoons. In facultative lagoons aerobic condition will prevail due to aeration by the wind action and at the bottom of the lagoon anaerobic condition will prevail. Within the lagoon the generation of biomass occurs and accumulates at the bottom of the lagoon which is always in anaerobic condition. The CH4 is generated at the bottom of the lagoon and it travels upwards through the aerobic zone near the surface it is oxidized. The oxidation ponds are of different categories namely, aerobic, facultative and anaerobic ponds. In Puducherry there are four oxidation ponds having an area of 52,392 Sqm and an average depth of 1.2 m and the capacity of all the 4 oxidation ponds is 12.5 Mld. The average BOD removal efficiency in the oxidation ponds is from 50% to 60%. The emission of Greenhouse gases from the Oxidation ponds include CO2 and CH4 as the top surface of the oxidation pond is in aerobic zone and the bottom of the pond is in anaerobic zone. The stabilization ponds typically emit 85 g/m2 day of CO2 and 86 g/m2 day of CH4, with nitrous oxide levels significantly lower [17] [18] .
3.1.4. Upflow Anaerobic Sludge Blanket (UASB)
In an UASB reactor approximately 60% - 65% of the influent COD is converted to CH4, depending on the temperature and the characteristics of the sewage. The advantages of conversion of COD to CH4 are the reduction of the oxygen demand in the aerobic post-treatment unit and the CH4 can be utilized for the generation of heat and electricity. Though the UASB reactor has the advantage of having energy self-sufficient sewage treatment plant (STP) about (20% - 40%) of the CH4 produced in UASB reactors remains dissolved in the effluent. In most of the USABs the CH4 is emitted into the atmosphere instead of recovery and this leads to the reduction of the potential energy generation from the utilization of the produced biogas and emission of potent greenhouse gas (GHG) in to atmosphere [19] . The uncontrolled greenhouse gas emissions shall be avoided and non-flaring of captured CH4 shall be prohibited. If instead, all the energy is used, with the increasing energy prices and tradable CO2 credits, anaerobic sewage treatment will become an affordable investment for many developing countries.
3.1.5. Sequencing Batch Reactor (SBR)
The sequencing batch reactor technology is one of the most commonly used biological wastewater treatment processes at both municipal and industrial wastewater treatment plants. Even though there are many variations of activated sludge biological wastewater treatment processes, all the reactions take place in the same reactor and the reactor serves as aeration and sedimentation tank [20] . In SBR, primary sedimentation, biodegradation and biomass separation take place within a single reactor. SBR is a time-oriented technology instead of space-oriented, which can be operated with great flexibility. Ease operation of SBR could be realized by controlling bacterial species which cause filamentous bulking, remove nutrient or hazardous organics.
3.1.6. Greenhouse Gases (GHG)
Carbon dioxide (CO2): The main source of CO2 in wastewater is from treatment process and electricity consumption. During aerobic process in activated sludge process, the emission of CO2 is from the breakdown of organic matter in the aeration tank. During anaerobic process the organic matter is converted in to biomass and again in to CO2 and CH4 through endogenous respiration. The other source of CO2 is from sludge digestion and combustion of digester combustion gas [21] .
Methane (CH4): The main source of CH4 is during the anaerobic treatment of wastewater as well as from its sludge. The extent of emission of CH4 depends on the quantity of degradable organic material in the wastewater, the temperature, and the type of treatment system. The rate of CH4 emission increases with the increase in temperature. When the temperature is below 15˚C the methanogens are not active and there is almost no production of CH4.
The Total CH4 emissions from domestic wastewater can be calculated as follows:
where,
BODi is the total organic content in domestic wastewater in the calculation year (TPY);
EF is the emission factor (kg CH4 (kg BOD));
R is the amount of recycled CH4 in the calculation year (CH4).
where,
Bo, is the maximum CH4 generation capacity.
M is the CH4 correction for domestic wastewater.
Nitrous Oxide (N2O): The main source of N2O is from the degradation of nitrogen components in the wastewater. Direct emissions of N2O may be generated during both nitrification and denitrification process. Nitrification is an aerobic process during which the ammonia and other nitrogen compounds are converted into nitrates (NO3) and. Denitrification is the process which occurs under anoxic conditions in which the nitrate is converted in to nitrites NO2 and again in to nitrogen gas N2. Both processes can occur in the plant and in the water body that is receiving the effluent [21] .
3.2. Estimation of GHG Emissions from Wastewater and Sludge Treatment Units
The Research Triangle Institute International (RTI) has submitted a report to the U.S. Environmental Protection Agency, in which the method of estimating CO2, CH4 and N2O emissions from biological wastewater treatment systems based on IPCC has been recommended. The equations provide a general means of estimating the CO2 and CH4 emissions directly from any type of wastewater treatment process assuming all organic carbon removed from the wastewater is converted to either CO2, CH4 or new biomass. There are different methods to quantify the life cycle greenhouse gases emissions. However the method adopted in this study is as per report of RTI International, submitted to the USEPA based on IPCC.
3.2.1. Estimation of CH4 and CO2 Emissions from Wastewater
In wastewater treatment process all organic carbon removed from the wastewater is converted to CO2, CH4, or new biomass. Aerobic wastewater treatment systems produce primarily CO2 during the process of wastewater treatment, whereas in anaerobic systems a mixture of CH4 and CO2 is produced. The estimation of direct emission of CO2 and CH4 can be done by the following equations [2] .


The biomass yield λ can be determined from the following equation and when the biomass generation rate cannot be assessed, default values for the biomass yield provided in Table 4 and Table 5 can also be used.

3.2.2. Estimation of CH4 and CO2 Emissions from Sludge
1) When the sludge is digested on-site, then there will be additional CO2 and CH4 and this is applicable for all sludge digesters. The estimation of GHG can be done by the following equations, when:
a) The sludge digester is the only biological treatment process at the facility.
b) Additional waste streams are fed to the sludge digester.
c) Other physical/chemical treatment process are conducted on the sludge prior to the digester that alter the mass of carbon entering the digester.


2) For most sludge digesters, the only solids entering the unit are those generated in the wastewater treatment system and for these cases, the following equations can be used to determine the sludge digester’s emissions based on the feed to the wastewater treatment process.


where,
CO2 = CO2 emission rate (Mg CO2/hr);
CH4 = CH4 emission rate (Mg CH4/hr);
Qww = Wastewater influent flow rate (m3/hr);
OD = Oxygen demand of influent wastewater to the biological treatment unit determined as either; BOD5 or COD (mg/L = g/m3);
EffOD = Oxygen demand removal efficiency of the biological treatment unit;


MCFWW = methane correction factor for wastewater treatment unit;

Qs = Waste sludge stream flow rate (m3/hr);
Qww = Wastewater influent flow rate (m3/hr);
MLVSSs = Mixed liquor volatile suspended solids concentration of the waste sludge stream (mg/L);
λ = Biomass yield (g C converted to biomass/g C consumed in the wastewater treatment process).
3.2.3. Estimation of N2O Emissions
The amount of nitrogen present in the influent wastewater will determine the N2O generation potential. The treatment process will also affect the magnitude of the N2O emissions. N2O emissions for both aerobic and anaerobic processes using an average value for the percent of influent TKN emitted as N2O.
Table 4. Default values for methane correction factor (MCF) and biomass yield (λ).
Source: IPCC (2006).
Table 5. Correction factors for different measurement method.
Source: IPCC (2006).

where,
N2OWWTP = N2O emissions generated from WWTP process (Mg N2O/hr);
Qi = Wastewater influent flow rate (m3/hr);
TKNi = Amount of TKN in the influent (mg/L = g/m3);

= 0.0050 g N emitted as N2O/g TKN [22] .
Source: Research Triangle Institute report to USEPA.
3.3. Emissions of GHG from Industries
In respect of emissions of an industrial plant there Scopes have been defined by the United Nations. As per the GHG Protocol Initiative 2004, the Scope 1 is the direct GHG emissions which includes GHG emissions that occur from the own sources of the company. The Scope 2 includes the emissions that occur from the use of electricity and the generation of purchased electricity bought and consumed and the emissions from Scope 1. Due to the use of electricity of the plant the emissions during the production of electricity are to be included to the emissions of the plant. Scope 3 includes the emissions from Scopes 1 and 2 and the emissions that occur during the production of the chemicals that are used in the pants [8] .
To estimate the GHG emissions of the wastewater treatment plants (WWTP) in a comparable way the considered emissions have to be listed. The selected boundaries are from Scope 3 and are listed below (Bridle Consulting, 2007):
1) CO2 and N2O emissions at biotreatment, endogenous respiration, BOD oxidation nitrification CO2 credit and nitrogen removal;
2) Energy use of plant, for aeration, mixing and pumping which leads to CO2 emissions;
3) Sludge digestion, biogas CH4 and CO2;
4) Sludge disposal, truck emissions trip to reuse/disposal site, CO2 emissions mineralization;
5) Power credit by use of biogas;
6) GHG emissions from chemical use.
3.4. Treatment and Discharge Systems and CH4 and N2O Generation Potential
The treatment systems that provide anaerobic environments will generally produce CH4 whereas systems that provide aerobic environments will normally produce little or no CH4 [1] . In lagoons without mixing or aeration, their depth is a critical factor in CH4 production. Shallow lagoons, less than 1 meter in depth, generally provide aerobic conditions and little or no CH4 is likely to be produced. Lagoons deeper than about 2 - 3 meters will generally provide anaerobic environments and significant CH4 production can be expected.
Maximum CH4 Producing Capacity for Domestic Wastewater
The potential of CH4 that could be generated from the domestic wastewater can be determined based on BOD or COD as recommended by the Intergovernmental Panel on Climate Change (IPCC). The annual methane emissions from domestic wastewater can be expressed as (IPCC, 2002): [1] .
where:
CH4 Emissions = CH4 emissions in inventory year, kg CH4/yr;
TOW = total organics in wastewater in inventory year, kg BOD/yr;
S = organic component removed as sludge in inventory year, kg BOD/yr;
Ui = fraction of population in income group i in inventory year;
Ti,j = degree of utilization of treatment/discharge pathway or system, j, for each income group fractioning inventory year;
i = income group: rural, urban high income and urban low income;
j = each treatment/discharge pathway or system;
EFj = emission factor, kg CH4/kg BOD;
R = amount of CH4 recovered in inventory year, kg CH4/yr.
The emission factor is a function of the maximum CH4 producing potential (Bo) and the methane correction factor (MCF).

where,
EFj = emission factor, kg CH4/kg BOD;
j = each treatment/discharge pathway or system;
Bo = maximum CH4 producing capacity, kg CH4/kg BOD;
MCFj = methane correction factor (fraction).
Generally, the country specific data may be used to determine the emission factor. In the case of non-availability of country specific data, default values may also be used. Accordingly, default maximum CH4 producing capacity for domestic wastewater can be determined [23] .
The maximum CH4 producing capacity for domestic wastewater
= 0.60 kg CH4/kg BOD;
= 0.25 kg CH4/kg COD.
3.5. Control Measures for Reduction of GHG
3.5.1. GHG Emissions and Reduction Strategies
In order to control the emission of GHG in domestic wastewater the CH4 may be recycled and reused during the process. In anaerobic treatment system, the CH4 may be recycled to replace the wastewater or sludge oxidation treatment system. The COD discharge is the oxygen required for the chemical oxidant to oxidize the organic pollutants in the water. Generally the amount of CH4 generated from treated wastewater with high COD or BOD concentration is more than that generated from that with lower concentrations. COD/N ratio in the reactor seems to have an effect on N2O emission. During denitrification, in the activated sludge process a relatively low COD/N ratio is the main parameter leading to N2O production. The N2O increase with the decrease of NH3 and thus during aerobic digestion the nitrification plays an important role in N2O emissions [24] .
Research with the objective of reducing the GHGs emissions in the wastewater and waste treatment and disposal field was a hot topic in line with the commencement of Kyoto Protocol in 2008 until 2012. The reduced GHGs emission either from the wastewater treatment process improvement or effluent and biomass recycling will generate Certified Emission Reduction (CER) for sale or export. Generally, measurements intended to reduce GHGs emissions in the wastewater treatment section could be achieved through the following aspects.
3.5.2. Proper Wastewater Treatment
The emission of GHG depends on the selection of wastewater treatment process based on the wastewater composition, organic loading rate, and the anticipated effluent quality. Normally anaerobic wastewater treatment processes are preferred as they could reduce GHGs emission by energy recycling in the form of CH4. A study showed that the anaerobic wastewater treatments are applicable when level of the biochemical oxygen demand (BOD) concentrations of the wastewater is more than 300 mg/L [25] [26] . In a particular situation, various anaerobic treatment processes have different GHGs reduction potential. During the treatment of low strength wastewater the CH4 may be dissolved in the effluent leading to significant loss of CH4. The CO2 and CH4 generated due to improper wastewater treatment reduced the possibility of carbon sequestration and energy recovery [27] [28] and the GHG emissions can be reduced by the regulation and control of process and operational parameters such as oxidation reduction potential, DO and COD/N ratio.
3.5.3. Control of Wastewater Treatment Plants
Performance of control strategies with adjusted flow of different combinations of Qw values can enable a reduction of GHG emissions. The predominant source of reduction in operating costs is the reduction of sludge produced for disposal, not reduction in pumping costs. The energy cost actually increase due to increased aeration requirements to maintain the desired level. But the reduction of GHG emissions due to the reduction in energy required for pumping is negligible. A high SRT increases direct non-N2O emissions from the bioreactor and indirect emissions resulting from electricity use [16] .
DO control strategy offers superior performance with regard to GHG emissions, operational costs and effluent quality. Increasing the SRT, can result in reduction of emission and cost but direct non-N2O emissions are increased. Developing control strategies to provide the greatest possible energy recovery may not always be preferable with regard to reducing GHG emissions and operational costs, since the effects of reduced energy recovery can be offset by the reduction in cost and emissions associated with sludge disposal, and a greater effluent quality may be achieved.
3.5.4. Recycling of Treated Wastewater and Excess Sludge
The reduction in the total emission of CH4 could be achieved by the biogas reuse. The CH4 can also be used as a source of renewable energy for the generation of electricity. It may be utilized as raw material to produce organic acids or biodegradable plastic. Further the aerobically treated sludge could be used as manure or organic fertilizer and for soil reclamation.
3.5.5. Systematic Strategies for the Future Wastewater Treatment
The cost-effective and energy-saving technology with low solid waste and GHGs emission shall be preferred. The excess sludge is rich in carbon, nitrogen and phosphorus, and micronutrients which could be used as raw material for industrial production. The excess sludge could be reduced for further treatment by recycling, for reducing the emission of GHG and for cost reduction. The goal intended to reduce the GHGs emissions from wastewater and excess sludge disposal could be achieved through the proper treatment of wastewater, choices for rational wastewater treatment process and recycling of treated wastewater and excess sludge.
3.6. Sample Collection and Testing
The samples of both the influent and effluent were collected at regular interval and were tested as per the standard testing methods. The physiochemical and biological parameters like pH, temperature, TDS, TSS, BOD, COD, N as NO3 and P as PO4 were tested for both the influent and effluent. However in this study the average values of the influent and effluent BOD from the SBR, oxidation pond and UASB were adopted for the estimation of the emissions of GHG. Similarly, samples were collected from the SBR and tested for the BOD, MLVSS, TKN and other parameters to study the variation of the biomass yield coefficient in the estimation of GHG emissions.
4. Results and Discussion
In this study, the emissions of GHG in Puducherry from the oxidation ponds of 12.5 Mld, UASB of 2.5 Mld (2 Nos) and one SBR of 17 Mld were determined. Only the on-site sources for the emissions were considered in this study. The emissions of GHGs in wastewater and sludge in SBR, UASB and Oxidation ponds in Puducherry were determined based on the IPCC guidelines.
Estimation of CO2, CH4 and N2O from the SBR, UASB and Oxidation Ponds
The estimation of CO2 in the aerobic treatment of wastewater in SBR was calculated using the Equation (3.1). There is no emission of CH4 during aerobic process. The emissions of CO2 and CH4 during on site sludge digestion in the sludge drying bed due to the sludge digester’s emissions based on the feed to the wastewater treatment process by using the Equations (3.6) and (3.7) and the same is designated as SBR (A1). Similarly the emissions of CO2 and CH4 during on site sludge digestion were calculated based on the MLVSS and the volume of the sludge wasted by considering the Equations (3.4) and (3.5), since chemical treatment process are carried out on the sludge prior to the digester that alter the mass of carbon entering the digester and the same is designated as SBR (A2). The emission of N2O from the sludge digestion was also calculated using the Equation (3.8).
The emissions of CO2 and CH4 during the anaerobic treatment of wastewater in the UASB and additional emissions of CO2 and CH4 during on site sludge digestion in the sludge drying bed were calculated. In the case of oxidation ponds the emissions of CO2 and CH4 from the aerobic zone and the additional emissions of CO2, CH4 and N2O from the sludge digestion in the anaerobic zone were calculated and the details of estimation of CO2, CH4 and N2O. The estimation of GHG in UASB, Oxidation Ponds and SBR (A1) are shown in Table 6.
The emissions of CO2 and CH4 in SBR are influenced by the biomass yield coefficient λ and the efficiency of the BOD removal. The biomass yield coefficient is the ratio of the g C converted to biomass to the g C consumed in the wastewater treatment process. It is influenced by the volume of the sludge and the mixed liquor volatile suspended solids (MLVSS) concentration of the waste sludge stream (mg/L). The variation of CO2 emission from the wastewater is shown in Figure 6 and the variation of CO2 from the sludge is shown in Figure 7.
The emissions of CO2, CH4 and N2O for different values of biomass yield coefficient (λ) were calculated as given in Table 7. The variation of biomass yield coefficient and the emissions CO2 from wastewater (CO2-WW) and CO2 (CO2-Sludge), CH4 (CH4-Equ CO2), N2O (N2O-Equ CO2) and total equivalent CO2 from sludge in terms of equivalent CO2 are shown in Figure 8. It may be seen that as the biomass yield coefficient increases the emission of CO2 from wastewater decreases. But the total equivalent CO2, CO2 from sludge, CH4-Equ CO2, and N2O-Equ CO2 increase as the biomass yield coefficient increases.
The total emissions of CO2 and equivalent CO2 of CH4 and N2O for the biomass yield coefficients of 0.1 to 0.65 are shown in Figure 9.
The emissions of CO2, CH4 and N2O from various treatment plants and the equivalent CO2 of GHG and the total equivalent CO2 are shown in Table 8. The total emissions of equivalent CO2 from the UASB and Oxidation ponds are 9532 tons per year, whereas the total emissions of equivalent CO2 from the SBR is 7649 tons per year, which is only 80% of the total CO2 emissions. However,
Table 6. Estimation of CO2, CH4 and N2O from UASB, oxidation ponds and SBR.
Table 7. Emissions of GHG for various biomass yield coefficient.
further study in the determination of CO2 emissions showed that when other physical/chemical treatment process are conducted on the sludge prior to the digester, the emissions of CO2 could be reduced to 5793 tons per year, which is only 60% of the total equivalent CO2 emissions. The emissions of equivalent CO2 per unit of organic load i.e., per kg of BOD for each plant were also compared. The equivalent emissions of CO2per for the SBR were 2.83 kg per kg of BOD, whereas the CO2 emissions for the UASB and oxidation ponds were 6.17 and 3.86 kg per kg of BOD respectively. From these, it can be seen that the
Figure 6. Variation of CO2 emission (Mg/Hr) from wastewater in SBR.
Figure 7. Variation of CO2 emission (Mg/Hr) from sludge in SBR.
Figure 8. Emission of GHG (Mg/Hr) vs. biomass yield (λ).
performance of the SBR is better than the performance of the UASB and oxidation ponds in the emission of GHG.
Figure 9. Total emission of GHG-equivalent of kg CO2.
Table 8. Emissions of GHG in UASB, SBR and oxidation ponds.
5. Conclusion
The emissions of GHG from the UASB, oxidation ponds and SBR in Puducherry were estimated. The emissions of CO2, CH4 and N2O from all these treatment plants were also estimated based on the guidelines of IPCC and RTI-USEPA and the total equivalent CO2 was computed and analyzed. The impact of biomass yield coefficient on the emissions of GHG was also examined and their variations were also studied. The total emissions of equivalent CO2 from the SBR of 17 Mld capacity are less than20% of the total CO2 emissions of CO2 from the UASB and oxidation ponds of 17.5 Mld capacity. Further study revealed that by modifying the treatment process of the sludge from the SBR, by altering the waste sludge flow rate and the MLSS, the total emissions of equivalent CO2, could be reduced and it is possible to achieve a reduction of about 40% of total equivalent CO2 emissions. The study also established that the emissions of CO2 from the SBR are the least and that the SBR performs well and more efficient in terms of reduction of GHG emissions when compared to that of the UASB and oxidation ponds.
Cite this paper
Vijayan, G., Saravanane, R. and Sundararajan, T. (2017) Carbon Footprint Analyses of Wastewater Treatment Systems in Puducherry. Computational Water, Energy, and Environmental Engineering, 6, 281-303. https://doi.org/10.4236/cweee.2017.63019
References
- 1. Doorn, M.R.J., Towprayoon, S., Vieira, S.M.M., Irving, W., Palmer, C., Pipatti, R. and Wang, C. (2006) Intergovernmental Panel on Climate Change (IPCC) Guidelines for National Greenhouse Gas Inventories.
- 2. United States Environmental Protection Agency (USEPA), Research Triangle International (RTI) (2010) Greenhouse Gas Emissions Estimation Methodologies for Biogenic Emissions from Selected Source Categories.
- 3. Chai, C., Zhang, D., Yu, Y., Feng, Y. and Wong, M.S. (2015) Carbon Footprint Analyses of Mainstream Wastewater Treatment Technologies under Different Sludge Treatment Scenarios in China. Water, 7, 918-938.
- 4. Das, S. (2011) Estimation of Greenhouse Gases Emissions from Biological Wastewater Treatment Plants at Windsor. Electronic Theses and Dissertations, Paper 77.
- 5. Trends in Global CO2 Emissions: 2014 Report—The Hague: PBL Netherlands Environmental Assessment Agency. European Commission, Joint Research Centre, Ispra.
- 6. Pahuja, N., Pandey, N., Mandal, K. and Bandyopadhyay, C. (2014) GHG Mitigation in India: An Overview of the Current Policy Landscape. Working Paper, World Resources Institute, Washington DC. http://www.wri.org/publication/ghg-mitigation-ind-policy
- 7. MoEF (2010) India Greenhouse Gas Emissions (2007) Report of Ministry of Environment and Forests, Government of India.http://envfor.nic.in/
- 8. Snip, L.J.P. (2010) Quantifying the Greenhouse Gas Emissions of Waste Water Treatment Plants. Thesis Project Systems and Control, MES (Environmental Sciences), Wageningen, The Netherlands.
- 9. Ma, Z.-Y., Feng, Gao, Q.-X., Lu, Y.-N., Liu, J.-R. and Li, W.-T. (2015) CH4 Emissions and Reduction Potential in Wastewater Treatment in China. Advances in Climate Change Research, 6, 216-224.
- 10. U.S. EPA (1983) An Emerging Technology, Sequencing Batch Reactors. A Project Assessment, U.S. Environmental Protection Agency.
- 11. Arceivala, S.J. and Asolekakar, S.R. (2008) Wastewater Treatment for Pollution Control and Reuse. Third Edition, Tata McGraw-Hill.
- 12. U.S. EPA (1986) Design Manual, Summary Report Sequencing Batch Reactors. EPA/625/8-86/011.
- 13. U.S. EPA (1999) Wastewater Technology Fact Sheet Sequencing Batch Reactors. U.S. Environmental Protection Agency, EPA 832-F-99-073.
- 14. U.S. EPA (2000) Wastewater Technology Fact Sheet Package Plants. U.S. Environmental Protection Agency, EPA 832-F-00-016.
- 15. The New England Interstate Water Pollution Control Commission (2005) Manual for Sequencing Batch Reactor Design and Operational Considerations.
- 16. Sweetapple, C., Fu, G. and Butler, D. (2014) Multi-Objective Optimisation of Wastewater Treatment Plant Control to Reduce Greenhouse Gas Emissions. Water Research, 55, 52-62.
- 17. Butler, E., Hung, Y.-T., Al Ahmad, M.S., Yeh, R.Y.-L., Liu, R.L.-H. and Fu, Y.-P. (2015) Oxidation Pond for Municipal Wastewater Treatment. Applied Water Science, 7, 31-51.
- 18. Hernandez-Paniagua, I.Y., Ramirez-Vargas, R., Ramos-Gomez, M.S., Dendooven, L., Avelar-Gonzalez, F.J. and Thalasso, F. (2014) Greenhouse Gas Emissions from Stabilization Ponds in Subtropical Climates. Environmental Technology, 35, 727-734. https://doi.org/10.1080/09593330.2013.848910
- 19. Heffernana, B., Blancb, J. and Spanjersc, H. (2012) Evaluation of Greenhouse Gas Emissions from Municipal UASB Sewage Treatment Plants. Journal of Integrative Environmental Sciences, 9, 127-137.https://doi.org/10.1080/1943815X.2012.696546
- 20. Guo, J., Ma, F., Qu, Y., Li, A. and Wang, L. (2012) Systematical Strategies for Wastewater Treatment and the Generated Wastes and Greenhouse Gases in China. Frontiers of Environmental Science & Engineering, 6, 271-279.
- 21. Gupta, D. and Singh, S.K. (2015) Energy Use and Greenhouse Gas Emissions from Wastewater Treatment Plants. International Journal of Environmental Engineering, 7, 1-10.https://doi.org/10.1504/IJEE.2015.069251
- 22. Chandran, K. (2010) Greenhouse Nitrogen Emission from Wastewater Treatment Operations: Interim Report. Water Environment Research Foundation (WERF), Report No. U4R07a.
- 23. Doorn, M.R.J., Strait, R., Barnard, W. and Eklund, B. (1997) Estimate of Global Greenhouse Gas Emissions from Industrial and Domestic Wastewater Treatment. Final Report, EPA-600/R-97-091, Prepared for United States Environmental Protection Agency, Research Triangle Park, NC, USA.
- 24. Caniani, D., Esposito, G., Gori, R. and Mannina, G. (2015) Towards a New Decision Support System for Design, Management and Operation of Wastewater Treatment Plants for the Reduction of Greenhouse Gases Emission. Water, 7, 5599-5616.
- 25. Cakir, F.Y. and Stenstrom, M.K. (2005) Greenhouse Gas Production: A Comparison between Aerobic and Anaerobic Wastewater Treatment Technology. Water Research, 39, 4197-4203.
- 26. Monteith, H.D., Sahely, H.R., MacLean, H.L. and Bagley, D.M. (2005) A Rational Procedure for Estimation of Greenhouse-Gas Emissions from Municipal Wastewater Treatment Plants. Water Environment Research, 77, 390-403.https://doi.org/10.2175/106143005x51978
- 27. Préndez, M. and Lara-González, S. (2008) Application of Strategies for Sanitation Management in Wastewater Treatment Plants in Order to Control/Reduce Greenhouse Gas Emission. Journal of Environmental Management, 88, 658-664.
- 28. Rosso, D. and Stenstrom, M.K. (2008) The Carbon-Sequestration Potential of Municipal Wastewater Treatment. Chemosphere, 70, 1468-1475.














