Crystal Structure Theory and Applications
Vol. 2 No. 3 (2013) , Article ID: 37258 , 8 pages DOI:10.4236/csta.2013.23017
A Hirshfeld Surface Analysis and Crystal Structure of 2’-[1-(2-Fluoro-Phenyl)-1H-tetrazol-5-Yl]-4-MethoxyBiphenyl-2-Carbaldehyde
1Department of Studies in Physics, University of Mysore, Mysore, India
2Department of Studies in Chemistry, University of Mysore, Mysore, India
3Department of Physics, Faculty of Science, An Najah National University, Nabtus West Bank, Palestinian Territories
Email: *lokanath@physics.uni-mysore.ac.in
Copyright © 2013 S. Madan Kumar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 3, 2013; revised June 5, 2013; accepted July 2, 2013
Keywords: crystal structure; intermolecular interactions; Hirshfeld surfaces
ABSTRACT
The title compound, C21H15FN4O2 is synthesized and characterized by 1H NMR, LC-MS and finally confirmed by single crystal X-ray diffraction method. This molecule crystallizes in the monoclinic crystal system and space group P21/c, with crystal parameters a = 9.4386(5) Å, b = 20.8082(1) Å, c = 9.4338(6) Å, β = 99.566(2)0, Z = 4 and V = 1826.98(19) Å3. The mean planes of fluro-phenyl moiety makes a dihedral angle of 21.51 (7)0 with biphenyl moiety. The molecules are connected by hydrogen bonds of the type C---H...O and C---H...F. In addition, crystal structure is stabilized with π … π (exhibits intramolecular interaction) and C---O... π interactions. The intercontacts in the crystal structure are analyzed using Hirshfeld surfaces computational method.
1. Introduction
Tetrazoles and its derivatives are the most important in the field of medicinal chemistry and found wide spectrum of applications in coordination chemistry because of their multiple coordination status, acting as ligands to metal ions and for the construction of novel metal-organic frameworks [1-3]. And they exhibit biological activities like antibacterial [4,5], antifungal and anticonvulsant [6], analgesic [7], antitubercular activity [8] and anti-cancer activity [9]. Also, 1,5-disubstituted tetrazoles used as anti-inflammatory and anti-hypertensive agents [10,11], such as Losartan [12,13]. Biphenyl tetrazoles have also demonstrated activities as stimulators of growth hormone release [14], metallo-protease inhibitors [15,16] and chloride channel blockers [17]. And, the 5-substituted 1H-tetrazole moiety has been used in the drug discovery as a bioisotere for the corboxylic acid group [18]. In addition, tetrazole compounds are used as new energetic materials because of their good thermal stability due to the presence of aromatic ring system (5-Azido-1H-tetrazole) [19]. Synthesizing the organic compounds in the Suzuki-Miyaura cross-coupling is one of the powerful methods for aromatic C-C bond formation [20]. We report here, the synthesis, spectroscopic studies, structural studies by X-ray diffraction method and analysis of intercontacts by Hirshfeld surfaces computational method of 2’-[1-(2-Fluoro-phenyl)-1H-tetrazol-5-yl]-4-methoxybiphenyl-2-carbaldehyde.
2. Experimental
All reagents were purchased as reagent grade and used without further purification. The reaction was monitored and determination of product was accomplished by TLC technique. The melting point was determined on SELACO-650 hot stage apparatus. Elemental analysis (C, H, N) were determined with Vario-EL instrument. 1H NMR spectra were recorded on a bruker DRX 300 MHz spectrometer using DMSO-d6 as solvent and TMS as internal standard. Chemical shifts are given in δ (ppm).
3. Synthesis and Crystallization of the Title Compound
The title compound is obtained using the Suzuki-Miyaura coupling (Figure 1) of the compound 1-(2-Flurophenyl)-1H-tetrazole (1mmol), 1), with 2-formyl-4-methoxy phenyl boronic acid (1 mmol) in presence of sodium carbonate (15 mmol) and palladium catalyst in a mixture of dimethyl ether (DME) and water in the ratio 3:1. Then the mixture was degasified by bubbling with nitrogen for 15 minutes. After degasify PdCl2 (PPh3)2 [dikis] (0.05 mmol) was added. The resultant mixture was heated at 80˚C under nitrogen atmosphere for 5 hours. After completion of reaction (monitored by TLC), the reaction mixture was concentrated under reduced pressure to remove DME. Then residue was dissolved with ethyl acetate (25 mL), washed with 0.1 N hydrochloric acid (2 * 25 mL), followed by brine solution (2 * 25 mL). Then, the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford crude 2’-[1-(2-Fluorophenyl)-1H-tetrazol-5-yl]-4-methoxy-biphenyl-2-carbaldehyde. 2), which was purified by column chromatography over silica gel (60 - 120 mesh) using Hexane: Ethyl acetate mixture in 8:2 ratios as eluent. The pure compound 2 was crystallized in ethyl acetate and hexane to obtain colorless single crystals.
4. Spectral Analysis
1H NMR (CDCl3, 400 MHz): δ 9.90 (s, 1H), 7.57 - 7.52 (m, 2H), 7.35 - 7.32 (m, 2H), 7.20 (s, 1H), 7.14 (t, J = 6.0 Hz, 2H), 6.98 (t, J = 7.8 Hz, 2H), 6.69 - 6.60 (m, 2H), 3.80 (s, 3H) (Figure 2). Mass, calculated: 374.36 found: 375 (M+ + 1) Elemental analyses, calculated: C, 67.37; H, 4.04; F, 5.07; N, 14.97; O, 8.55. Found: C, 67.57; H, 4.28; F, 5.02; N, 14.773; O, 8.76 (Figure 3). Melting point (˚C): 103 - 105 (Uncorrected).
5. Crystal Structure Determination
A good single crystal of the title compound with dimension 0.30 × 0.35 × 0.35 mm was chosen for X-ray diffraction study. Data collection and cell refinement were carried out using Bruker Kappa ApexII CCD diffractometer [21] with MoKα radiation. The absorption correction was applied using multi-scan technique for data collection. The lattice parameters were determined by the least-squares methods on the basis of all reflections with F2 > 2σ (F2). The structure was solved by the direct methods using SHELXS-97 [22,23]. All the non-hydrogen atoms were revealed in the Fourier map itself. Full-matrix least squares refinement using SHELXL-97 [22,23] with isotropic temperature factors for all the atoms was done. Refinement of non-hydrogen atoms with anisotropic parameters was started at this stage. The hydrogen atoms were placed at chemically acceptable positions and were allowed to ride on their parent atoms. About 165 parameters were refined with 3213 unique reflections which saturated the residuals to R1 = 0.0375 and wR2 = 0.1071. The details of the crystal data and refinement are given in table 1. Table 2 lists the hydrogen bonds. All the figures (ORTEP, packing and hydrogen bonding) were plotted using MERCURY [24]. Hirshfeld surface analyses were carried out and finger print plots were plotted using CRYSTALEXPLORER [25]. Electrostatic potentials were calculated using TONTO [26,27].
6. Results and Discussion
The dihedral angle between mean planes of fluoro-phenyl moiety and benzeze ring (C2/C3/C4/C5/C6/C7 attached with methoxy and carbaldehyde species) is 8.03(8)0. And, the mean planes of rings making dihedral angle with each other is as follows; the tetrazole ring (N1/N2/N3/N4/C15) makes 49.16(11)0 with benzene ring (C14/C13/C12/C11/C10/C9), 57.38(10)0 with fluro-phenyl moiety and 54.86(10)0, with phenyl moiety [(C2/ C3/C4/C5/C6/C7) attached with methoxy and carbaldehyde species]. Similarly, the benzene ring (C14/C13/ C12/C11/C10/C9) makes a dihedral angle of 64.67(10)0 with fluoro-phenyl moiety. Also, it makes an angle of 56.86(10)0 with phenyl moiety (C2/C3/C4/C5/C6/C7) attached with methoxy and carbaldehyde species. The overall geometry of the title compound is similar to 1-(4-nitrophenyl)-1H-tetrazol-5-amine and {(E)-[1-(4-ethoxyphenyl)-1H-tetrazol-5-yl] iminomethyl} dimethylamine [28].
Figure 4 represents the ORTEP diagram of the title molecule. The molecules in the unit cell are connected by hydrogen bonds C10-H10...F1, C12-H12...O2 and C20- H20...O2 (Table 2). And, figure 5 shows the packing of the molecules are arranged in the fishing net pattern. The observed weak interactions p...p and C---O...p helps in crystal structure stabilization. The intramolecular p...p interactions exists between centroid (Cg4: C16/C17/C18/ C19/C20/C21) of fluoro-phenyl moiety and benzene ring (Cg2: C2/C3/C4/C5/C6/C7) with a distance 3.7806(10) Å [x, y, z] (Figure 6). And, inter molecular p…p exists between face to face (Cg4 and Cg4) interactions with a distance of 3.6875(11) Å [2 − x, −y, 1 − z]. In addition to this C---O...p (Cg4) interaction exists between carbaldehyde moiety (C8-O2) and Cg4 with a distance of 3.9551(15) Å [x, y, z].
7. Hirshfeld Surface Analysis
The intermolecular interactions of the title compound are quantified using Hirshfeld surface analysis. This approach is a graphical tool for visualization and understanding of intermolecular interactions [25]. Here, we estimate the intermolecular contacts, which are shown in figure 7. The chart indicates that the contribution of inter-contacts to the Hirshfeld surfaces, H...H (36%), N...H (19%), C...H (16%), O...H (14%), F...H (7%) and others (C...C, N...N, C...O, N...F; 8%). These inter-contacts
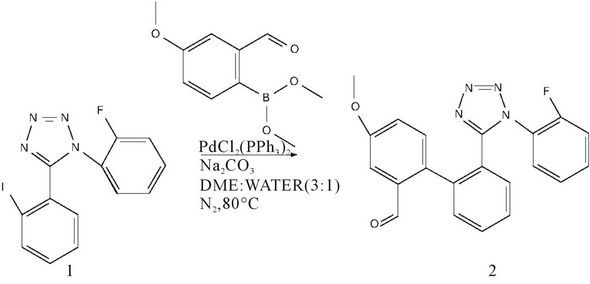
Figure 1. Schematic diagram and synthesis pathway of the title compound.
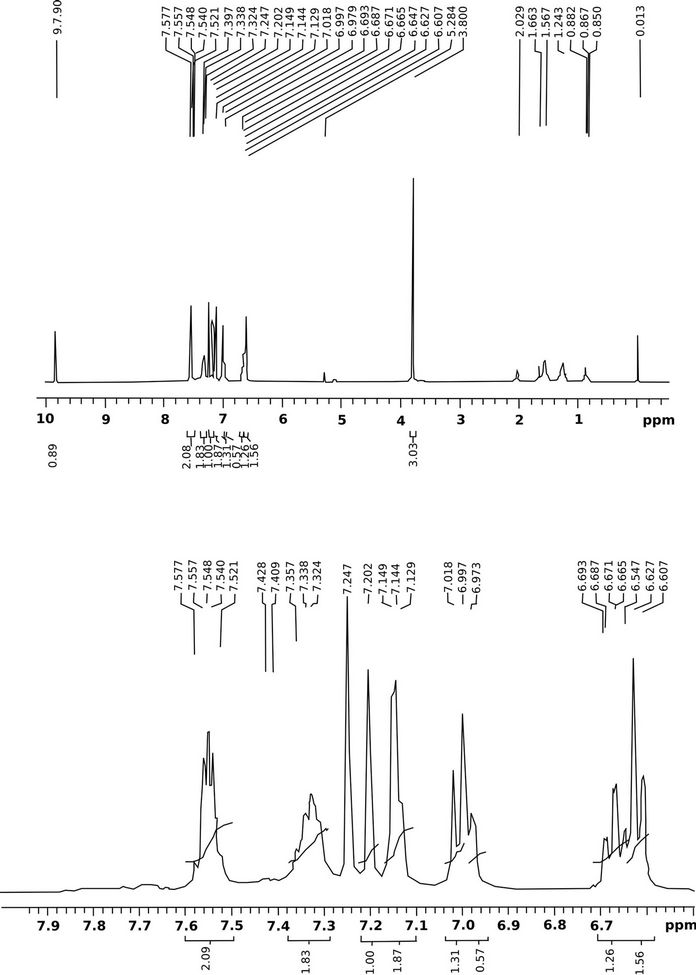
Figure 2. 1H NMR spectra of the title compound.
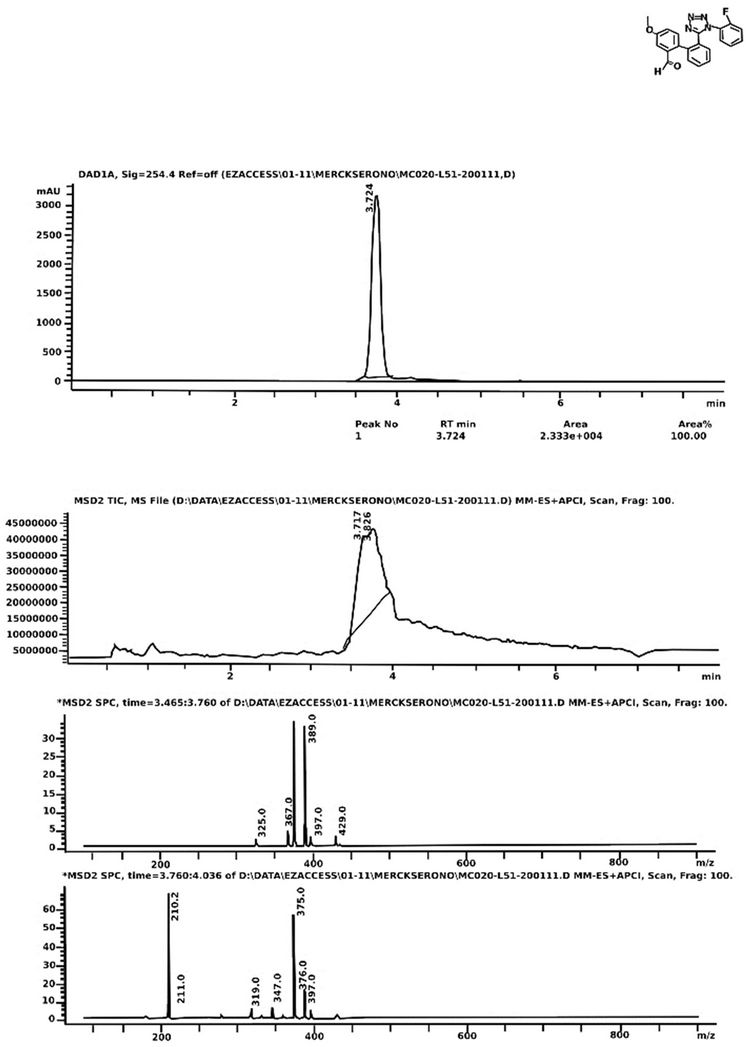
Figure 3. LC-MS spectra of the title compound.
are highlighted by conventional mapping of dnorm on molecular Hirshfeld surfaces are shown in figure 8. The red spots over the surface indicate the intercontacts involved in the hydrogen bonds. Further, intercontacts were plotted with fingerprint plots (Figure. 9). H...H intercontacts, (Figure 9(a)) shows large surfaces, whereas the O...H plot (Figure 9(d)) shows the presence of O...H contact with the two characteristic wings. The N...H contact plot is shown in figure 9(b). The intercontacts F...H (Figure 9(e)) showing two narrow pointed wings provide evidence for C-H...F non-classical hydrogen bonds. And, C...H plot reveals the information of inter molecular hydrogen bonds.
The electrostatic potential is mapped on Hirshfeld surface using STO-3G basis set at the Hartree-Fock theory over the range of ± 0.025 au (Figure 10). The positive electrostatic potential (blue region) over the surface indicates hydrogen donor potential, whereas the hydrogen bond acceptors are represented by negative electrostatic potential (red region) [27]. The crystal geometries were


Table 1. Crystal data, data collection and structure refinement.
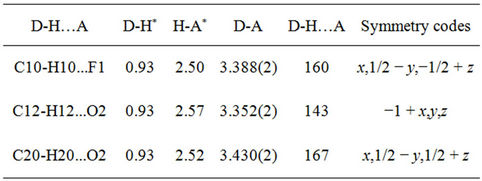
Table 2. Hydrogen bonds [Å, 0].
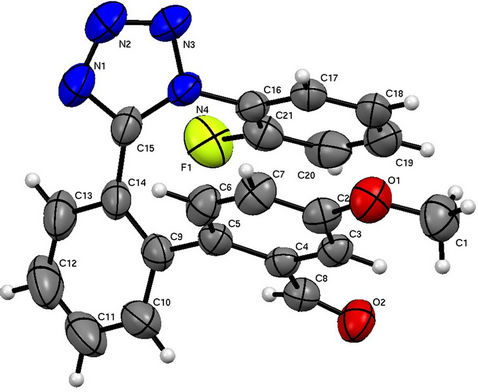
Figure 4. Molecular structure of the title compound showing the atomic numbering system. Displacement ellipsoids are drawn at the 50% probability.

Figure 5. Packing diagram of the title molecule along b-axis. Dotted lines represents hydrogen bonds.
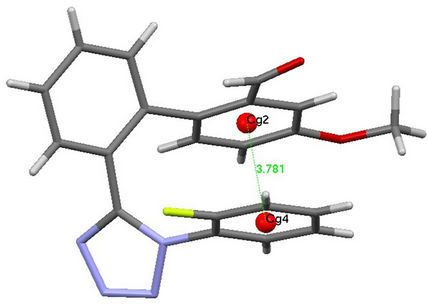
Figure 6. Intramolecular interaction between Cg2 and Cg4. Dotted lines (green) represents interaction.
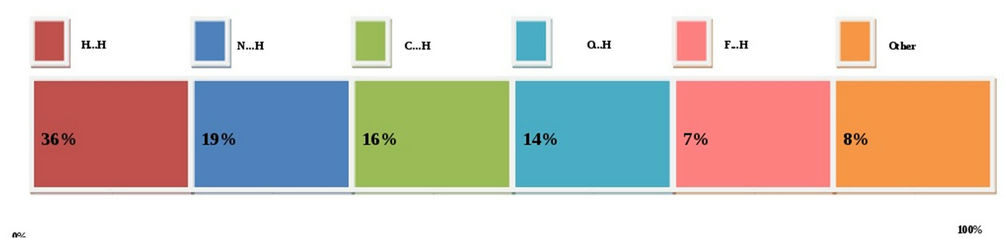
Figure 7. Hirshfeld surface: Percentage of various intermolecular contacts contributed to the Hirshfeld surface.
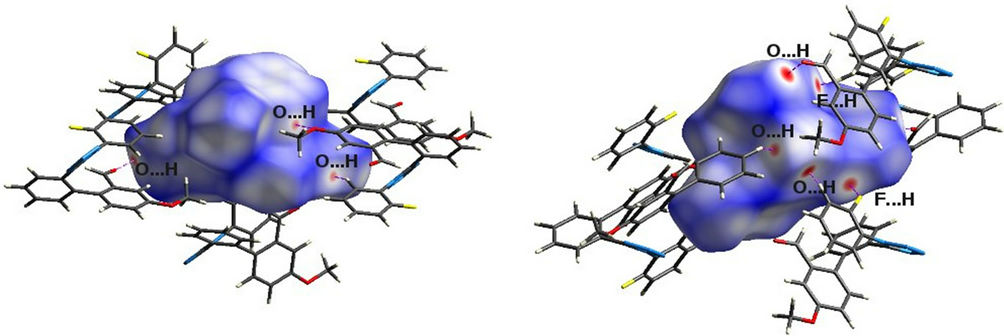
Figure 8. dnorm mapped on Hirshfeld surface for visualizing the intercontacts of the title compound. Color scale in between −0.18 au (blue) to 1.4 au (red). Dotted lines (magenta) represent hydrogen bonds.
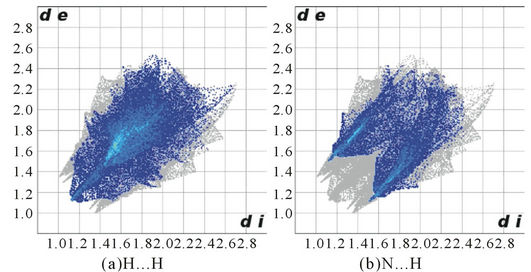

Figure 9. Fingerprint of the title compound, (a) H...H, (b) C...H, (c) C...H, (d) O...H and (e) F...H. The outline of the full fingerprint is shown in gray. di is the closest internal distance from a given point on the Hirshfeld surface and de is the closest external contacts.
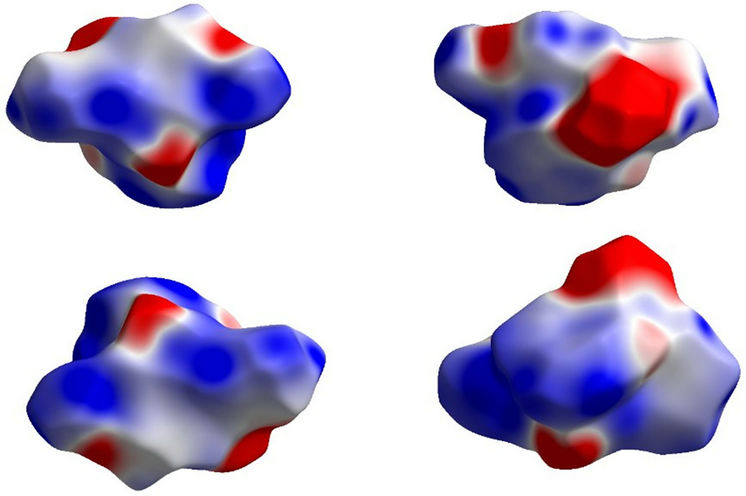
Figure 10. Electrostatic potential mapped on Hirshfeld surface (different orientation) with ± 0.25 au. Blue region corresponds to positive electrostatic potential and red region to negative electrostatic potential.
used as input to the TONTO [26] integrated with Crystal explorer [25].
8. Conclusion
The dihedral angle between fluro-phenyl moiety and biphenyl moiety is 21.51 (7)0. The molecules are connected by C-H...O and C-H...F hydrogen bonds. In addition, the short contacts of the type p...p and C---O...p help in crystal stabilization. The Hirshfeld surface analysis with finger plots and electrostatic potential map reveals the percentage of intermolecular contacts and distribution of electrostatic potential of the title compound.
9. Acknowledgments
Madan Kumas S. thanks UGC-BRS and University of Mysore for providing fellowship. MPS gratefully acknowledge the financial support from University Grants Commission, New Delhi, India.
REFERENCES
- A. R. Katritzky, C. Cai and N. K. Meher, “Efficient Synthesis of 1,5-Disubstituted Tetrazoles,” Synthesis, Vol. 8, 2007, pp. 1204-1208. doi:10.1055/s-2007-966001
- X.-S. Wang, Y. Z. Tang, X-F, Huang, Z.-R. Qu, C.-M. Che, C. W. H. Chan and R.-G. Xiong, “Syntheses, Crystal Structures, and Luminescent Properties of Three Novel Zinc Coordination Polymers with Tetrazolyl Ligands,” Inorganic Chemistry, Vol. 44, No. 15, 2005, pp. 5278- 5285. doi:10.1021/ic050354x
- R.-G. Xiong, X. Xue, H. Zhao, X.-Z. You, B. F. Abrahams and Z.-L. Xue, “Novel, Acentric Metal-Organic Coordination Polymers from Hydrothermal Reactions Involving in Situ Ligand Synthesis,” Angewandte Chemie International Edition, Vol. 41, No. 20, 2005, pp. 3800- 3803. doi:10.1002/1521-3773(20021018)41:20<3800::AID-ANIE3800>3.0.CO;2-3
- K. Kemajl, V. Idriz, H. Arben, G. Sevdije, I. Muharrem and M. Sefkija, “Fabrication of 3D Hepatic Tissues by Additive Photopatterning of Cellular Hydrogels,” The FASEB Journal, Vol. 21, No. 3, 2007, pp. 790-795. doi:10.1096/fj.06-7117com
- V. V. Mulwad, R. B. Pawar and A. C. Chaskar, “Synthesis and Antibacterial Activity of New Tetrazole Derivatives,” Journal of Korean Chemical Society, Vol. 52, No. 3, 2008, pp. 249-256. doi:10.5012/jkcs.2008.52.3.249
- R. S. Upadhayaya, S. Jain, N. Sinha, N. Kishore, R. Chandra and S. K. Arora, “Synthesis of Novel Substituted Tetrazoles Having Antifungal Activity,” European Journal of Medicinal Chemistry, Vol. 39, No. 7, 2004, pp. 579- 592. doi:10.1016/j.ejmech.2004.03.004
- A. Rajasekaran, N. Sankar, A. Murugesh, Kalasalingam and A. Rajagopal, “Antibacterial, Antifungal and Anticonvulsant Evaluation of Novel Newly Synthesized 1[2-- (1H-Tetrazol-5-yl)ethyl]-1H-benzo[d][1,2,3]triazoles,” Archives of Pharmacal Research, Vol. 29, No. 7, 2006, pp. 535-540. doi:10.1007/BF02969261
- J. Adamec, K. Waisser, J. Kunes and J. Kaustova, “A Note on the Antitubercular Activities of 1-Aryl-5-benzylsulfanyltetrazoles,” Pharmaceutical & Medicinal Chemistry (Weinheim), Vol. 338, No. 8, 2005, pp. 385-389. doi:10.1002/ardp.200400967
- A. O. De, Souza, M. T. Pedrosa, J. B. Alderete, A. F. Cruz, M. A. Prado, R. B. Alves and C. L. Silva, “Cytotoxicity, Antitumoral and Antimycobacterial Activity of Tetrazole and Oxadiazole Derivatives,” Pharmazie, Vol. 60, No. 5, 2005, pp. 396-397.
- R. J. Herr, “5-Substituted-1H-tetrazoles as Carboxylic Acid Isosteres: Medicinal Chemistry and Synthetic Methods,” Bioorganic and Medicinal Chemistry, Vol. 10, No. 11, 2002, pp. 3379. doi:10.1016/S0968-0896(02)00239-0
- L. V. Myznikov and G. I. Koldobskii, “Drugs in the Tetrazole Series (Review),” Chemistry of Heterocyclic Compounds, Vol. 43, No. 1, 2007, pp. 1-9. doi:10.1007/s10593-007-0001-5
- R. D. Smith, C. S. Sweet, A. Goldberg and P. B. W. M. Timmermans, “Losartan Potassium Cozaar (TM) a Nonpeptide Antagonist of Angiotensin-II,” Drugs Today, Vol. 32, 1996, pp. 1-12.
- K. Dickstein, P. B. M. W. M. Timmermans and R. Segal, “Losartan: A Selective Angiotensin II Type 1 (AT1) Receptor Antagonist for the Treatment of Heart Failure,” Expert Opinion on Investigational Drugs, Vol. 7, No. 11, 1998, pp. 1897-1914. doi:10.1517/13543784.7.11.1897
- R. G. Smith, K. Cheng, W. R. Schoen, S. S. Pong, G. Hickey, T, Jacks, B. Butler, W. W. S. Chan, L. Y.-P, Chaung, F. Judith, J, Taylor, M. J. Wyvratt and M. H. Fisher, “A Nonpeptidyl Growth-Hormone Secretagogue,” Science, Vol. 260, No. 5114, 1993, pp. 1640-1643. doi:10.1126/science.8503009
- B. G. Green, J. H. Toney, J. W. Kozarich and S. K. Grant, “Inhibition of Bacterial Peptide Deformylase by Biaryl Acid Analogs,” Archives of Biochemistry and. Biophysics, Vol. 375, No. 2, 2000, pp. 355-358. doi:10.1006/abbi.1999.1673
- J. H. Toney, P. M. D. Fitzgerald, N. Grover-Sharma S. H. Olson, W. J. May, J. G. Sundelof, D. E. Vanderwall, K. A. Cleary, S. K. Grant, J. K. Wu, J. W. Kozarich, D. L. Pompliano and G. G. Hammond, “Antibiotic Sensitization Using Biphenyl Tetrazoles as Potent Inhibitors of Bacteroides Fragilis Metallo-Beta-Lactamase,” Chemistry & Biology, Vol. 5, No. 4, 1998, pp. 185-196. doi:10.1016/S1074-5521(98)90632-9
- P. Christophersen and B. H. Dahl, WO Patent, 0024707, 2000.
- J. Stierstorfer, M. T. Klapotke, A. Hammerl and R. D. Chapman, “5-Azido-1H-Tetrazole—Improved Synthesis, Crystal Structure and Sensitivity Data,” Journal of Inorganic and General Chemistry, Vol. 634 No. 6-7, 2008, pp. 1051-1057. doi:10.1002/zaac.200800003
- J. Spencer, H. Patel, J. J. Deadman, R. A. Palmer, L. Male, J. S. Coles, G. O. Uzoh and S. L. Price, “The Unexpected But Predictable Tetrazole Packing in Flexible 1-Benzyl-1H-Tetrazole,” Crystal Engineering Communications, Vol. 14, No. 20, 2012, pp. 6441-6446. doi:10.1039/c2ce25940k
- N. Miyaura, “Organobom Compounds,” Topics in Current Chemistry, Vol. 219, 2002, pp. 11-59.
- Bruker, “APEX2 and SAINT-Plus,” Bruker AXS Inc., Madison, 2004.
- G. M. Sheldrick, “SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement,” University of Gottingen, Gottingen, 1997.
- G. M. Sheldrick, “A Short History of SHELX,” Acta Crystallographica A, Vol. 64, Part 1, 2008, pp. 112- 122. doi:10.1107/S0108767307043930
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, “Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures,” Journal of Applied Crystallography, Vol. 41, Part 2, 2008, pp. 466-470. doi:10.1107/S0021889807067908
- S. K. Wolff, D. J. Grimwood, J. J. McKinnon, D. Jayatilaka and M. A. Spackamn, “Crystal Explorer 3.0,” University of Westren Australia, Perth, 2007.
- D. Jayatilaka, D. J. Grimwood, A. Lee, A. Lemay, A. J. Russel, C. Taylo, S. K. Wolff, Cassam-Chenai and A. Whitton, “TONTO—A System for Computational Chemistry,” 2005.
- M. A. Spackmann, J. J. McKinnon and D. Jayatilaka, “Electrostatic Potentials Mapped on Hirshfeld Surfaces Provide Direct Insight into Intermolecular Interactions in Crystals,” Crystal Engineering Communications, Vol. 10, No. 4, 2008, pp. 377-388. doi:10.1039/b715227b
- A. S. Lyakhov, A. N. Vorobiov, L. S. Inashkevich and P. N. Gapoink, “Two Derivatives of 1,5-Disubstituted Tetrazoles: 1-(4-Nitrophenyl)-1H-tetrazol-5-amine and {(E)- [1-(4-ethoxyphenyl)-1H-tetrazol-5-yl]iminomethyl}dimethylamine,” Acta Crystallographica, Vol. C64, Part 8, 2008, pp. o414-o416. doi:10.1107/S0108270108019707
NOTES
*Corresponding author.

