Advances in Breast Cancer Research
Vol.2 No.4(2013), Article ID:36732,8 pages DOI:10.4236/abcr.2013.24022
Subcutaneous Trastuzumab (Herceptin®): A UK Time and Motion Study in Comparison with Intravenous Formulation for the Treatment of Patients with HER2-Positive Early Breast Cancer
1Maidstone and Tunbridge Wells NHS Trust, Maidstone, UK
2Nottingham University Hospitals NHS Trust, Nottingham, UK
3Brighton and Sussex University Hospitals NHS Trust, Brighton, UK
4Roche Products Ltd., Welwyn Garden City, UK
5pH Associates Ltd., Marlow, UK
6Velindre Cancer Centre, Cardiff, UK
Email: fran@phassociates.com
Copyright © 2013 Russell Burcombe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 1, 2013; revised August 27, 2013; accepted September 2, 2013
Keywords: Trastuzumab; Breast Neoplasms; Administration Subcutaneous; Administration Intravenous
ABSTRACT
Aim: Firstly, to quantify active healthcare professional (HCP) time and costs associated with subcutaneous (SC) administration of trastuzumab (Herceptin®) compared with the standard intravenous infusion (IV) in the treatment of patients with HER2-positive early breast cancer within the adjuvant PrefHer trial setting; secondly, to measure patient time in the care unit and patient infusion chair time for both routes of administration. Methods: A UK multi-centre prospective, observational Time and Motion study was conducted alongside the PrefHer trial (ClinicalTrials.gov id: NCT01401166). Trained observers measured the duration of each SC and IV related task that HCPs undertook and recorded patient time in the chemotherapy unit and infusion chair. The type and quantity of medical consumables used with each route of administration were also collected. Twenty-four patient episodes were recorded (12 SC, 12 IV). Mean total administration time was calculated as the mean sum of task times, both for IV and SC formulations. The mean cost of each route of administration was calculated as the mean cost of HCP time plus the mean cost of consumables used. HCP time was costed using Personal Social Services Research Unit. Consumables were costed using hospital pharmacy data and online sources. Results: Mean active HCP time for IV administration was 92.6 minutes compared with 24.6 minutes for SC administration. The mean cost for IV preparation and administration was £144.96 (£132.05 of HCP time and £12.92 of consumables) versus £33.15 (£31.99 of HCP time and £1.17 of consumables) for SC administration. Mean time spent in the care unit and in the infusion chair was 94.5 minutes and 75 minutes respectively for IV, and 30.3 minutes and 19.8 minutes for SC. SC administration of trastuzumab could translate to a time saving of 68 minutes (versus IV) with a total cost saving of £111.81 per patient episode. This equates to a potential saving of £2012.58 over a full course of adjuvant treatment (18 cycles). Conclusion: Substituting IV infusion with SC administration of trastuzumab may lead to a substantial reduction in active HCP time, patient chair and unit time, consumable use and overall costs. The reduced patient chair and unit time could provide increased capacity within existing resources.
1. Introduction
Breast cancer is the most common female malignancy in England and Wales, with around 44,000 new cases diagnosed in 2011 [1,2].
Breast cancer incidence increased by 90% between 1971 and 2010, whilst mortality rates fell by 37% in the same time period. Survival rates have risen steadily, as a result of earlier detection and improved treatment [3], leading to increased demands on healthcare resources. This in turn places increased pressure on capacity in oncology units.
Breast cancer can be characterised by growth depending on oestrogen, progesterone and human epidermal growth factors. Human epidermal growth factor receptor-2 (HER2)-positive breast cancer confers a worse prognosis. Trastuzumab (Herceptin®), a humanized IgG1 monoclonal antibody, selectively binds to the extracellular domain of the HER2 protein and has a negative effect on tumour cell growth.
Trastuzumab is a standard therapy for HER2-positive metastatic and early breast cancer (EBC), being administered either as a 3-weekly adjuvant regimen for 18 cycles in EBC or in combination with palliative chemotherapy in metastatic disease (MBC).
Trastuzumab requires an intravenous (IV) loading infusion with over 90 minutes followed, if well tolerated, by subsequent doses over 30 minutes [4]. Prolonged adjuvant or maintenance metastatic therapy therefore requires considerable HCP resource utilisation. It is common clinical practice to insert indwelling venous catheters for patients requiring prolonged IV infusion therapy. In addition to the costs of theatre and anaesthetist time, indwelling venous catheters may result in complications such as infection or thrombosis [5], which proves both costly to treat and undesirable for patients.
A subcutaneous (SC) formulation for trastuzumab has been granted a CHMP positive opinion with European Marketing authorisation expected by September 2013, as an alternative to the 3-weekly IV infusion in both EBC and MBC. A key excipient in the SC formulation is a recombinant hyaluronidase enzyme (rHuPH20), which breaks down subcutaneous hyaluronic acid fibres and in turn improves delivery of SC injections [6]. No data on the healthcare professional (HCP) time and resource use required for SC and IV trastuzumab infusion are currently available.
The increased demand for systemic anti-cancer therapy over the past decade has created capacity and cost pressures on National Health Service (NHS) oncology treatment units [7].
This study was designed to describe the healthcare resources, costs and patient time associated with IV and SC administration of trastuzumab in patients with HER2- positive EBC participating in the PrefHer clinical study (Protocol Number: MO22982) conducted at selected UK and international hospitals (ClinicalTrials. gov id: NCT01401166). PrefHER is a randomised, multicentre, multinational cross-over study to evaluate patient preference and healthcare professional satisfaction with SC administration, compared to IV administration, of trastuzumab as an adjuvant therapy in patients with HER2- positive EBC. It is a two-cohort switch study (involving 400 patients in total) comparing trastuzumab SC administration, via hand-held syringe or a single-use injection device (SID), with trastuzumab IV administration. The primary endpoint of PrefHER is the proportion of patients indicating an overall preference for SC over IV.
2. Method
2.1. Study Design
This was a non-interventional, prospective, multi-centre descriptive research study, using a time and motion direct observation methodology conducted as a sub-study of the PrefHer clinical trial. The time and motion study was conducted in four UK centres: Maidstone, Nottingham, Brighton, and Cardiff.
2.2. Subjects
Data were collected by direct observation of the HCPs involved in the PrefHer clinical trial. The study was primarily designed to observe tasks performed by HCPs. Staff inclusion criteria stipulated that the HCP: 1) was a member of the care team with responsibility for the management of patients who had consented to the PrefHer clinical trial; and 2) gave consent to being observed.
Patients were required to: 1) be participating in the PrefHer clinical trial; and 2) give consent to the presence of the observer during administration of trastuzumab.
Patients were recruited by HCPs who were part of the care team conducting the PrefHer clinical trial. Patients were approached during a routine visit early in their participation in the PrefHer clinical trial and given information about the sub-study. Their consent was sought, by a member of the clinical team, at the start of a subsequent trastuzumab administration visit for an observer to be present during that visit.
The study protocol was approved by National Research Ethics Service Committee London – Chelsea (reference 11/LO/1830). Local Trust management approval was provided by the Research & Development department of each participating NHS hospital trust. All participants provided written informed consent, and all completed the study.
2.3. Study Sample
Twenty-four patients were recruited (12 SC and 12 IV trastuzumab). The SC formulation required minimal preparation and therefore preparation and administration were recorded in the same patient episode. IV preparation was measured separately to administration giving a total of 36 observed patient episodes (Table 1).
2.4. Data Collection, Management and Analysis
SC injection and IV infusion data were collected during the PrefHer study time frame only. No observations for IV infusions occurring outside the PrefHer study (i.e. routine clinical practice) were conducted, so that any bias was similar for both IV and SC administration. Trained observers monitored the time required by HCPs to complete the tasks associated with the preparation and administration of trastuzumab SC and IV (including medication preparation, medication administration and patient monitoring). Start and stop times for each task were measured to the second. The type and amount of consumable supplies used (e.g. syringes, needles, alcohol swabs, etc) were also recorded. All data were collected by the same two observers. A list of infusion/injection related tasks was agreed with nursing staff at the clinical care centres prior to commencing data collection and data were collected in respect of these tasks alone (Tables 2 and 3). Patient time at the care unit and patient chair time was also recorded. The following data were collected for each task associated with an episode of trastuzumab administration: 1) route of trastuzumab administration; 2) first or subsequent dose of trastuzumab; 3) task; 4) HCP (profession and grade) performing the task; 5) consumable supplies (item and quantity) used; 6) start time; and 7) stop time. For some tasks, the HCP may have briefly carried out other activities and may not have been present with the patient all the time. However, the patient remained the focus of their care and therefore the recorded time is assumed to be 100% active HCP time, as specified in the study protocol. The following were collected for each observed episode of trastuzumab administration for the sub-study: 1) arrival time in the unit; 2) route of trastuzumab administration; 3) start time of “chair time” (the time the patient spends in the treatment room chair); 4) end time of “chair time”; and 5) time of departure from the unit. Reference costs were applied to each observed activity. HCP time was costed using unit costs taken from UK National Health Service (NHS) reference costs, using Personal Social Services Research Unit (PSSRU) Unit Costs of Health & Social Care 2011 [8]. Consumables were costed using hospital pharmacy data and online sources. Analysis was limited to inclusion of only those items with an individual cost of £0.05 or more. The cost of trastuzumab was not included in the analysis; the study scope was limited to non-drug costs as the cost of the SC formulation, although not set at the
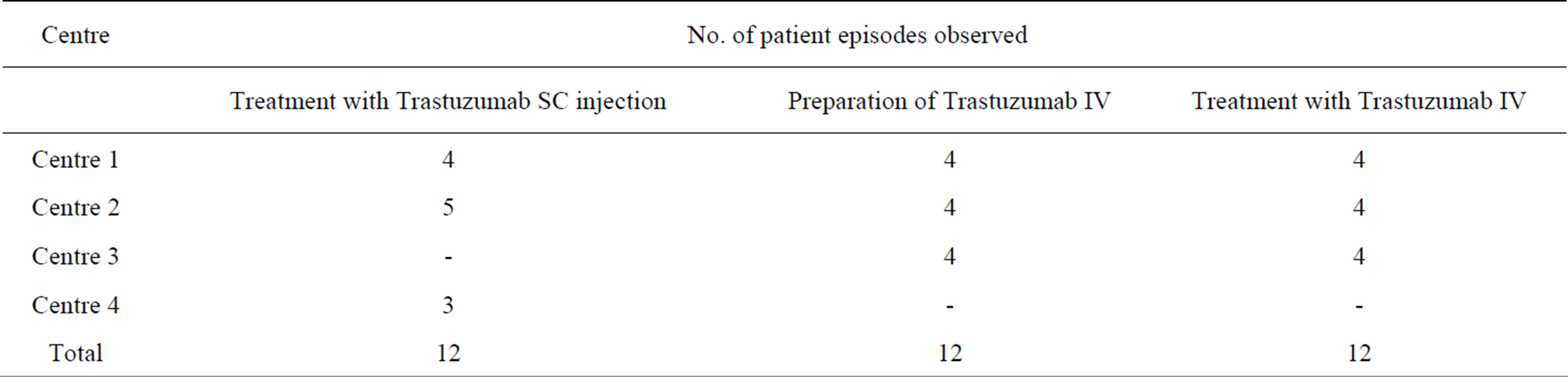
Table 1. Patient episodes observed by centre.
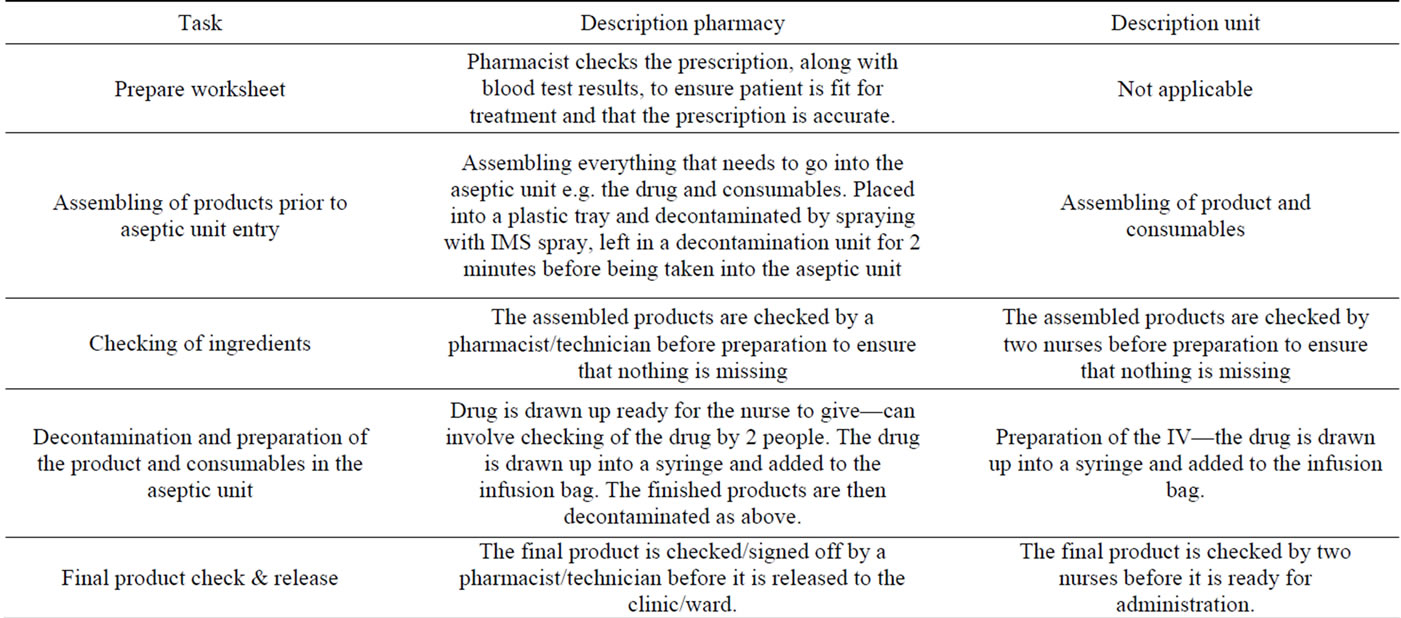
Table 2. Preparation tasks.
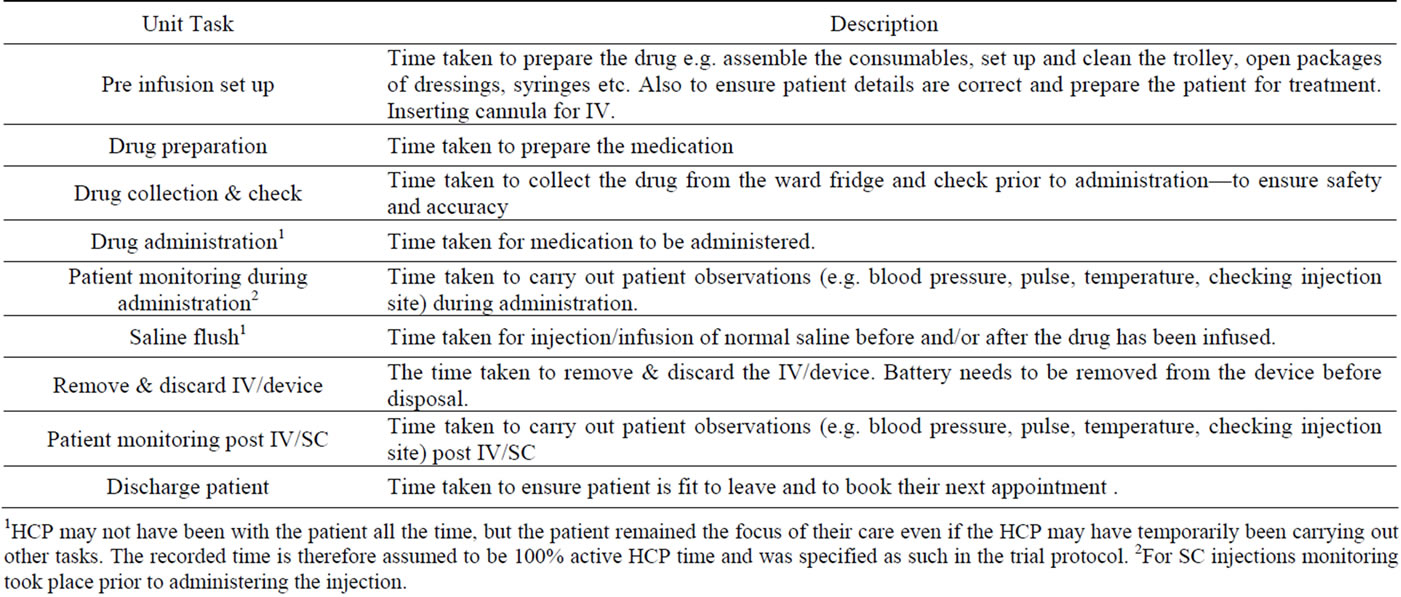
Table 3. Administration tasks.
1HCP may not have been with the patient all the time, but the patient remained the focus of their care even if the HCP may have temporarily been carrying out other tasks. The recorded time is therefore assumed to be 100% active HCP time and was specified as such in the trial protocol. 2For SC injections monitoring took place prior to administering the injection.
time of the study, is expected to be comparable to the cost of IV for a patient of mean weight. Data were collected between February 2012 and February 2013. Prior to conducting the analysis of the final dataset, additional quality checks were performed to ensure completeness and accuracy of the data.
Missing values that occurred during collection of observational data were tabulated as part of the data analysis. Clarifications for missing data and other data issues were requested from the observers or from each site as appropriate. No imputation of missing data was performed.
2.5. Pharmacovigilance Procedures
Commercial trial sponsors are required to adhere to European pharmacovigilance regulations. All pharmacovigilance requirements were met by the PrefHer clinical trial protocol. No additional or investigational treatments were provided as a result of this sub-study protocol. Procedures for safety reporting as described in the PrefHer study protocol were followed.
2.6. Objectives
Primary objective: To quantify the costs associated with healthcare resource utilisation for administration of trastuzumab SC in the treatment of patients with HER2- positive EBC within the PrefHer trial setting (for all UK sub-study sites—pooled data).
Secondary objectives:
● To quantify the costs associated with healthcare resource utilisation for preparation and administration of trastuzumab IV infusion in the treatment of patients with HER2-positive EBC within the PrefHer trial setting (for all UK sub-study sites—pooled data).
● To document the “active” HCP time required per patient for SC and IV preparation and administration processes (for all UK sub-study sites).
● To measure the quantity of medical supplies required per patient for SC and IV preparation and administration processes (for all UK sub-study sites).
● To record the patient time in the care unit for IV & SC administration of trastuzumab (for all UK substudy sites).
● To record the patient “chair time” for administration of IV & SC trastuzumab (for all UK sub-study sites).
2.7. Endpoints
Primary endpoint:
● Mean (SD) cost of resources (HCP time and consumables) used in the administration of trastuzumab SC injection (pooled UK data).
Secondary Endpoints: (pooled UK data):
● Mean (SD) per patient cost of resources (HCP time and consumables) used in the IV preparation and administration of trastuzumab.
● Distributions of cost of resources for IV and SC trastuzumab preparation and administration. Mean (SD) and distribution of per patient observed HCP time for each pre-specified task related to trastuzumab SC and IV preparation and administration. Mean (SD) and distribution of per patient observed HCP time for all tasks associated with trastuzumab SC and IV preparation and administration.
● Mean (SD) and distribution of per patient quantity of consumables used related to trastuzumab SC and IV preparation and administration.
● Mean (SD) and distribution of time per patient spent in the care unit for SC and IV administration of trastuzumab.
● Mean (SD) and distribution of patient chair time for SC and IV administration of trastuzumab.
This study was designed to be descriptive and not to detect statistically significant differences in time for IV infusion versus SC injection process. Descriptive analyses were conducted, assuming that data were normally distributed (distributions, mean, standard deviation, median, minimum, and maximum) and 95% confidence intervals were calculated for each endpoint.
At one centre (4 patient episodes) the trastuzumab SC injection was prepared in pharmacy (as opposed to on the chemotherapy unit in the other eight patient episodes). For consistency of analysis between all 12 SC patient episodes, the cost of resources required to prepare the SC injection in pharmacy was recorded within the administration tasks (specifically “Drug preparation”).
Initially when calculating overall mean resource costs the data were stratified according to location of SC injection preparation to determine whether this made any significant difference to the mean overall cost. As no significant difference in cost was found, all data have been analysed together.
The intention was to analyse the data for initial and subsequent treatments separately on the assumption that initial treatments might be expected to take longer than subsequent treatments. However, only one initial treatment was observed and as the HCP time taken and consumables used were within the range of those recorded for subsequent doses, all dose episodes were analysed together.
3. Results
3.1. Time Savings
The mean amount of HCP time (min, max, SD) required for SC trastuzumab administration was 24.6 mins (11 - 45, SD 13.2) compared to 58.1 mins (50 - 67, SD 5.8) for IV administration (Table 4).
IV administration also required an additional 34.5 mins (21 - 67, SD 13.6) of HCP time for completion of preparation tasks (Table 5). Drug administration and saline flush were the tasks which took the longest time to complete.
Patients receiving IV trastuzumab spent more time on the unit than those receiving SC treatment: mean 94.5 minutes, versus 30.3 minutes (Figures 1 and 2). Over a course of 18 infusions this represents an additional patient time of 19 hours and 16 minutes compared with SC administration.
The mean patient chair time for administration of trastuzumab was also longer for IV administration: 75.0 minutes versus 19.8 minutes.
3.2. Cost Savings
Administration of IV & SC trastuzumab on the unit was predominantly carried out by NHS Band 6 nurses (deputy ward/unit manager, ward team leader, senior staff nurse) and Band 5 nurses (staff nurse, registered nurse, registered practitioner). NHS nursing staff are employed at one of eight pay bands, with the level of responsibility and pay increasing from Band 1 to Band 8. In a small number of cases Health Care Assistants (Bands 2 & 3) completed less skilled tasks.
The preparation of IV trastuzumab in pharmacy required input from both NHS technicians and pharmacists. IV trastuzumab does not require preparation in aseptic conditions and at one site the preparation was carried out on the unit by Band 6 nurses. Where the SC injection
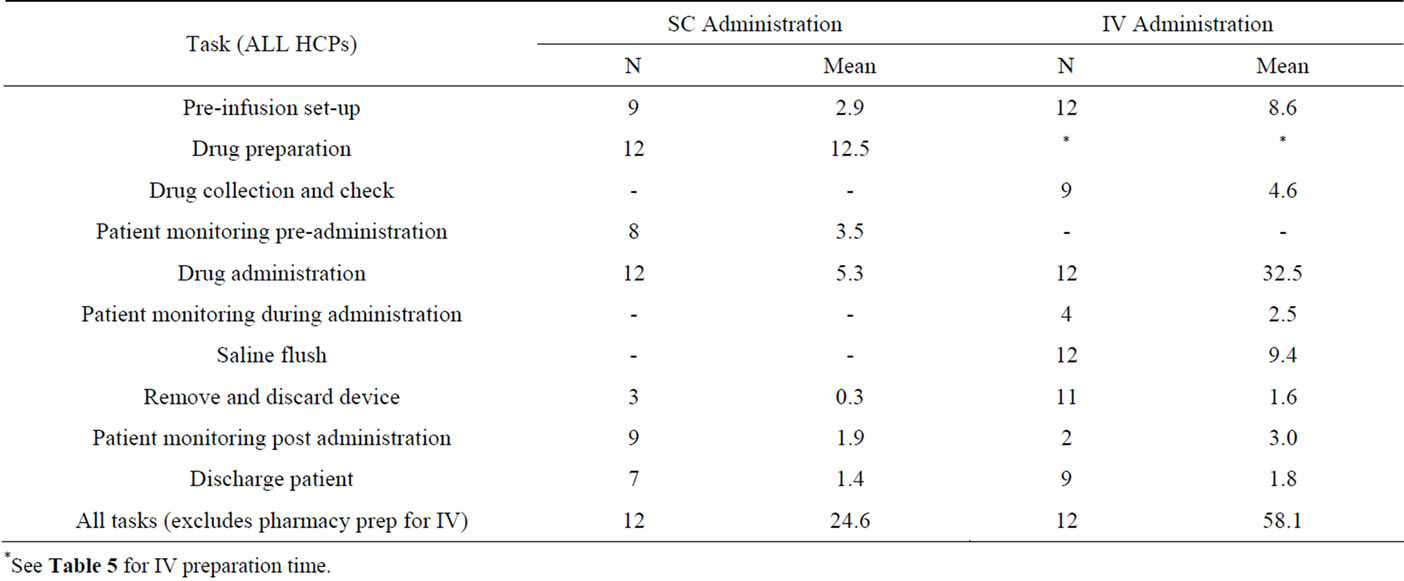
Table 4. HCP time (mins) per patient episode for each pre-specified task related to trastuzumab SC injection and IV infusion administration.
*See Table 5 for IV preparation time.
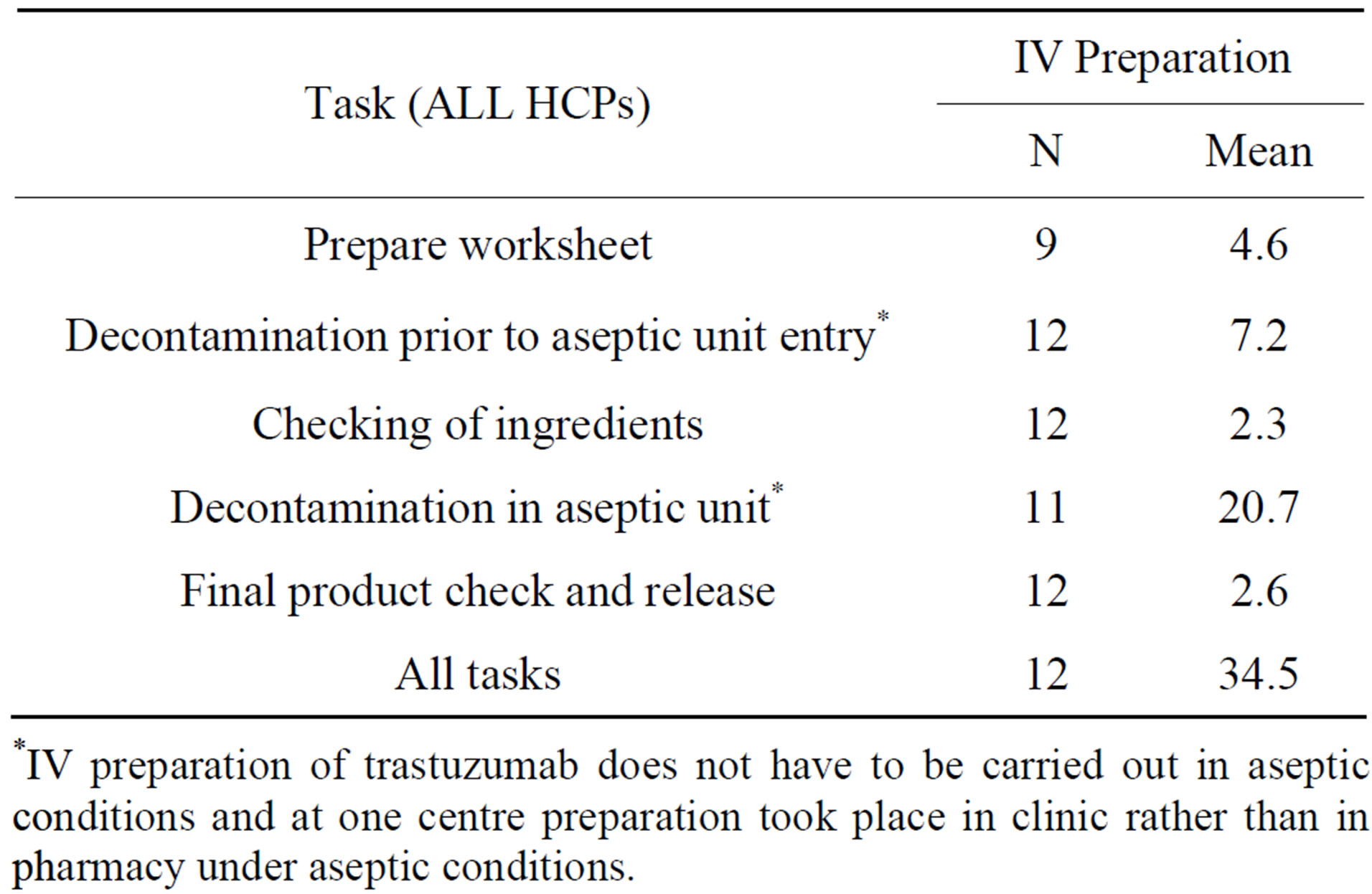
Table 5. HCP time (mins) per patient episode for each pre-specified task related to trastuzumab IV preparation.
*IV preparation of trastuzumab does not have to be carried out in aseptic conditions and at one centre preparation took place in clinic rather than in pharmacy under aseptic conditions.
was prepared in pharmacy this required input from both technicians and pharmacists.
The mean (95% CI) overall cost for SC injection admnistration was £33.15 (£27.07 to £39.23) (Table 6).
The mean (95% CI) cost of resources per patient episode for preparation of IV trastuzumab was £22.07 (£19.21 to £24.93) and for administration £122.89 (£114.47 to £131.31). The mean total cost of preparation and administration was therefore £144.96 (Table 6). The mean cost of consumables was considerably less than the mean cost of HCP time for both IV and SC trastuzumab administration.
The mean costs, for IV administration, for HCP time and consumables were £132.05 and £12.92 respectively, compared to £31.99 and £1.17 for SC treatment (Table 6). The costs for administration of SC injection were lower than for IV administration for all episodes.
The difference in cost per patient episode between IV and SC administration of trastuzumab was £111.81 per SC injection administration (95% confidence interval of this difference: £100.44 to £123.56 (P < 0.001)).
4. Discussion
4.1. Main Findings
The total preparation and administration time to administer IV trastuzumab was approximately three times longer than the total time required for SC administration. For capacity planning purposes, it may be possible to administer SC trastuzumab to approximately two patients in the time it would take to administer IV treatment to one patient. This excludes IV preparation time, as this is carried out in pharmacy.
The shorter mean patient chair time and time spent on the unit with SC injection could create increased capacity and an increased number of available appointments within the unit.
It costs £22.07 more to prepare IV trastuzumab in the
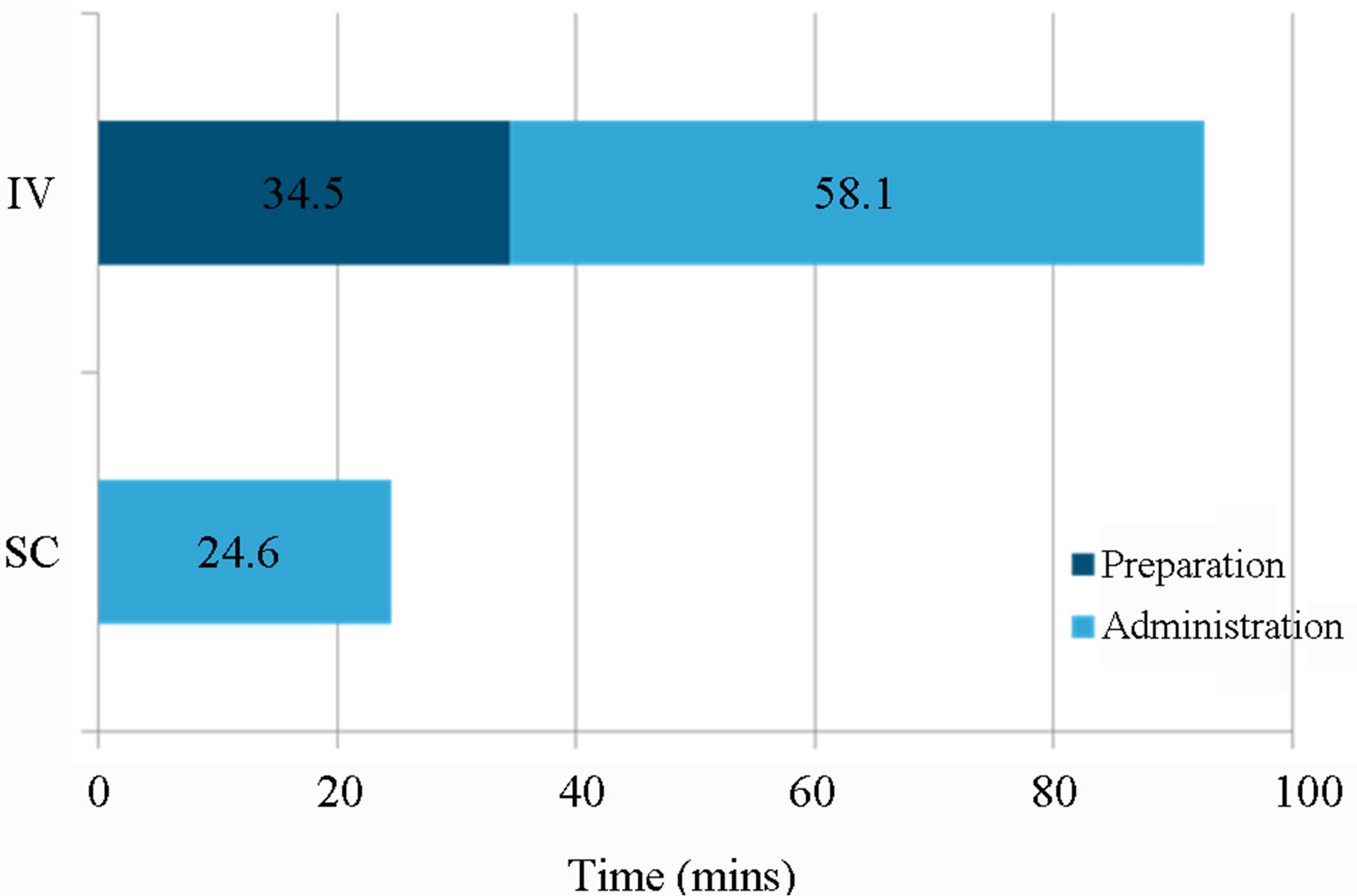
Figure 1. Mean HCP time for preparation and administration of IV vs. SC trastuzumab.
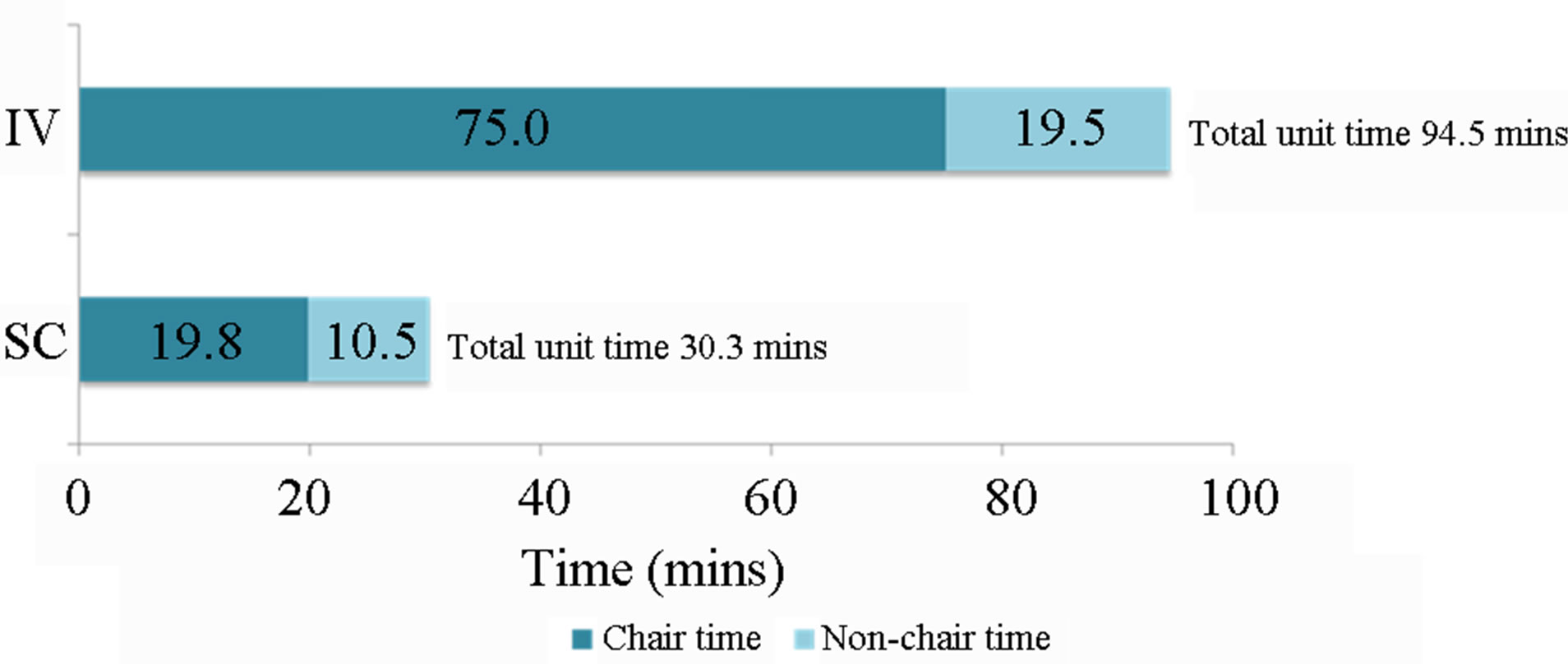
Figure 2. Mean patient time on the unit for IV vs SC administration of trastuzumab.
pharmacy/unit, and £89.74 more to administer IV treatment, compared to SC injection. Delivery of SC trastuzumab saves £111.81 per patient episode (i.e. per cycle) compared to IV administration.
Over a full course (18 cycles) of treatment, trastuzumab administration by subcutaneous injection represents a saving of £2012.58 per patient compared to IV administration.
Extrapolating these data to the population of UK EBC patients presents the opportunity to deliver substantial cost savings: if all 5484 EBC patients eligible for trastuzumab in the UK in 2012 [9] received subcutaneous therapy (i.e. a 100% conversion rate from IV to SC) over a full course of treatment (18 cycles in EBC) and assuming similar acquisition costs for the IV and SC formulations, the savings to the NHS would be approximately £11,000,000.
Further it is reasonable to assume that the preparation and administration times observed in this study would also be applicable to MBC patients eligible for treatment with trastuzumab. Therefore in line with the expected marketing authorisation of the SC formulation, which also includes MBC, factoring in the 2205 patients with MBC who receive on average 17.2 cycles over a course of treatment [10], the total potential savings to the NHS would be valued in excess of £15,000,000.
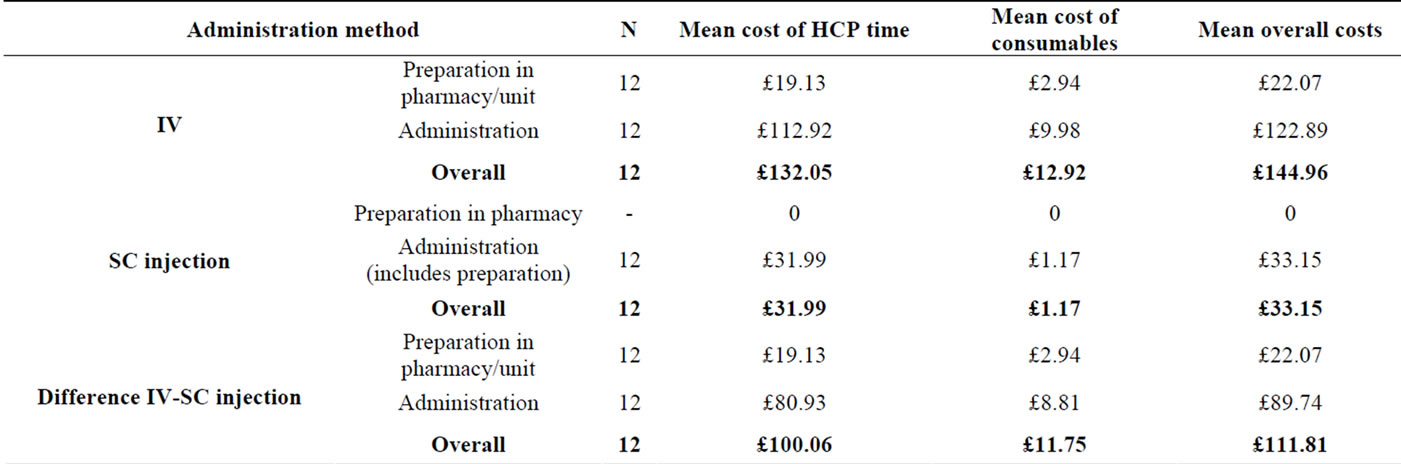
Table 6. Comparison of resource costs per patient episode in the administration of IV vs. SC trastuzumab.
4.2. Limitations
SC injection and IV infusion data were collected from patients participating in the PrefHer study only. These data may not reflect normal clinical practice though one might speculate that treatment times in a clinical trial setting may, if anything, be slightly longer. However, as no observations of IV infusions in normal clinical practice were conducted, any bias is likely to be similar for both IV and SC administration.
Many patients in normal clinical practice have an indwelling venous catheter inserted to facilitate repeated IV infusions. These devices are costly and resource intensive in terms of insertion, maintenance, flushing and operating theatre/anaesthetist/HCP time. Indwelling catheters also give rise to potential complications such as infection and thrombosis, which in turn place additional pressures on healthcare resource utilisation. This study did not measure resource use associated with indwelling venous catheters.
The acquisition cost of the two formulations of trasuzumab was not considered in this analysis. While the cost of the SC formulation is expected to be set at parity with the cost of IV for a patient of mean weight, there may be additional savings from using the SC formulation arising from reduced wastage due to its fixed dosing, in contrast to the weight-based dosing required for the IV route, which can result in wastage of partly-used vials.
It seems likely therefore that the cost and time savings demonstrated will be an underestimate of the total savings achievable in practice with the SC formulation.
Some UK healthcare providers administer systemic cancer therapies in the patient’s own home. SC trastuzumab might potentially reduce HCP contact time in the home setting and, in turn, increase efficiency by increasing the number of care episodes possible per day.
With ever increasing demands on chemotherapy units and oncology pharmacy staff, SC trastuzumab offers the potential to treat more patients with the same resources in less time.
5. Conclusions
Significant cost savings, in terms of HCP time and consumable use, may be realised by adopting SC trastuzumab in favour of IV infusion.
The time savings associated with SC administration could potentially increase capacity in busy oncology units, and at the same time resulted in a better patient experience.
In the UK, a move from IV to SC trastuzumab administration may result in substantial efficiency savings for the National Health Service.
6. Acknowledgements
This study was funded by Roche Products Limited. Roche personnel contributed to the study design and provided support with data collection, data analysis and manuscript preparation, via the contracted services of pH Associates Limited.
REFERENCES
- Office for National Statistics, London, “Cancer Statistics Registrations England (Series MB1) No 42, 2011,” 2013. http://www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations--england--series-mb1-/index.html
- Welsh Cancer Intelligence and Surveillance Unit Cardiff, “Cancer Incidence in Wales, 2007-2011,” 2013. http://www.wales.nhs.uk/sites3/page.cfm?orgid=242&pid=66227
- Office for National Statistics, “Breast Cancer: Incidence, Mortality and Survival, 2010. Female Breast Cancer in England: Incidence and Mortality,” 2012. http://www.ons.gov.uk/ons/dcp171780_280355.pdf
- “Herceptin (Trastuzumab) Summary of Product Characteristics,” 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf
- S. Vescial, A. K. Baumgartner, V. R. Jacobs, M. KiechleBahat, A. Rody, S. Loibl and N. Harbeck, “Management of Venous Port Systems in Oncology: A Review of Current Evidence,” Annals of Oncology, Vol. 19, No. 1, 2008, pp. 9-15. http://dx.doi.org/10.1093/annonc/mdm272
- S. Hamizi, G. Freyer, N. Bakrin, E. Henin, A. Mohtaram, O. Le Saux and C. Falandry, “Subcutaneous Trastuzumab: Development of a New Formulation for Treatment of HER2-Positive Early Breast Cancer,” OncoTargets and Therapy, Vol. 6, 2013, pp. 89-94.
- Department of Health, “Improving Outcomes: A Strategy for Cancer,” 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/135516/dh_123394.pdf.pdf
- Personal Social Services Research Unit (PSSRU) Unit “Costs of Health & Social Care,” 2011. http://www.pssru.ac.uk/archive/pdf/uc/uc2011/uc2011.pdf
- Roche Products Ltd., “Herceptin (Trastuzumab) Algorithm, RXUKHERC00419,” Data on File, 2012.
- Roche Products Ltd., “Herceptin Mean Number of Doses in 1L HER2+ mBC. RXUKDONF00248,” Data on File. 2012.

