Advances in Aging Research
Vol.2 No.1(2013), Article ID:28503,6 pages DOI:10.4236/aar.2013.21005
Effect of aging on the relationship between capillary supply and muscle fiber size
![]()
1Department of Engineering Science, Bioscience and Technology Program, University of Electro-Communications, Tokyo, Japan; *Corresponding Author: kano@pc.uec.ac.jp
2Research Center for Physical Fitness, Sports and Health, Toyohashi University of Technology, Toyohashi, Japan
Received 2 November 2012; revised 1 December 2012; accepted 15 December 2012
Keywords: Skeletal Muscle; Endurance Training; Resistance Training; Capillary Luminal Diameter
ABSTRACT
A quantitative analysis of capillary supply to skeletal muscle is important for understanding the upper limit of the capacity for delivery of oxygen and substrates to muscle cells. It has been well documented that the number of capillaries is altered by several factors including development, aging, and alteration of muscle activity level such as exercise training and inactivation. There is, however, a contradiction in animal studies for aging-related change in the number of capillaries. Human studies using biopsy technique also displayed an inconsistency on that point, in which capillary supply was not influenced or decreased with aging. This review discussed an inconsistency among studies for aging-related change in muscle capillary supply. In conclusion, the relationship between capillary supply and muscle fiber size is similar for both young and elderly population, and the morphological balance between capillaries and each muscle fiber was maintained with advancing age.
1. INTRODUCTION
Capillaries exchange gasses (e.g., oxygen and carbon dioxide) and various substances (e.g., substrates and metabolic products). For this reason, capillaries are also called “exchange vessels”. Capillaries have a structure that is quite suitable for exchanging substances; their single-layer structure is composed of vascular endothelial cells with the smallest thickness of the cell membrane of only 1.5 μm [1]. Normally, two or three endothelial cells mutually contact to form the lumen. These structural characteristics are suitable for going in and out of substances [2]. In addition, the properties are deeply related to the morphological change of the capillary network. The proliferation of vascular endothelial cells composing capillaries means an increase in the number of capillaries; in contrast, degradation of vascular endothelial cells means the disappearance of capillaries [3-6]. The increase and decrease in the number of vessels is a morphological aspect peculiar to capillaries. In this paper, we will describe the morphological changes in capillaries due to aging, and the effects of endurance and resistance training during the aging period.
2. EFFECTS OF MUSCLE FIBER COMPOSITION AND SIZE
First, we describe the layout of capillaries and muscle fibers as anatomical characteristics. The micrograph in Figure 1 depicts a cross-sectional image of the skeletal muscle of a rat, in which capillaries with their lumen open can be observed. The capillaries are arranged so that they surround the muscle fiber, and many muscle fibers are surrounded by three to six capillaries. The number of capillaries surrounding the muscle fiber depends on the composition and the size of the fiber. Basically, more capillaries are observed on slow-twitch fibers than on fast-twitch fibers. Among the fast twitch fibers, Type IIA fiber has more capillaries than Type IIB
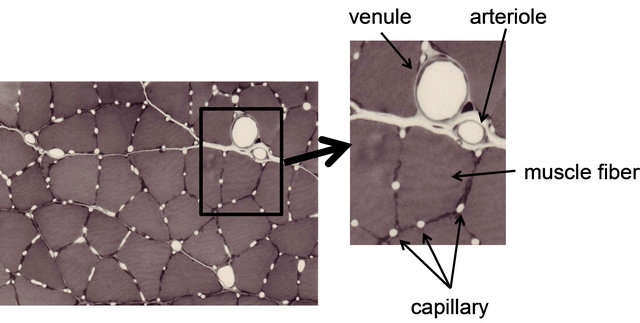
Figure 1. Photomicrograph of microcirculation network in skeletal muscle. This photograph is traversal image of rat skeletal muscle after perfusion-fixation. Dilatation blood vessels and capillaries can be observed. Several capillaries are arranged around each muscle fiber.
fiber does. Green et al. [7] investigated the number of capillaries surrounding each type of muscle fiber of the vastus lateralis muscle of young men and found 5.38 capillaries on Type I fiber, 5.54 capillaries on Type IIA fiber, and 4.20 capillaries on Type IIB fiber. Ahmed et al. [8] investigated the vastus lateralis muscle of young men and women and found a significant correlation between the number of capillaries and the size of muscle fiber. Furthermore, they reported the same correlation for Type I, Type IIA, and Type IIB fibers. These results suggest that the number of capillaries surrounding the muscle fiber is regulated by both the it can be assumed that the number of capillaries is influenced by both the composition and size of muscle fiber. Recently, it is indicated that capillary supply of individual fibers is related to fiber size and oxidative capacity in human and rat skeletal muscle [9].
3. AGING AND SKELETAL MUSCLE CAPILLARIZATION
Various changes take place in the muscular tissue with age [10-13]. The remarkable morphological features are atrophy of individual muscle fiber, a decrease in the number of muscle fibers, and a decrease in the function of muscle that accompanies them. It is commonly assumed that skeletal muscle atrophy occurs because the amount of physical activity decreases with aging and the degree of disuse progresses. Other factors are the lowered function of the neuromuscular junction and a decrease in motor units [14,15]. From the viewpoint of histochemistry in particular, the atrophy of fast-twitch muscle fiber, which is classified as Type II fiber, is remarkable [16]. On the other hand, slow-twitch fibers that were enlarged by the action of compensation can also be observed [17]. However, Carter et al. [18] suggest that the slow twitch muscle undergoes large phenotypic alterations in very old age. Thus, it is necessary to determine what change in capillarization occurs with age. The size of the adjacent muscle fiber and the composition of muscle fiber, which represent metabolic and shrinkage properties, are related to capillaries. Therefore, it can be predicted that skeletal muscle atrophy and changes in metabolic properties cause a decrease in the number of capillaries. However, previous studies that used human and animals had conflicting findings on changes in the number of capillaries due to aging [19]. The following section reviews these studies.
3.1. Studies on Human Skeletal Muscle
Coggan et al. [20] reported changes in the number of capillaries in ten males and females in their aged 20 years and 60 years, and found that the density of capillaries decreased by 25% in the aged groups of both males and females. Similarly, a study that investigated changes in the number of capillaries on a time scale over a period of 12 years (from aged 65 to aged 77 years) [21] indicated that the density of capillaries decreased by 20%. On the other hand, according to reports by Proctor et al. [22] and Denis et al. [23], the number of capillaries of subjects in their aged 60 years did not differ from that of subjects in their aged 20 years. However, Proctor et al. [22] reported that the number of capillaries on Types IIA and IIB fibers decreased significantly in elderly people. This study confirmed that muscular atrophy takes place in Types IIA and IIB fibers in the elderly. These results indicate that, while no remarkable decrease in the number of capillaries was observed in the muscle as a whole, a decrease in capillaries could be observed in only the fast-twitch fibers where atrophy of muscle fibers took place.
3.2. Studies on Animal Skeletal Muscle
Many findings have been reported in previous studies using experiments on animals [9,24-28]. Brown [25] and Mitchell et al. [26] investigated changes in the number of capillaries with age in the soleus muscle (mostly occupied by slow-twitch fibers) and the extensor digitorum longus muscle (mostly fast-twitch fibers) of a rat and reported that no decrease in the number of capillaries with age was observed in either type of muscle. However, Degens et al. [27] demonstrated the disappearance of capillaries is related to aging in the plantaris muscle of a rat. In contrast, Davidson et al. [28] reported an increase in the number of capillaries with age in the soleus muscle and extensor digitorum longus muscle of mice. Although in this report the authors pointed out the involvement of angiogenic factors or of the decreased down-regulation of those factors as the cause of the increase in the number of capillaries, the mechanism has not yet been clarified. Kano et al. [24] investigated capillaries of an aged rat for individual types of muscle fibers and clarified that the number of capillaries surrounding the muscle fiber is regulated by muscle fiber size, regardless of age (Figures 2 and 3). Furthermore, this relationship was recognized in both slow-twitch fibers (the soleus muscle) and fas-twitch fibers (the plantaris muscle). As described previously, in the aging period, selective atrophy does occur in some muscle fibers, but some muscle fibers are enlarged by compensatory action on the atrophic muscles (see Figure 2). When viewed on the level of individual muscle fibers, this phenomenon is like a mixture of physical inactivity and resistance training; in this case, evaluation of the number of capillaries in the entire muscle may lead to contradictory results. Therefore, evaluating the number of capillaries in the aged period for each type of muscle fiber will be useful for clarifying the effect of training and the aging phenomenon.
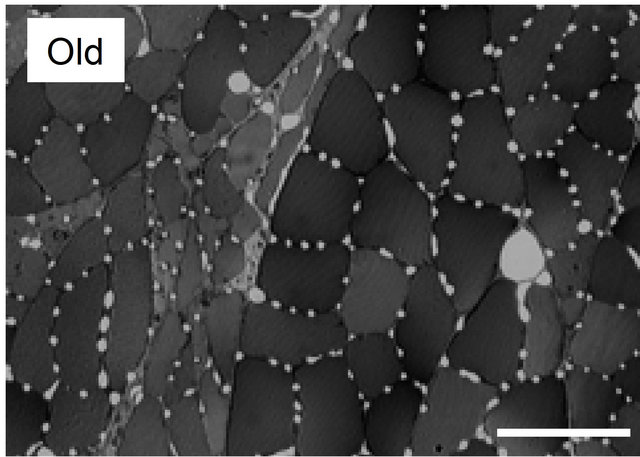
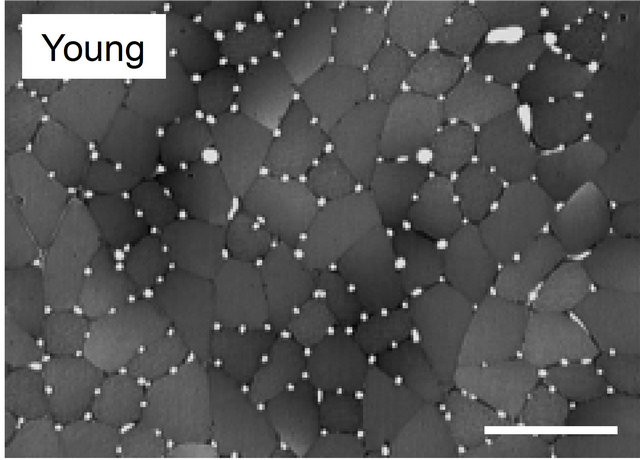
Figure 2. Light micrographs of transversely sectioned perfusion-fixed muscle for plantaris muscle in young and old Wistar rats. Appearance of atrophied and hypertrophied fibers was observed in the muscles of old rats, that is a characteristic feature of early stage of aging, so that fiber size was distributed in a wider range in old animals than in young ones. Bar = 100 μm.
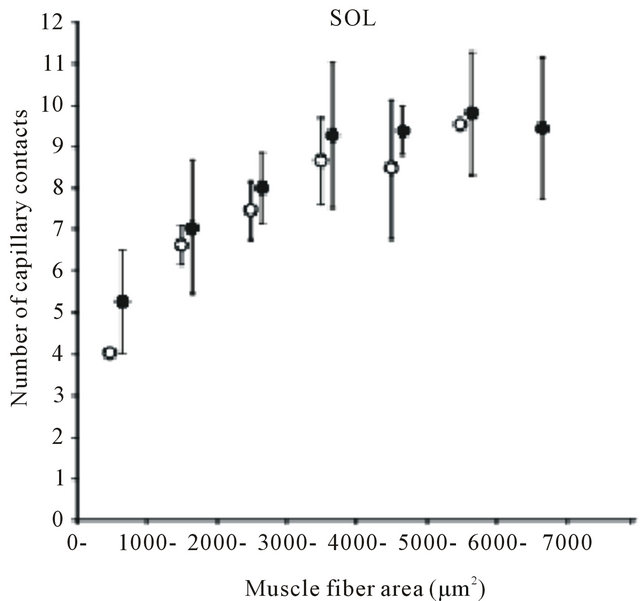
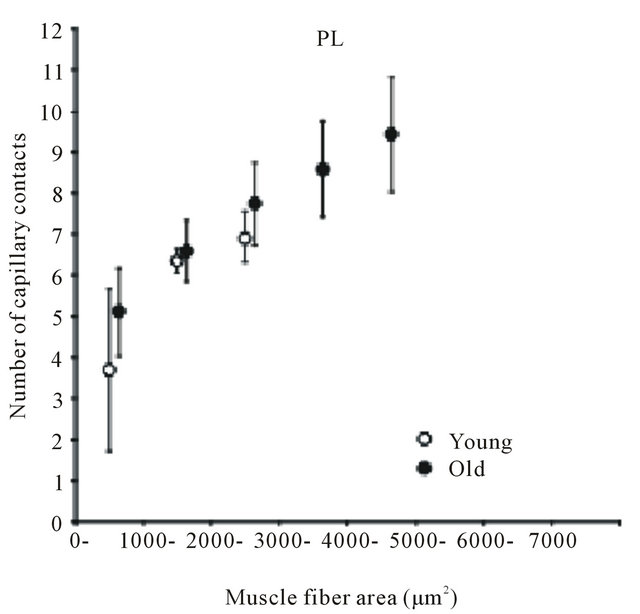
Figure 3. Diagram showing relation between number of capillaries around a fiber and muscle fiber area. Each point represents the mean value of all fibers having a cross-sectional area within a 1000 μm2 interval. SOL: soleus muscle. PL: plantaris muscle. Results are expressed as means ± SD.
Furthermore, the size of the capillary lumen is a morphological factor that influences blood flow resistance and oxygen diffusion ability. Some research has indicated that the size of capillary lumens in young rats is not homogeneous [29-31]. Takahara et al. [31], using the microcorrosion casts method, reported capillary diameters ranging from 3.1 μm to 13.2 μm. Our previous studies, using the perfusion fixation method, indicated capillary diameters ranging from 2 μm to 10 μm [29,30]. We have reported that capillary luminal size decreases in atrophied skeletal muscles of tail-suspended rats, i.e. reduced mechanical stress on hind limbs [29]. The narrower capillaries in the atrophied muscle could be related to an increase in the blood flow resistance. In skeletal muscle of older rats, the mean of capillary luminal diameter did not statistically differ among young and old rats. Also, the range and distribution of the capillary luminal size are similar for young and old rats [24]. These data indicate that the capillary luminal area is stable with aging. These different findings between the tail-suspended and the older rats may be related to the hemodynamics of locomotory muscles. McDonald et al. [32] reported that blood flow to the antigravity soleus muscle was reduced during hind-limb suspension. In contrast, resting blood flow is maintained in skeletal muscle of old rats [33]. It has been suggested that capillary growth is initiated by mechanical factors related to blood flow [34]. Therefore, it is likely that variable blood flow contributes to the morphological changes of capillary lumens.
4. EXERCISE TRAINING AND ADAPTATION OF CAPILLARIES IN THE AGED PERIOD
In considering what response the capillaries of muscular tissue have to endurance training and resistance training in the aged period, one conclusion is that the “adaptability of capillaries is maintained in the aged period”. Most research indicates that capillaries develop at the same level as in the adolescence in response to training in the aged period [19,35,36]. Proctor et al. [22] investigated 60-year-old men who habitually performed endurance training. They found that the density of capillaries of these men was significantly higher than that of the control group of the same age, and that the observed value was also higher than that of young people who performed endurance training. However, as a matter of great interest, no effect of training was recognized in the number of capillaries surrounding Type IIa fibers. Similarly, while atrophy of slow-twitch fibers was suppressed in elderly people who were training, atrophy of fasttwitch fibers with age was recognized. These results confirmed that endurance training in the aged period does not involve fast-twitch fibers, and this fact should be considered when determining the method of training during the aged period.
Hepple et al. [37] investigated elderly males aged 65 to 73 years, and observed the enlargement of muscle fibers and a new generation of capillaries when resistance training of three sessions per week was performed for nine weeks at a resistance of 6 to 12 RM. The increase in the number of capillaries at this time corresponded to the degree of enlargement of muscle fibers.
5. CONCLUSION
The present article reviews the relationship between aging and capillaries from the viewpoint of anatomy. A significant relationship exists between the size of muscle fibers and the morphological features of capillaries; Figure 4 depicts these relationships schematically. A strong correlation exists between the metabolic demands of oxygen and nutrients (i.e. the cross-sectional area of muscle fibers) and its supply limit (i.e. the number of capillaries). While many muscle fibers cause atrophy with age, the number of capillaries also decreases. However, the number of capillaries increases on the muscle fibers that are enlarged by compensatory response. The relationship between supply and demand is thus clearly established even in muscular tissue of an elderly period. Endurance training increases the volume of mitochondria without increasing the cross-sectional area of muscle fibers. In this case, the metabolic demand may be regulated by the volume of mitochondria, rather than muscle fibers. As a result, the ability to supply energy to muscle fibers increases, and aerobic performance capacity improves. Furthermore, resistance training occurs for muscle fiber hypertrophy, depending on the contents of the
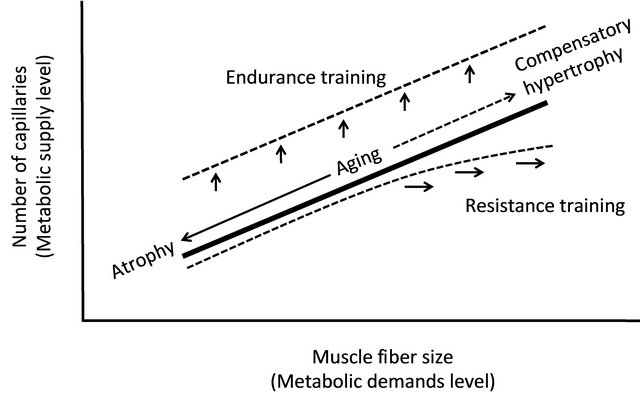
Figure 4. Relationship between the size of muscle fibers and the number of capillaries. A significant correlation exists between the metabolic demands level (i.e. the size of muscle fibers) and its supply limit (i.e. the number of capillaries). Endurance training increases the number of capillaries without increasing the cross-sectional area of muscle fibers. Morphological balance between capillary and muscle fiber are maintained by resistance training. When an extremely hypertrophy occurs, however, the supply from capillaries becomes insufficient for enlarging muscle fibers.
training program, but this situation occurs when the supply from capillaries becomes insufficient for enlarging muscle fibers. In this case, a decrease in endurance potential can be expected. However, it is difficult in the aged period to perform the degree of training that causes extreme enlargement of muscle fibers; therefore, the studies that report an insufficient increase in the number of capillaries for enlarging muscle fibers are limited to those addressed to body builders [38,39]. Thus, it seems that resistance training during the aged period accompanies the development of capillaries.
![]()
![]()
REFERENCES
- Hernandez, N., Torres, S.H., Finol, H.J. and Vera, O. (1999) Capillary changes in skeletal muscle of patients with essential hypertension. Anatomical Record, 256, 425- 432. doi:10.1002/(SICI)1097-0185(19991201)256:4<425::AID-AR9>3.0.CO;2-X
- Vink, H. and Duling, B.R. (1996) Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circulation Research, 79, 581-589. doi:10.1161/01.RES.79.3.581
- Hudlicka, O. (1985) Development and adaptability of microvasculature in skeletal muscle. Journal of Experimental Biology, 115, 215-228.
- Brown, M.D. and Hudlicka, O. (2003) Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: Involvement of VEGF and metalloproteinases. Angiogenesis, 6, 1-14. doi:10.1023/A:1025809808697
- Hudlicka, O. and Brown, M.D. (2009) Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: Role of shear stress, nitric oxide and vascular endothelial growth factor. Journal of Vascular Research, 46, 504-512. doi:10.1159/000226127
- Egginton, S. (2009) Invited review: Activity-induced angiogenesis. Pflügers Archeiv, 457, 963-977. doi:10.1007/s00424-008-0563-9
- Green, H., Goreham, C., Ouyang, J., Ball-Burnett, M. and Ranney, D. (1999) Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. American Journal of Physiology, 276, R591-596.
- Ahmed, S.K., Egginton, S., Jakeman, P.M., Mannion, A.F. and Ross, H.F. (1997) Is human skeletal muscle capillary supply modelled according to fibre size or fibre type? Experimental Physiology, 82, 231-234.
- Wüst, R.C., Gibbings, S.L. and Degens, H. (2009) Fiber capillary supply related to fiber size and oxidative capacity in human and rat skeletal muscle. Advances in Experimental Medicine and Biology, 645, 75-80. doi:10.1007/978-0-387-85998-9_12
- Sakuma, K. and Yamaguchi, A. (2010) Molecular mechanisms in aging and current strategies to counteract sarcopenia. Current Aging Science, 3, 90-101. doi:10.2174/1874609811003020090
- Narici, M.V. and Maffulli, N. (2010) Sarcopenia: Characteristics, mechanisms and functional significance. British Medical Bulletin, 95, 139-159. doi:10.1093/bmb/ldq008
- Thompson, L.V. (2009) Age-related muscle dysfunction. Experimental Gerontology, 44, 106-111. doi:10.1016/j.exger.2008.05.003
- Faulkner, J.A., Larkin, L.M., Claflin, D.R. and Brooks, S.V. (2007) Age-related changes in the structure and function of skeletal muscles. Clinical and Experimental Pharmacology and Physiology, 34, 1091-1096. doi:10.1111/j.1440-1681.2007.04752.x
- Luff, A.R. (1998) Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Annals of the New York Academy of Sciences, 854, 92-101. doi:10.1111/j.1749-6632.1998.tb09895.x
- Larsson, L. (1995) Motor units: Remodeling in aged animals. Journal of Gerontology A: Biological Science and Medical Science, 50, 91-95.
- Lexell, J. (1995) Human aging, muscle mass, and fiber type composition. Journal of Gerontology A: Biological Science and Medical Science, 50, 11-16.
- Hepple, R.T., Ross, K.D. and Rempfer, A.B. (2004) Fiber atrophy and hypertrophy in skeletal muscles of late middle-aged Fischer 344 x Brown Norway F1-hybrid rats. Journal of Gerontology A: Biological Science and Medical Science, 59, 108-117.
- Carter, E.E., Thomas, M.M., Murynka, T., et al. (2010) Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Experimental Gerontology, 45, 662-670. doi:10.1016/j.exger.2010.04.001
- Harris, B.A. (2005) The influence of endurance and resistance exercise on muscle capillarization in the elderly: A review. Acta Physiologica Scandinavica, 185, 89-97. doi:10.1111/j.1365-201X.2005.01461.x
- Coggan, A.R., Spina, R.J., King, D.S., et al. (1992) Skeletal muscle adaptations to endurance training in 60 to 70- yr-old men and women. Journal of Applied Physiology, 72, 1780-1786.
- Frontera, W.R., Hughes, V.A., Fielding, R.A., Fiatarone, M.A., Evans, W.J. and Roubenoff, R. (2000) Aging of skeletal muscle: A 12-yr longitudinal study. Journal of Applied Physiology, 88, 1321-1326.
- Proctor, D.N., Sinning, W.E., Walro, J.M., Sieck, G.C. and Lemon, P.W. (1995) Oxidative capacity of human muscle fiber types: Effects of age and training status. Journal of Applied Physiology, 78, 2033-2038.
- Denis, C., Chatard, J.C., Dormois, D., Linossier, M.T., Geyssant, A. and Lacour, J.R. (1986) Effects of endurance training on capillary supply of human skeletal muscle on two age groups (20 and 60 years). Journal of Physiology (Paris), 81, 379-383.
- Kano, Y., Shimegi, S., Furukawa, H., Matsudo, H. and Mizuta, T. (2002) Effects of aging on capillary number and luminal size in rat soleus and plantaris muscles. Journal of Gerontology A: Biological Science and Medical Science, 57, B422-427.
- Brown, M. (1987) Change in fibre size, not number, in ageing skeletal muscle. Age and Ageing, 16, 244-248. doi:10.1093/ageing/16.4.244
- Mitchell, M.L., Byrnes, W.C. and Mazzeo, R.S. (1991) A comparison of skeletal muscle morphology with training between young and old Fischer 344 rats. Mechanisms of Ageing and Development, 58, 21-35. doi:10.1016/0047-6374(91)90117-I
- Degens, H., Turek, Z., Hoofd, L., van’t Hof, M.A. and Binkhorst, R.A. (1993) Capillarisation and fibre types in hypertrophied m. plantaris in rats of various ages. Respiration Physiology, 94, 217-226. doi:10.1016/0034-5687(93)90049-G
- Davidson, Y.S., Clague, J.E., Horan, M.A. and Pendleton, N. (1999) The effect of aging on skeletal muscle capillarization in a murine model. Journal of Gerontology A: Biological Science and Medical Science, 54, B448-451. doi:10.1093/gerona/54.10.B448
- Kano, Y., Shimegi, S., Takahashi, H., Masuda, K. and Katsuta, S. (2000) Changes in capillary luminal diameter in rat soleus muscle after hind-limb suspension. Acta Physiologica Scandinavica, 169, 271-276. doi:10.1046/j.1365-201x.2000.00743.x
- Kano, Y., Shimegi, S., Masuda, K., Ohmori, H. and Katsuta, S. (1997) Morphological adaptation of capillary network in compensatory hypertrophied rat plantaris muscle. European Journal of Applied Physiology and Occupational Physiology, 75, 97-101. doi:10.1007/s004210050132
- Takahara, Y., Senda, M., Hashizume, H., Yagata, Y. and Inoue, H. (1996) Capillary architecture in the skeletal muscles in the rat hind limb. Acta Medica Okayama, 50, 211-218.
- McDonald, K.S., Delp, M.D. and Fitts, R.H. (1992) Fatigability and blood flow in the rat gastrocnemius-plantaris-soleus after hindlimb suspension. Journal of Applied Physiology, 73, 1135-1140.
- Irion, G.L., Vasthare, U.S. and Tuma, R.F. (1988) Preservation of skeletal muscle hyperemic response to contraction with aging in female rats. Experimental Gerontology, 23, 183-188. doi:10.1016/0531-5565(88)90005-8
- Hudlicka, O. (1998) Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation, 5, 7-23.
- Terjung, R.L., Zarzeczny, R. and Yang, H.T. (2002) Muscle blood flow and mitochondrial function: Influence of aging. International Journal of Sport Nutrition and Exercise Metabolism, 12, 368-378.
- McCall, G.E., Byrnes, W.C., Dickinson, A., Pattany, P.M. and Fleck, S.J. (1996) Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. Journal of Applied Physiology, 81, 2004- 2012.
- Hepple, R.T., Mackinnon, S.L., Thomas, S.G., Goodman, J.M. and Plyley, M.J. (1997) Quantitating the capillary supply and the response to resistance training in older men. Pflügers Archeiv, 433, 238-244. doi:10.1007/s004240050273
- Bell, D.G. and Jacobs, I. (1990) Muscle fibre area, fibre type & capillarization in male and female body builders. Canadian Journal of Sport Science, 15, 115-119.
- Tesch, P.A., Thorsson, A. and Kaiser, P. (1984) Muscle capillary supply and fiber type characteristics in weight and power lifters. Journal of Applied Physiology, 56, 35- 38.

